Abstract
Schistosoma spindale and Schistosoma indicum are the most prevalent causes of visceral schistosomosis among bovines of India. The infection causes morbidity, mortality, reduced productivity and poor subsequent reproductive performance and it is economically significant in producing animals. The high prevalence of S. spindale among slaughtered bovines of South India warrants the need to devise improved diagnostics for specific and timely detection of infection for appropriate treatment and control. The present study focused on analysing the excretory–secretory proteins of S. spindale and identification of the immunodominant polypeptide fractions of this antigen. The major polypeptide antigens corresponding to approximately 28 and 66 kDa size could be detected as highly immunogenic during natural infections in cattle. Consistent blotting pattern was observed with known schistosome positive bovine sera and specificity of the antigen was also ascertained with sera of amphistome and strongyle positive animals.
1. Introduction
Schistosomosis has been recognized as one of the major parasitic diseases of producing animals and human beings. As many as 10 different species of schistosomes infect cattle, the most important being Schistosoma bovis, Schistosoma mattheei and Schistosoma curassoni in Africa and Schistosoma spindale, Schistosoma indicum, Schistosoma nasalis and Schistosoma japonicum in Asia. De Bont and Vercruysse (Citation1997) speculated that at least 165 million cattle are infected with schistosomes worldwide, while another 530 million live in endemic areas. In South India, the prevalence of S. spindale was estimated to be 68% in Karnataka (Sumanth et al. Citation2004) and 48% in Kerala (Lakshmanan et al. Citation2011), based on abattoir surveys. The pathogenesis of the disease is strongly related to host immunity to schistosomes. Chronically infected cattle develop immunity against schistosomes thus leading to reduced, as well as, fluctuating egg excretion in the faeces (Aradaib et al. Citation1995). Moreover during acute infection, eggs are often masked by the high mucus content of the faeces and they easily hatch out in the presence of water. An important implication of both is that routine coprological examination succeeds in detecting eggs in a minority of cases only. This has undoubtedly led to the underestimation of infection among the dairy cattle of Kerala State, as is evident from the Animal Disease Surveillance Report (AHD Citation2008) which documented schistosomosis in only 0.38% of total cases of parasitism in cattle of Kerala. This data are based on coprological examination and are exist a wide difference between abattoir surveys. Besides pathology in animals, it is long known that cercariae of S. spindale are a common cause of dermatitis in human beings in Asia, especially in India and that the fresh water snail, Indoplanorbis exustus, is a major source of infection (Nithiuthai et al. Citation2004). In this context, ante-mortem detection of intestinal schistosomosis among cattle population of the State is, undoubtedly, the need of the hour. An efficient diagnostic tool providing unequivocal evidence of infection in living animals is, perhaps, the key to formulate and deliver control measures to the target population. The utility of excretory–secretory (ES) proteins as diagnostic and vaccine candidates for schistosomosis has been a focus of medical research since long. These proteins include products actively secreted through secretory pathways, digestive enzymes emanating from the intestine of adult worms, as well as, the uterine contents released by female worms along with eggs, also have been reported to be a rich source of potential immunogens (Hewitson et al. Citation2009). ES proteins of S. spindale have neither been characterized nor incorporated as diagnostic antigen candidates in any serodiagnostic assays. Hence a study was designed to study the protein and immunoprofile of ES antigens (ESAs) of S. spindale.
2. Materials and methods
2.1. Antigen preparation
Adult S. spindale worms were recovered from the mesentery of cattle slaughtered in local abattoir as per the method of Lakshmanan et al. (Citation2011). The adult schistosomes were morphologically identified as S. spindale based on the tegumental features and eggs (Kumar Citation1999). ESAs of S. spindale were prepared based on the method of Liu et al. (Citation2009) with some modifications. Eight hundred adult worms were soaked in 2 ml of 1X PBS for 2 hours at room temperature followed by overnight incubation at 4°C. The viability of the worms was checked under a stereozoom microscope to ensure that all worms were intact and live. After removing the flukes under sterile conditions, the culture fluid was centrifuged at 10,000 × g for 30 min at 4°C and the supernatant used as ESA. The protein concentration of ESA was estimated using protein estimation kit by Lowry’s method (Merck GeNeiTM, Bangalore) in a spectrophotometer (Lambda 750, Perkin Elmer) at 660 nm and was further aliquoted and stored at −20°C.
2.2. Serum samples
Sera collected from slaughtered animals in which the mesenteries harboured adult schistosomes served as known positive bovine sera (n = 30). The serum collected from worm negative animals with a history of being reared in confined farms of non-endemic areas served as known negative control (n = 30). Besides, samples collected from cattle infected with amphistomes and strongyles, as revealed in routine coprological examination, served as specificity controls (n = 10).
2.3. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE)
The antigen was analysed in one-dimensional SDS–PAGE as per Laemmli (Citation1970) in a vertical electrophoresis apparatus (Vertical Minigel system, Merck GeNei, Bangalore). The standard medium range protein molecular weight marker (Merck GeNei, Bangalore) was run along with the samples in separate wells. The polypeptide fractions of the ESA were resolved in 12% gel and stained Coomassie Brilliant Blue. The protein profile was analysed using Gel Analyzer, Version 2010a.
2.4. Western blotting
The duplicate unstained portion of this gel was transferred manually onto nitrocellulose membranes (NCM) as per the technique described by Towbin et al. (Citation1979), with minor modifications. Capillary transfer of the resolved proteins onto NCM was achieved by manual blotting overnight at room temperature. The transfer of proteins was checked by reversible staining using Ponceau-S stain. After blocking with 5% non-fat milk, the NCM strips were treated with 1:100 dilution each of known schistosome positive bovine sera, known negative sera and specificity control sera. Later, NCM strips were washed with 0.05% Tween-20 in 1X PBS (PSBT) and incubated at 37°C for one hour with horse radish peroxidase conjugated rabbit anti-bovine IgG (Merck GeNei, Bangalore), at a dilution of 1:2000 in blocking buffer. The strips were visualized for the development of blot patterns by immersing in chromogenic visualization solution, containing diaminobenzidine, with mild rocking at room temperature. The reaction was terminated by washing the membrane with distilled water which was then air dried and photographed.
3. Results
The protein content of the ESA preparation was estimated for different concentration of live adult schistosomes to arrive at the optimum worm concentration. The relationship between protein concentration and worm count per ml of PBS could be explained by the logarithmic equation as follows:
The protein concentrations of ESA prepared by incubating adult schistosomes in 1 ml PBS (pH 7.4) were found to increase with the worm concentration up to 200 worms per ml PBS. The protein concentrations were estimated after six months of storage in −20°C and were found to be unaltered.
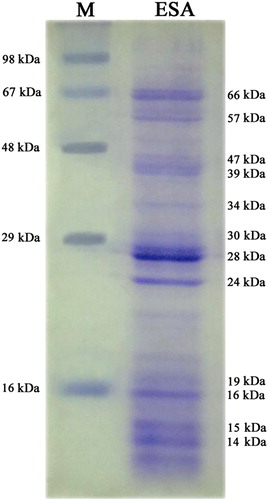
One-dimensional electrophoretic separation of ESA yielded several bands ranging from 14 to 66 kDa upon Coomassie Brilliant Blue staining. Five polypeptides with approximate molecular weights of 16, 24, 28, 39 and 66 kDa were the main bands detected, of which, 28 kDa size was the most abundant one. Furthermore, minor bands of approximately 14, 15, 19, 30, 34, 47 and 57 kDa were also identified ().
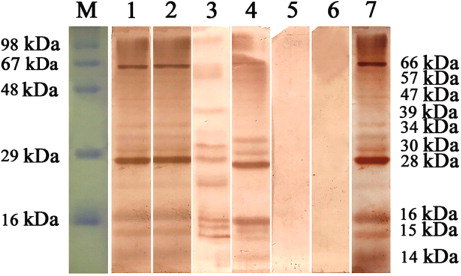
When blotting was performed with positive bovine sera (n = 30), two antigens with molecular weights of 28 and 66 kDa were strongly recognized (, lanes 1–4 and 7). In addition, antigens of 14, 15, 16, 30, 34, 39, 47 and 57 kDa showed lower reactivity pattern with the positive sera (n = 24). The intensity of the bands could be visually graded as ++++ (28 kDa), +++ (66 kDa) and + (reduced intensity bands). To ascertain the specificity of ESA, immunoblotting was done using this antigen with known schistosome negative sera and sera from cattle positive for mixed amphistome and strongyle infection. Bovine amphistomosis and strongylosis were selected since these were the most predominant helminth parasitic infection among adult bovines of Kerala. Immunoblots revealed consistent bands with schistosome positive sera, while known negative sera and sera from amphistome and strongyle positive animals failed to yield positive blots (, Lane 6).
4. Discussion
Analysis of schistosome antigens contributes to a better understanding of immunoprophylaxis and design of improved immunodiagnostics. Collaborative studies on antigens of human schistosomes have proved that defined or characterized antigens showed advantage over crude antigens in immunodiagnosis of S. japonicum (Mott et al. Citation1987). ES proteins of S. spindale have not been analysed yet. The ESAs of several other Schistosome spp. were prepared by different in vitro culture techniques in artificially supplemented media (Call et al. Citation1995; Cutts & Wilson Citation1997; Perez-Sanchez et al. Citation2006; El-Ridi & Tallima Citation2009). But we adopted an alternate strategy of incubating adult flukes in PBS (pH 7.4) for definite time–temperature combinations. This protocol was followed, since the excretion and secretion behaviour of the parasites could be altered due to removal from natural host tissues and in vitro maintenance in chemical mixture, as reviewed by Morphew et al. (Citation2007). Liu et al. (Citation2009) had also characterized the ES proteome of adult S. japonicum flukes by adopting a similar strategy with further concentration and purification. The protein concentrations of ESA were found to increase with the worm concentration up to 200 worms per ml PBS. Further increase in worm concentration had no effect on protein concentration which could be due to the fact that adult flukes were not provided with nutrient supplementation to support their metabolism and detoxification mechanisms. Nevertheless, all adult flukes remained intact and live after 24 hours.
Several protein fractions of molecular weights corresponding to approximately 14–66 kDa could be detected by SDS–PAGE of S. spindale ESA. Call et al. (Citation1995) had reported several bands ranging from 10 to 30 kDa in SDS–PAGE gels and more than 100 reactive bands ranging from 14 to 200 kDa, upon immunoblotting of S. mansoni ESA. Characterization of ES proteome of S. bovis had also shown that 28 kDa glutathione S-transferase (GST), 47/53 kDa surface protein-fluke, 22.6 kDa tegumental antigen and serum protein inhibitors were the antigenic components while, 15 kDa fatty acid binding protein (FABP), 16/19 kDa superoxide dismutase, 59 kDa lysozyme, 66/80 kDa thioredoxin glutathione reductase were the non-antigenic components (Perez-Sanchez et al. Citation2006).
In the present study, 28 and 66 kDa were found to be the most immunodominant fractions of S. spindale ESA when blotted with infected bovine serum. The 28 kDa fraction could correspond to the 28 kDa GST reported earlier by Perez-Sanchez et al. (Citation2006) for S. bovis. The other immunodominant polypeptide of approximately 66 kDa could correspond to the dominant 67 kDa protein in ESA of S. mansoni demonstrated to be localized in the gastrodermis of parasite gut by Cutts and Wilson (Citation1997). Less prominent bands in the immunoblot profile obtained in the present work included 14, 15, 16, 30, 34, 39, 47 and 57 kDa. Thaumaturgo et al. (Citation2001) identified a highly immunogenic 14 kDa component in S. mansoni ESA and later Liu et al. (Citation2009) characterized a 14 kDA FABP in ES proteome of S. japonicum, which along with GST were reviewed to be potent anti-schistosome vaccine candidates. A consistent immunoblotting pattern obtained with all known schistosome positive sera and the absence of such blotting pattern with known schistosome negative as well as with amphistome and strongyle positive bovine sera, further confirmed the diagnostic specificity of ESA. Further, the results also suggested the possibility of probing S. spindale ESA for protective vaccine candidates. The present study is a preliminary attempt to analyse the immunogenic fractions in ESA of S. spindale. Only limited work has been attempted in immunodiagnosis of S. spindale using whole worm antigens (Sumanth et al. Citation2003, Citation2004; Divya et al. Citation2012; Murthy et al. Citation2013). The superiority of ESA in serodiagnosis has been observed by several workers for detecting other trematode infections as well viz., Clonorchis sinensis (Choi et al. Citation2003), Paragonimus sp. (Narain et al. Citation2005), F. hepatica (Awad et al. Citation2009), Opisthorchis felineus (Gomez-Morales et al. Citation2013) and Paramphistomum cervi (Anuracpreeda et al. Citation2013). The present findings of lack of cross reactivity strongly favour the incorporation of ESA in different diagnostic assays for S. spindale. The work also throws light into the possibility of development of an enzyme immunotransfer blot assay using ESA as a specific diagnostic tool for detection of S. spindale antibodies among bovines in field conditions.
Acknowledgements
The authors acknowledge the facilities provided by Kerala Veterinary and Animal Sciences University, Wayanad and Dean, College of Veterinary and Animal Sciences, Mannuthy, Kerala to carry out the study.
Disclosure statement
All the authors hereby declare that there is no conflict of interest.
References
- [AHD] Department of Animal Husbandry. 2008. Animal disease surveillance annual report. Kerala: Department of Animal Husbandry, Government of Kerala; 156 p.
- Anuracpreeda P, Poljaroen J, Chotwiwatthananku C, Tinikul Y, Sobhon P. 2013. Antigenic components, isolation and partial characterization of excretion–secretion fraction of Paramphistomum cervi. Exp Parasitol. 133:327–333.
- Aradaib IE, Omer OH, Abbas BB, Bushara HO, Elmalik KH, Saad AM, Osburn BI, Taylor MG. 1995. Schistosoma bovis whole egg antigen did not protect zebu calves against experimental schistosomiasis. Prevent Vet Med. 21:339–345. 10.1016/0167-5877(94)00385-V
- Awad WS, Ibrahim AK, Sahb FA. 2009. Using indirect ELISA to assess different antigens for the serodiagnosis of Fasciola gigantica infection in cattle, sheep and donkeys. Res Vet Sci. 86:466–471. 10.1016/j.rvsc.2008.08.009
- Call JL, Pilcher JB, Freeman GL, Tsang VCW. 1995. Serum-free culturing of adult Schistosoma mansoni in dialysis bags for the production of excretory–secretory antigens. J Parasitol. 81:742–746. 10.2307/3283965
- Choi MH, Park C, Li S, Hong ST. 2003. Excretory–secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Kor J Parasitol. 41:35–39.
- Cutts L, Wilson RA. 1997. The protein antigens secreted in vivo by adult male Schistosoma mansoni. Parasitology. 114:245–255. 10.1017/S0031182096008438
- De Bont J, Vercruysse J, 1997. The epidemiology and control of cattle schistosomiasis. Parasitol Today. 13:255–262. 10.1016/S0169-4758(97)01057-0
- Divya SP, Lakshmanan B, Subramanian H. 2012. Prevalence of schistosomiasis in cattle. Indian Vet J. 89:81–82.
- El-Ridi R, Tallima H, 2009. Schistosoma mansoni ex vivo lung-stage larvae excretory–secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 27:666–673. 10.1016/j.vaccine.2008.11.039
- Gomez-Morales MA, Ludovisi A, Amati M, Pozio E. 2013. Validation of an excretory/secretory antigen based ELISA for the diagnosis of Opisthorchis felineus infection in human from low trematode endemic areas. PLOS ONE. 8:2267.
- Hewitson JP, Grainger JR, Maizels RM. 2009. Helminth immunoregulation: the role of secreted proteins in modulating host immunity. Mol Biochem Parasitol. 167:1–11. 10.1016/j.molbiopara.2009.04.008
- Kumar, V. 1999. Trematode infections and diseases of man and animals. Dordrecht: Kluwer Academic Publishers; 362 pp.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. 10.1038/227680a0
- Lakshmanan B, Rauoof A, Fawaz M, Subramanian H. 2011. Abattoir survey of Schistosoma spindale infection in Thrissur. J Vet Anim Sci. 4:53–54.
- Liu F, Cui SJ, Hu W, Feng Z, Wang ZG, Han ZG. 2009. Excretory–secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Mol Cell Proteomics. 8:1236–1251.
- Morphew RM, Wright HA, LaCourse EJ, Woods DJ, Brophy PM. 2007. Comparative proteomics of excretory–secretory proteins released by the liver fluke Fasciola hepatica in sheep host bile and during in vitro culture ex host. Mol Cell Proteomics. 6:963–972. 10.1074/mcp.M600375-MCP200
- Mott KE, Dixon H, Carter CE, Garcia E, Ishii A, Matsuda H, Mitchell G, Owhashi M, Tanaka H, Tsang VC. 1987. Collaborative study on antigens for immunodiagnosis of Schistosoma japonicum infection. Bull World Health Org. 65:233–244.
- Murthy GSS, D’Souza PE, Isloor KS. 2013. Evaluation of a polyclonal antibody based sandwich ELISA for the detection of faecal antigens in Schistosoma spindale infections in bovines. J Parasite Dis. 37:47–51.
- Narain K, Devi KR, Mahanta J. 2005. Development of enzyme-linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J Med Res. 121:739–746.
- Nithiuthai S, Anantaphruti MT, Waikagul J, Gajadhar A. 2004. Waterborne zoonotic helminthiasis. Vet Parasitol. 126:167–193.
- Perez-Sanchez R, Ramajo-Hernandez A, Ramajo-Martin V, Oleaga A. 2006. Proteomic analysis of the tegument and excretory–secretory products of adult Schistosoma bovis worms. Proteomics. 6:S226–S236.
- Singh A, Singh A, Chaudhri SS. 2004. Visceral schistosomiasis of domestic animals in India: humoral immune status of infected sheep and goats against major polypeptide antigens of Schistosoma indicum and S. spindale. Parasite Immunol. 26:167–175. 10.1111/j.0141-9838.2004.00697.x
- Sumanth S, D’Souza PE, Jagannath MS. 2003. Immunodiagnosis of nasal and visceral schistosomiasis in cattle by Dot-ELISA. Indian Vet J. 80:495–498.
- Sumanth S, D’Souza PE, Jagannath MS. 2004. A study of nasal and visceral schistosomiasis in cattle slaughtered at an abattoir in Bangalore, South India. Revue Scientifique et Technique de l Office International Des Epizooties. 23:937–942.
- Thaumaturgo N, Vilar MM, Diogo CM, Edelenyi R, Tendler M. 2001. Preliminary analysis of Sm14 in distinct fractions of Schistosoma mansoni adult worm extract. Memórias do Instituto Oswaldo Cruz. 96:79–83.
- Towbin H, Staehelint T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci. 76:4350–4354. 10.1073/pnas.76.9.4350