Abstract
Chlorpyrifos is widely used to control agricultural pests associated with fruit, nut and vegetable crops, despite its toxic effects and potential brain alterations in aquatic organisms. This study was carried out to determine the in vivo and in vitro effects of chlorpyrifos on rainbow trout brain acetylcholinesterase (AChE) enzyme activity. The fish were exposed to 2.25 µg/L (25% of 96 h LC50), 4.5 µg/L (50% of 96 h LC50) and 6.75 µg/L (75% of 96 h LC50) of chlorpyrifos for 24, 48, 72 and 96 h. In vitro studies, inhibition constants (Ki ) and half-maximal inhibitory concentration (IC50) values for chlorpyrifos were determined by Lineweaver–Burk graphs and by plotting activity percentage vs. [I], respectively. Enzyme activities were determined by a colorimetric method. In fish exposed to chlorpyrifos 2.25 and 4.5 µg/L, enzyme inhibition was not observed (p > 0.05); however, chlorpyrifos exposure to 6.75 µg/L for 72 and 96 h caused a significant decrease in enzyme activity (p < 0.05). The IC50 value of chlorpyrifos was found to be 30 µg/L. These results show that chlorpyrifos may cause direct cellular injury in the brain, and suggest that AChE may be used as a bioindicator for toxicological studies.
1. Introduction
Aquatic ecosystems are often affected by contaminants released through various sources (Ghorashi et al. Citation2013). The increase of agricultural and industrial production has resulted in the exposure of freshwater systems to pollutants present in wastewater releases. These pollutants enter the food chain and can cause toxicity in aquatic organisms. Fish are used to assess the health of aquatic environment and physiological changes occurring as a result of pollution in an aquatic environment (USEPA Citation1992; Salazar-Lugo et al. Citation2011). Various non-target organisms, such as fish, exposed to pesticides and water pollution may experience adverse biological effects including biochemical alterations (Bernet et al. Citation1999; Burkepile et al. Citation2000). The biochemical half-life of these toxic substances in the environment is long and may lead to physiological changes resulting in tissue damage (Roy et al. Citation2006; Xing et al. Citation2012; Mishra & Devi Citation2014). Thus, given the important role of fish in the human food chain, the adverse effects of pesticides on fish (Oruç Citation2010).
Chlorpyrifos is an organophosphate insecticide used to control agricultural pests including termites, mosquitoes, associated with fruit, nut and vegetable crops and has been listed as a pesticide of potential concern by the US National Oceanic and Atmospheric Administration (Bernet et al. Citation1999; PMRA Citation2000; Verrin et al. Citation2004). It has been reported that chlorpyrifos causes contamination of surface and ground water and several studies have shown that chlorpyrifos is toxic to fish by inhibiting brain acetylcholinesterase (AChE) activity (Singh & Singh Citation2008; Xing et al. Citation2012; Mishra & Devi Citation2014; Xing et al. Citation2014). AChE (EC 3.1.1.7) is responsible for cholinesterase activity in the nervous system of vertebrates. Chlorpyrifos may disrupt the enzyme structure by attacking the active serine hydroxyl group of AChE (Carr & Chambers Citation2001; Boelsterli Citation2003). Thus inactivating the enzyme, and hindering the hydrolysis of the hydroxyl group. AChE inhibition is therefore irreversible and leads to the accumulation of neurotransmitter acetylcholine and, finally, to neurotoxicity (Thompson & Richardson Citation2004). AChE is thus an enzyme biomarker in pesticide-exposed fish (Da Cuna et al. Citation2011).
However, very little information is available regarding the effects of chlorpyrifos on rainbow trout brain. Such studies would provide much needed information required for an appropriate risk assessment of chlorpyrifos. This study was designed to investigate the in vivo and in vitro effects of chlorpyrifos on AChE activity of rainbow trout brain.
2. Materials and methods
2.1. Experimental design
Rainbow trout with an average weight of 170 ± 6.25 and average length of 18.51 ± 0.84 were obtained from Ataturk University (Erzurum, Turkey), Faculty of Fisheries and Inland Water Fish Breeding and Research Centre. Fish were placed in 400 L fibreglass tanks with dechlorinated tap water (temperature 10–12°C, pH 7.4 ± 0.2, dissolved oxygen 7.5 ± 0.5 mg/L, water hardness 167.6 ± 4.52 mg/L CaCO3, SO4 −2 = 0.36 mg/L, PO4 −3 = trace, NO3 − = 1.59 mg/L and NO2 − = trace), with each tank containing 15 fish. Fish were acclimated for 15 days under laboratory conditions and were fed 2.5% of their body weight with commercial trout pellets. The commercial formulation of chlorpyrifos [480 g/L chlorpyrifos, O,O-diethyl-O-(3,5,6-trichlor-2-pyridyl) phosphorothioate] was purchased from a distributor company (Turkey) and was dissolved in distilled water. The chlorpyrifos lethal dose (LC50) value for rainbow trout was defined as 9 µg/L (USEPA Citation1986). The concentrations used for this study were 25% (2.25 µg/L), 50% (4.5 µg/L) and 75% (6.75 µg/L) of LC50 value. Fish were exposed to nominal concentrations of chlorpyrifos for 96 h. At the end of the experimental period, control and exposed fish were randomly chosen from each tank and were sampled at 24, 48, 72 and 96 h. For each dose and time, four fish were euthanized by cervical section and the brain tissues were immediately removed. Tissues of each group were stored at –20°C until analysis of AChE enzyme activity.
2.2. Enzyme extraction
The brain tissues were transferred to the buffer solution (0.1 M KH2PO4/K2HPO4; pH 7.4) by adding Triton X-100 to a final concentration of 1% and stirring the mixture for 15 min at 4°C. The samples were homogenized in a homogenizer. Following this, the homogenates were centrifuged for 1 h at 100,000 × g (4°C) and the supernatants (crude extracts) were used in subsequent studies (Chhajlani et al. Citation1989; Rosenfeld et al. Citation2001).
2.3. In vivo assay
AChE activity was determined by following the procedure of Ellman et al. (Citation1961) with a slight modification. AChE catalyzes the hydrolysis of acetylthiocholine to acid and thiocholine. The formation of thiocholine was colorimetrically determined using 5,5′-dithiobis-(2-nitrobenzoic acid; DTNB), which reacts with the free thiol group of thiocholine. The molar extinction coefficient of DTNB (ε = 13,600 M−1 cm−1) was used to calculate enzyme activity (Kaya et al. Citation2013). Briefly, 0.5 mM DTNB (50 μL) in 1% sodium citrate, 0.5 M phosphate buffer (KH2PO4/K2HPO4; pH 8; 200 μL) and distilled water (650 μL) were added to the crude extract (50 μL) and the reaction was started by the addition of either 10 mM acetylthiocholine iodide (50 μL). Enzyme activity was determined by reading the changes in the absorbance at 412 nm for 5 min. The enzyme activity measured in the absence of acetylthiocholine iodide was considered as the control cuvette activity.
2.4. In vitro assay
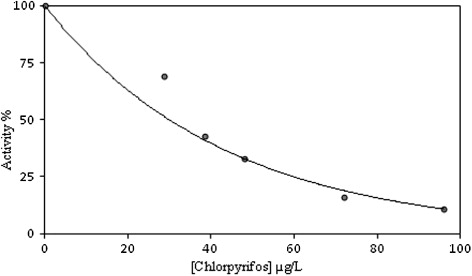
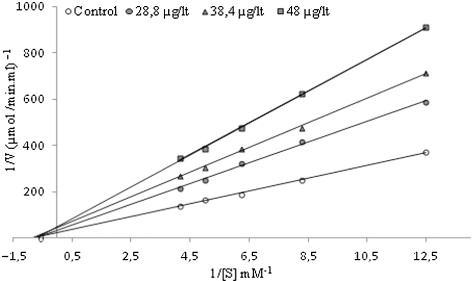
Inhibitory effects of chlorpyrifos on AChE enzyme activity were tested in triplicate at each concentration used. AChE enzyme activity was measured in the presence of different concentrations of inhibitors. Without inhibitor, control cuvette activity was taken as 100%. The inhibitor concentrations which produce 50% inhibition (IC50) were determined from the activity (%)-[Chlorpyrifos] graph. To determine the inhibitory constant (Ki ), three different inhibitor concentrations (28.8, 38.4 and 48 µg/L) were tested at five different substrate concentrations (0.08, 0.12, 0.16, 0.2 and 0.24 mM). Lineweaver–Burk curves were used for the determination of Ki value and type of inhibition (Lineweaver & Burk Citation1934).
2.5. Statistical analyses
All data were expressed as mean ± SEM. Statistical analysis of data was performed using a one-way analysis of variance and LSD test and analysed using SPSS version 10.0 software. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. In vivo AChE activity
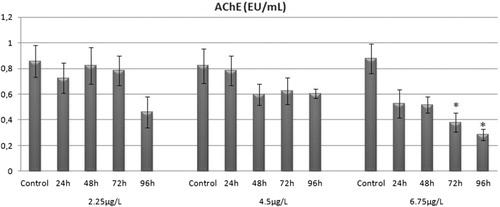
The effects of different doses of chlorpyrifos (2.25, 4.5 and 6.75 µg/L) and exposure times (24, 48, 72 and 96 h) on brain AChE activity of rainbow trout were demonstrated by in vivo studies. A time-dependent decrease in enzyme activity was observed a dose of at 2.25 and 4.5 µg/L chlorpyrifos, but no significant difference was observed (p > 0.05). Exposure to 6.75 µg/L chlorpyrifos for 72 and 96 h caused a significant decrease in brain AChE enzyme activity of rainbow trout (p < 0.05; ).
3.2. In vitro AChE activity
In the present study, Ki and IC50 parameters for chlorpyrifos as an inhibitor of brain AChE were determined. The inhibitor concentrations causing up to 50% inhibition were determined from the activity (%)-[Chlorpyrifos] graphs. As can be seen in and , the IC50 value for chlorpyrifos was determined as 30 µg/L. In addition, the Ki value was calculated from Lineweaver–Burk plots () as 44.2 ± 10.04 µg/L; chlorpyrifos exhibited non-competitive inhibition.
Table 1. IC50, Ki values and inhibition types of chlorpyrifos on the enzyme activity.
4. Discussion
Herein, the in vivo and in vitro changes in rainbow trout brain AChE enzyme activity, following acute exposure to chlorpyrifos, were determined. The effects of chlorpyrifos on rainbow trout brain have been scarcely studied, despite these being extensively reported in other fish species (Xing et al. Citation2010; Yen et al. Citation2011; Khalil et al. Citation2013; Mishra & Devi Citation2014). For example, Xing et al. (Citation2010) indicated that chlorpyrifos induced AChE inhibition in the brain of common carp. In another study, chlorpyrifos caused a significant decrease in brain AChE activity in Oryzias latipes (Khalil et al. Citation2013). Finally, Yen et al. (Citation2011) tested chlorpyrifos on larval zebrafish, observing an AChE inhibition in the fish brains.
In aquatic organisms exposed to organophosphorus pesticides, AChE inhibition has been observed to occur in neuromuscular junctions and cholinergic synapses (Oruç Citation2010). Nevertheless, the most important nervous system effects of chlorpyrifos is AChE inhibition (Xu et al. Citation2011). Chlorpyrifos can disrupt the structure of the enzyme by attacking the active serine hydroxyl group of AChE (Boelsterli Citation2003). The enzyme is thus inactivated and the hydroxyl group can no longer be hydrolysed. AChE inhibition is therefore irreversible and leads to the accumulation of acetylcholine, which finally causes neurotoxic changes, including the prevention of nerve impulses transmission and, interference of energy metabolism in the nervous system (Thompson & Richardson Citation2004; Da Cuna et al. Citation2011). Uner et al. (Citation2006) reported that AChE inhibition caused degradation of acetylcholine, resulting in excessive stimulation of cholinergic nerves. Herein, enzyme inhibition following low-dose exposure (2.25 µg/L and 4.5 µg/L) was not observed; however, chlorpyrifos exposure at 6.75 µg/L for 72 and 96 h caused a decrease in brain AChE enzyme activity of rainbow trout. In fish, a decrease in AChE activity causes acetylcholine accumulation within synapses, leading to the impairment of important functions such as swimming, feeding and general behaviour (Glusczak et al. Citation2006). Further, AChE inhibition may be associated with cell damage and lower AChE expression in the nervous system (Xing et al. Citation2013; Zhang de et al. Citation2013). It has been reported that AChE enzyme activity and its mRNA transcription decreased after exposure of chlorpyrifos in common carp brain (Xing et al. Citation2010).
The in vitro results indicated that the IC50 and Ki values for chlorpyrifos were 30 µg/L and 44.2 ± 10.04 µg/L, respectively. Chlorpyrifos was found to be a non-competitive inhibitor. In accordance with the present results, previous studies have demonstrated that chlorpyrifos has strong inhibitory effect on AChE activity. Assis et al. (Citation2012) investigated the effects of chlorpyrifos on brain AChE in tropical fish such as Arapaima gigas, Rachycentron canadum, Oreochromis niloticus and Electrophorus electricus. Ki constants for chlorpyrifos were determined as 2.69 × 10–2, 5.94 × 10–2, 0.161 and 2.18 × 10–4 μM, respectively. On the other hand, IC50 values were found to be 7.87, 30.24, 26.78 and 0.03 μM, respectively. The decrease in AChE was correlated with an increase in acetylcholine levels. During these conditions, catecholamines may increase and chlorpyrifos may interfere in vital processes such as the energy metabolism of nerve cells (Uner et al. Citation2006). Thus, these results show that AChE is a pesticide target and can be used as a bioindicator (Oruç Citation2010; Pérez et al. Citation2013). From the above results, it is clear that chlorpyrifos is a potent inhibitor of brain AChE enzyme activity in both in vivo and in vitro conditions. Nevertheless, several studies are required to further elucidate the inhibition mechanisms of AChE.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Assis CR, Linhares AG, Oliveira VM, França RC, Carvalho EV, Bezerra RS, de Carvalho LB Jr. 2012. Comparative effect of pesticides on brain acetylcholinesterase in tropical fish. Sci Total Environ. 15:141–150.
- Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T. 1999. Histopathology in fish: proposal to assess aquatic pollution. J Fish Dis. 22:25–34.
- Boelsterli UA. 2003. Mechanistic toxicology. London: Taylor & Francis.
- Burkepile DE, Moore MT, Holland MM. 2000. Susceptibility of five non-target organisms to aqueous diazinon exposure. Bull Environ Contam Toxicol. 64:114–121.
- Carr RL, Chambers JE. 2001. Toxic responses of the nervous system. In: Schlenk DM, Benson WH, editors. Target organ toxicity in marine and freshwater teleosts, vol. 2. London: Taylor and Francis; p. 27–95.
- Chhajlani V, Derr D, Earles B, Schmell E, August T. 1989. Purification and partial amino acid sequence analysis of human erythrocyte acetylcholinesterase. FEBS Lett. 247:279–282.
- Da Cuna RH, Rey Vazquez G, Piol MN, Guerrero NV, Maggese MC, Lo Nostro FL. 2011. Assessment of the acute toxicity of the organochlorine pesticide endosulfan in Cichlasoma dimerus (Teleostei, Perciformes). Ecotoxicol Environ Saf. 74:1065–1073.
- Ellman GL, Courtney KD, Andres V, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95.
- Ghorashi S, Shajeei H, Vaezi G, Shamoushaki MMN, Babakhani A. 2013. Histopathological studies on kidneys and gills of Onchorhynchus mykiss exposed to sublethal concentration of ethylenediaminetetraacetic acid (EDTA). Global Veterinaria. 10:121–127.
- Glusczak L, dos Santos Miron D, Crestani M, Braga da Fonseca M, de Araújo Pedron F, Duarte MF, Vieira VL. 2006. Effect of glyphosate herbicide on acetylcholinesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf. 65:237–241.10.1016/j.ecoenv.2005.07.017
- Kaya HB, Özcan B, Şişecioğlu M, Ozdemir H. 2013. Purification of acetylcholinesterase by 9-amino-1,2,3,4-tetrahydroacridine from human erythrocytes. Appl Biochem Biotechnol. 170:198–209.10.1007/s12010-013-0177-3
- Khalil F, Kang IJ, Undap S, Tasmin R, Qiu X, Shimasaki Y, Oshima Y. 2013. Alterations in social behavior of Japanese medaka (Oryzias latipes) in response to sublethal chlorpyrifos exposure. Chemosphere. 92:125–130.10.1016/j.chemosphere.2013.02.042
- Lineweaver H, Burk D. 1934. The determination of enzyme dissociation constants. J Am Chem Soc. 56:658–666.
- Mishra A, Devi Y. 2014. Histopathological alterations in the brain (optic tectum) of the freshwater teleost Channa punctatus in response to acute and subchronicexposure to the pesticide chlorpyrifos. Acta Histochem. 116:176–181.10.1016/j.acthis.2013.07.001
- Oruç EÖ. 2010. Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic Biochem Physiol. 96:160–166.
- Pérez J, Monteiro MS, Quintaneiro C, Soares AMVM, Loureiro S. 2013. Characterization of cholinesterases in Chironomus riparius and the effects of three herbicides on chlorpyrifos toxicity. Aquat Toxicol. 144–145:296–302.
- PMRA (Pest Management Regulatory Agency). 2000. Environmental assessment of chlorpyrifos. Ottawa: Environmental Assessment Division, Pest Management Regulatory Agency.
- Rosenfeld C, Kousba A, Sultatos LG. 2001. Interactions of rat brain acetylcholinesterase with the detergent Triton X-100 and the organophosphate paraoxon. Toxicol Sci. 63:208–213.
- Roy S, Chattoraj A, Bhattacharya S. 2006. Arsenic-induced changes in optic tectal histoarchitecture acetylcholinesterase–acetylcholine profile in Channa punctatus: amelioration by selenium. Comput Biochem Physiol C. 144:16–24.
- Salazar-Lugo R, Mata C, Oliveros A, Rojas LM, Lemus M, Villarroel E. 2011. Histopathological changes in gill, liver and kidney of neotropical fish Colossoma macropomum exposed to paraquat at different temperatures. Environ Toxicol Pharmacol. 31:2490–2495.
- Singh PB, Singh V. 2008. Pesticide bioaccumulation and plasma sex steroids in fishes during breeding phase from north India. Environ Toxicol Pharmacol. 25:342–350.
- Thompson, CM, Richardson RJ. 2004. Anticholinesterase toxicology. In: Marrs TC, Ballantyne B, editors. Pesticide toxicology and international regulation. West Sussex: John Wiley & Sons Ltd; p. 89–127.
- Uner N, Oruç EO, Sevgiler Y, Sahin N, Durmaz H, Usta D. 2006. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol. 21:241–245.
- USEPA. 1986. Ambient water quality criteria for chlorpyrifos. Washington, DC: Office of Water Regulations and Standards, Criteria and Standards Division.
- USEPA. 1992. Ground water and drinking water 810/K-92-001. Cincinnati (OH): U.S. Environmental Protection Agency. Available from: http://www.epa.gov/safewater/consumer/2ndstandards.html.
- Verrin SM, Begg SJ, Ross PS. 2004. Pesticide use in British Columbia and the Yukon: an assessment of types, applications and risks to aquatic biota. Can Tech Rep Fish Aquat Sci. 2517:xvi + 209 p.
- Xing H, Li S, Wang Z, Gao X, Xu S, Wang X. 2012. Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere. 88:377–383.
- Xing H, Wang J, Li J, Fan Z, Wang M, Xu S. 2010. Effects of atrazine and chlorpyrifos on acetylcholinesterase and carboxylesterase in brain and muscle of common carp [J]. Environ Toxicol Pharmacol. 30:26–30.
- Xing H, Wu H, Sun G, Zhang Z, Xu S, Li S. 2013. Alterations in activity and mRNA expression of acetylcholinesterase in the liver, kidney and gill of common carp exposed to atrazine and chlorpyrifos. Environ Toxicol Pharmacol. 35:47–54.10.1016/j.etap.2012.11.004
- Xing H, Zhang Z, Yao H, Liu T, Wang L, Xu S, Li S. 2014. Effects of atrazine and chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere. 104:244–250. 10.1016/j.chemosphere.2014.01.002
- Xu W, Jilong L, Houjuan X, Shiwen X. 2011. Review of toxicology of atrazine and chlorpyrifos on fish. J Northeast Agric Univ. 18:88–92.10.1016/S1006-8104(12)60031-2]
- Yen J, Donerly S, Levin ED, Linney EA. 2011. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol Teratol. 33:735–741.10.1016/j.ntt.2011.10.004]
- Zhang de L, Hu CX, Li DH, Liu YD. 2013. Zebrafish locomotor capacity and brain acetylcholinesterase activity is altered by Aphanizomenon flos-aquae DC-1 aphantoxins. Aquat Toxicol. 138–139:139–149.