Abstract
Boric acid (BA) is an essential nutrient for plants and many organisms, but it has become an environmental contaminant because of widespread use. Pesticide and its compounds are a serious threat to aquatic organisms. This study was carried out to determine the histopathological effects of acute exposure to BA concentrations in rainbow trout. The fish were exposed to 102 and 103 mg/L concentrations of BA. Tissues were sampled at 6, 12, 24, 48 and 96 h. Histopathological alterations occurring in tissues were common in both doses of BA. Gill tissues showed lamellar oedema, cellulary infiltration, lamellar disorganization, degenerative changes and lamellar thickening. Kidneys had glomerular oedema and glomerulonephritis, degeneration of the tubulary epithelium, interstitial fibrosis and a hyaline cast within the tubular lumens. Muscle tissues displayed interstitial oedema and degenerative and atrophic changes to varying degrees in the myofibrils. Our study shows that BA can be toxic for rainbow trout and cause histopathological damage in fish tissue.
1. Introduction
Boron is a naturally occurring compound found in low concentrations in natural surface waters and is an essential nutrient for plants and animals (Hu & Brown Citation1997; Nielsen Citation1997). Boron enters aquatic environments in varied ways such as anthropogenic and natural sources. The main sources of boron and its compounds are the weathering of clay sedimentary soils and volcanic activity (Nable et al. Citation1997; Polat et al. Citation2004). They are used for many different purposes in various products such as cleaners, detergents, personal care products, the manufacturing of ceramic, processes of metallurgy, glass and glass fibres, adhesives and industrial fluids (Schoderboeck et al. Citation2011). Boron is soluble in water and may cause toxic effects in aquatic animals. Fish are more sensitive than aquatic invertebrates to boron exposure during chronic exposures (Birge & Black Citation1977; Schoderboeck et al. Citation2011). The fish LC50 values (3.63 to >1000 mg B/L) show high variation due to test cases, life stages, fish size, fish species and test duration (Schoderboeck et al. Citation2011). The 96-h LC50 value of boron for rainbow trout is up to 1100 mg B/L (USEPA, Office of Prevention, Pesticides and Toxic Substances Citation1993b), but it has been reported that the LC50 value of boric acid (BA) is determined to be 138 mg/L and the lowest observed effect concentrations (LOECs) for rainbow trout are 0.10 mg/L (Loewengart Citation2001). In another study, LOECs for rainbow trout range between 0.001 and 0.008 mg B/L (Loewengart Citation2001). These values show that aquatic organisms like rainbow trout are very sensitive to boron treatment, and its compounds should be further investigated.
BA and its compounds are pesticides (USEPA, Office of Prevention, Pesticides and Toxic Substances Citation1993a), and additives for numerous industrial and pharmaceutical products and techniques (Turkez & Geyikoglu Citation2010). BA is found naturally in water and seas at 1–5 ppm concentrations, and it may change the biological functions of different animal species. Therefore, it is frequently used as a pesticide, but high doses of BA and boron compounds are toxic to fish such as rainbow trout and zebrafish (Rowe et al. Citation1998; Büyükgüzel et al. Citation2013).
Aquatic organisms may be exposed to herbicides in streams and ponds surrounded by agricultural areas. Rainbow trout are among these organisms, and it is one of the most studied fish species because of its importance as food (Ozaki et al. Citation2001). There is no information about the histopathological effects of BA in rainbow trout. Histological techniques are used to assess the unknown toxic effect of pollutants such as pesticides and heavy metals in the aquatic environment (Capkın et al. Citation2009). Therefore, it is important to investigate histopathological effects occurring after acute exposure to BA in juvenile rainbow trout. Thus, the objective of this study was to investigate the toxic effects of BA on the histopathological changes in the kidney, muscle and gill tissues of juvenile rainbow trout.
2. Materials and methods
2.1. Fish maintenance
Juvenile rainbow trout (Oncorhynchus mykiss) with an average weight of 3.3 ± 0.5 g were obtained from Atatürk University, Faculty of Fisheries and Inland Water Fish Breeding and Research Center. Fish were acclimatized for 30 days under laboratory conditions in dechlorinated tap water (12 h light–12h dark). During acclimation, fish were fed 2.5% of their body weight in commercial trout pellets. Water quality parameters were as follows: temperature 9.0 ± 2°C, pH 7.0 ± 0.2, dissolved oxygen 9.1 ± 0.3 mg/L, SO4 –2 = 0.33 mg/L, PO4 –3 = trace, NO3 – = 1.45 mg/L and NO2 – = trace. Two-thirds of the water in the aquarium was renewed every day.
2.2. Experimental design
BA (molecule formula H3BO3) was purchased from Sigma Chemical Co. It was dissolved in water, and the exposure concentrations of the boric acid in the stock solutions were 102 and 103 mg/L for each group. We chose lower and upper values of lethal concentrations for rainbow trout because both doses of BA may occur in an aquatic environment. The LC50 value for rainbow trout of BA was 138 mg/L (Loewengart Citation2001). After the acclimation, 30 fish in three groups were assigned to treatments into each glass aquarium containing 25 L of water. The first group was Control I (untreated fish). The other two groups (II, III) were exposed to 102 and 103 mg/L BA concentrations, respectively, under laboratory conditions. At the end of the exposure period, the control and all exposed fish were chosen from each aquarium randomly and were sampled at 6, 12, 24, 48 and 96 h. At the end of the experiment, fish were euthanized by cervical section, and the kidney, gill and muscle tissues were immediately removed. Muscles were taken from the dorsal side of the fish.
2.3. Histopathological process
Tissue samples from the gill, kidney and muscles were fixed in a 10% buffered formalin solution. After the routine histopathology process, paraffin sections in 5 µ were prepared and stained with haematoxylin and eosin. Histopathological lesions were semi-quantitatively assessed under the light microscope (Olympus BX51 with DP72 camera attachment system). For the histopathological score, six high-power (at 20X–40X magnification) fields were randomly chosen in two different fish (6 + 6 = total 12 areas). The scores were derived semi-quantitatively, considering the number of microscopic are with lesions and were reported as follows: none: − (0 lesion), mild: + (1–4 lesions), moderate: ++ (5–8 lesions) and severe: +++ (≥9 lesions).
3. Results
3.1. Control group
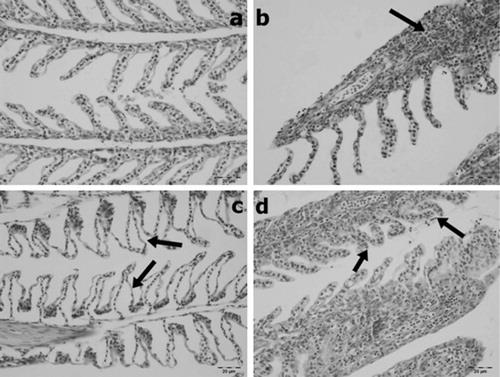
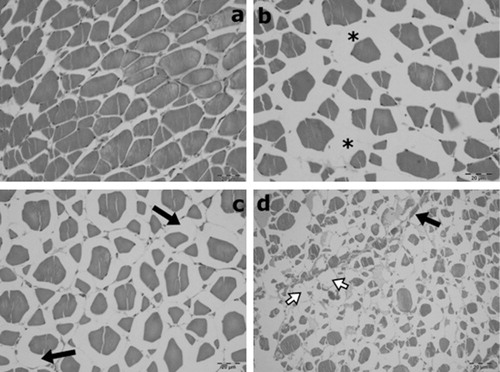
No histopathological changes were observed in the gill and muscle tissues of the control group ( and ).
3.2. Experimental groups
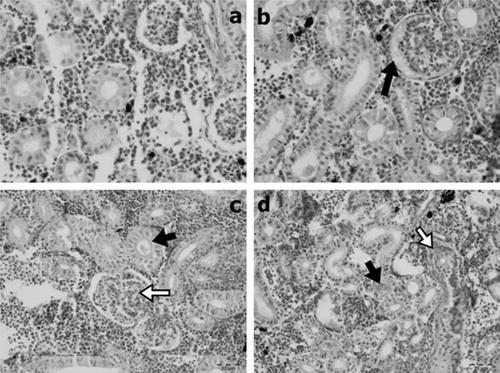
Lamellary oedema, cellulary infiltrations, thickening because of cellulary infiltrations or hypertrophy and clumping were observed in gill sections to varying degrees. Kidney tissues showed glomerular oedema, glomerulonephritis, degeneration of tubular epithelium, hyaline cast within the tubulary lumens and interstitial fibrosis to varying degrees. In muscle tissue, interstitial oedema as well as degenerative and atrophic changes in myocytes to varying degrees were observed.
Time- and dose-dependent comparisons of gill, kidney and muscle tissues were displayed in – and –.
Table 1. Time- and dose-dependant comparison of histopathology in gill tissue.
Table 2. Time- and dose-dependant comparison of histopathology in kidney tissue.
Table 3. Time- and dose-dependant comparison of histopathology in muscle tissue.
4. Discussion
BA is often used as a pesticide, and various non-target organisms are exposed to it (Büyükgüzel et al. Citation2013). Sub-lethal concentrations of pesticides cause biochemical, histological and physiological changes in many tissues of aquatic organisms (Boran et al. Citation2010). In this study, histological alterations were observed in the gill, kidney and muscle tissues of rainbow trout after acute exposure to BA. Similar results have been shown with other pesticides (Dalela et al. Citation1979; Ortiz et al. Citation2003; Altınok & Capkın Citation2007).
It is known that the gills are important organs in fish because they have important roles, including respiration, osmoregulatory functions and acid-base regulation (Ghate & Mulherkar Citation1979). Gills are considered to be one of the organs affected by water pollution, and it has been noticed that gills are target organs because of the effects of toxic pollutants (Ba-Omar et al. Citation2011). Toxic pollutants affect gill tissues, and they cause the disruption of the osmoregulatory function and reduce the oxygen consumption of aquatic organisms (Ghate & Mulherkar Citation1979; Peebua et al. Citation2008). It has been reported that pesticides lead to respiratory distress in the gill tissues of fish (Richmonds & Dutta Citation1989). In the present study, we observed that gill tissues were the most affected organ due to direct contact with toxic substances. In our study, lamellar oedema, cellulary infiltration, lamellar disorganization, degenerative changes in lamellar epithelium and lamellar thickening because of inflammation were observed in the gill tissues. These results showed that histopathological damages are direct responses to BA. Our results have shown to be similar to other pesticides in different fish species. Cengiz (Citation2006) reported lesions such as epithelial hyperplasia, oedema and fusion of the secondary lamellae in the gill tissues of common carp exposed to deltamethrin. Ba-Omar et al. (Citation2011) observed hypertrophy, epithelial lifting, desquamation and lamellar fusion in the gill tissues of Aphanius dispar exposed to the pesticide temephos.
The kidney is a critical organ that is affected by contaminants in the aquatic environment (Thophon et al. Citation2003; Iqbal et al. Citation2004). At the same time, kidneys are considered a good indicator of aquatic pollution (Cengiz Citation2006). Histopathological changes in the kidneys of fish exposed to chemical pollutants such as metals occurred. Hyaline droplets, reduction of Bowman’s space, tubule degeneration, dilation of capillaries and changes in the corpuscle were the main findings in fish exposed to chemical pollutants (Camargo & Martinez Citation2007). Thus it has been reported that renal lesions may be considered indicators of environmental pollution (Cengiz Citation2006). In this study, dose- and time-dependent lesions were observed in the histopathology of kidney tissues. These lesions were glomerular oedema, glomerulonephritis, degeneration of the tubulary epithelium, mild interstitial fibrosis 96 h after treatment with 103 mg/L BA and a hyaline cast within the tubular lumens. It has been suggested that the hyaline droplets found in renal tubules may be an indicator of the renal toxicity of pollutants (Chaudhuri et al. Citation1999). Ortiz et al. (Citation2003) reported the effects of lindane used as pesticide on fish, and their results showed lesions consisted of desquamation, tubular necrosis and the vacuolisation of tubular epithelial cells in the kidney. In another study, Altınok and Capkın (Citation2007) recorded that endosulfan showed histopathological effects such as exudates in tubules and between tubules, tubular necrosis, glomerular necrosis and renal tubular separation in the kidneys of rainbow trout. Interstitial fibrosis was observed only in the kidney sections of a fish. Fibrotic change may be associated with other chronic conditions. However, kidney fibrosis was reported in O. mykiss with the other histological changes in kidney tissue in the treatment of sub-lethal concentrations of ethylenediaminetetraacetic acid during 96 h (Ghorashi et al. Citation2013). Further studies are needed in kidney fibrosis regarding boric acid toxicity.
The histopathological effects of BA on muscle tissues have not been previously reported. The present study showed that boric acid caused interstitial oedema and degenerative and atrophic changes to varying degrees in the myofibrils of the muscle tissues. These results showed that BA may affect the muscle.
In conclusion, the results of this study suggest that BA can be toxic for rainbow trout and cause histopathological damage in fish tissue. In addition, histopathology may be used as a useful indicator for environmental pollutants and toxicity studies in fish.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Altinok I, Capkin E. 2007. Histopathology of rainbow trout exposed to sublethal concentrations of methiocarb or endosulfan. Toxicol Pathol. 35:405–410.10.1080/01926230701230353
- Ba-Omar TA, Al-Jardani S, Victor R. 2011. Effects of pesticide temephos on the gills of Aphanius dispar (Pisces: Cyprinodontidae). Tissue Cell. 43:29–38.10.1016/j.tice.2010.11.002
- Birge WJ, Black JA. 1977. Sensitivity of vertebrate embryos to boron compounds. EPA- 560/1-76-008, prepared by University of Kentucky, Lexington KY, under contract No. 68-01-3920. Washington (DC): Office of Toxic Substances, US EPA.
- Boran H, Altinok I, Capkin E. 2010. Histopathological changes induced by maneb and carbaryl on some tissues of rainbow trout, Oncorhynchus mykiss. Tissue Cell. 42:158–164.10.1016/j.tice.2010.03.004
- Büyükgüzel E, Büyükgüzel K, Snela M, Erdem M, Radtke K, Ziemnicki K, Zbigniew A. 2013. Effect of boric acid on antioxidant enzyme activity, lipid peroxidation, and ultrastructure of midgut and fat body of Galleria mellonella. Cell Biol Toxicol. 29:117–129.
- Camargo MMP, Martinez CBR. 2007. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotropical Ichthyology. 5:327–336.10.1590/S1679-62252007000300013
- Capkin E, Birincioglu S, Altinok I. 2009. Histopathological changes in rainbow trout (Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol Environ Saf. 72:1999–2004.
- Cengiz EI. 2006. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ Toxicol Pharmacol. 22:200–204.10.1016/j.etap.2006.03.006
- Chaudhuri BN, Kleywegt GJ, Bjorkman J, Lehman-McKeeman LD, Oliver JD, Jones TA. 1999. The structures of α2u-globuline and its complex with a hyaline droplet inducer. Acta Cryst Sect D. 55:753–62.
- Dalela RC, Bhatnagar MC, Tyagi AK, Verma SR. 1979. Histological damage of gills in Channa gachua after acute and subacute exposure to endosulfan and rogor. Mikroscopie. 35:301–307.
- Ghate HV, Mulherkar L. 1979. Histological changes in the gills of two freshwater prawn species exposed to copper sulphate. Ind J Exp Biol. 17:838–840.
- Ghorashi S, Shajeei H, Vaezi G, Shamoushaki MMN, Babakhani A. 2013. Histopathological studies on kidneys and gills of exposed to sublethal concentration of ethylenediaminetetraacetic acid (EDTA). Global Vet. 10:121–127.
- Hu H, Brown PH. 1997. Absorption of boron by plant roots. Plant Soil. 193:49–58.10.1023/A:1004255707413
- Iqbal F, Qureshi IZ, Ali M. 2004. Histopathological changes in the kidney of common carp, Cyprinus carpio following nitrate exposure. J Res Sci. 15:411–418.
- Loewengart G. 2001. Toxicity of boron to rainbow trout: a weight-of-the-evidence assessment. Environ Toxicol Chem. 20:796–803.10.1002/etc.5620200415
- Nable RO, Bañuelos GS, Paull JG. 1997. Boron toxicity. Plant Soil. 193:181–198.
- Nielsen FH. 1997. Boron in human and animal nutrition. Plant Soil. 193:199–208.
- Ortiz JB, De Canales MLG, Sarasquete C. 2003. Histopathological changes induced by lindane (gamma-HCH) in various organs of fishes. Sci Mar. 67:53–61.
- Ozaki A, Sakamoto T, Khoo S, Nakamura K, Coimbra MRM, Akutsu T, Okamoto N. 2001. Quantitative trait loci (QTLs) associated with resistance/susceptibility to infectious pancreatic necrosis virus (IPNV) in rainbow trout (Oncorhynchus mykiss). Mol Genet Genomics. 265:23–31.10.1007/s004380000392
- Peebua P, Kruatrachue M, Pokethitiyook P, Singhakaew S. 2008. Histopathological alterations of Nile tilapia, Oreochromis niloticus in acute and subchronic alachlor exposure. J Environ Biol. 29:325–331.
- Polat H, Vengosh A, Pankratov I, Polat M. 2004. A new methodology for removal of boron from water by coal and fly ash. Desalination. 164:173–188.
- Richmonds C, Dutta HM. 1989. Histopathological changes induced by malathion in the gills of bluegill Lepomis macrochirus. Bull Environ Contam Toxicol. 43:123–130.10.1007/BF01702248
- Rowe RI, Bouzan C, Nabili S, Eckhert CD. 1998. The response of trout and zebrafish embryos to low and high boron concentrations is U-shaped. Biol Trace Elem Res. 66:261–270.10.1007/BF02783142
- Schoderboeck L, Mühlegger S, Losert A, Gausterer C, Hornek R. 2011. Effects assessment: boron compounds in the aquatic environment. Chemosphere. 82:483–487.10.1016/j.chemosphere.2010.10.031
- Thophon S, Kruatrachue M, Upathan ES, Pokethitiyook P, Sahaphong S, Jarikhuan S. 2003. Histopathological alterations of white seabass, Lates calcarifer in acute and subchronic cadmium exposure. Environ Pollut. 121:307–320.10.1016/S0269-7491(02)00270-1
- Turkez H, Geyikoglu F. 2010. Boric acid: a potential chemoprotective agent against aflatoxin b1 toxicity in human blood. Cytotechnology. 62:157–165.10.1007/s10616-010-9272-2
- USEPA, Office of Prevention, Pesticides and Toxic Substances. 1993a. Reregistration eligibility decision (RED): boric acid and its sodium salts. Available from: www.pesticide.gov/pesticides; p. 18.
- USEPA, Office of Prevention, Pesticides and Toxic Substances. 1993b. EFED (Environmental Fate and Effects Division) Science Chapter for boric acid/sodium metaborate RED (Chemicals #011001, 011103; Case #0034). June 1.