ABSTRACT
The study was conducted to explore the possibilities of utilization of green cabbage (Brassica olerecea) in the preparation of chicken meatballs and their subsequent storage at 4 ± 1°C. The green cabbage was incorporated at various levels, namely 0%, 15% and 25% by replacing lean meat in the formulation. Crude protein, fat, ash and energy content of the chicken meatballs showed significantly (p < .05) decreasing trend with increasing levels of green cabbage. There was a significant (p < .05) increase in the moisture content, cooking yield, moisture retention and moisture protein ratio in treated products than control and recorded highest for T2. Chicken meatballs were aerobically packaged and assessed for storage quality under refrigerated (4 ± 1°C) conditions. Sensory attributes showed a significantly (p < .05) decreasing trend for both control as well as green-cabbage-incorporated chicken meatballs, whereas pH, total plate counts, yeast and mould count, free fatty acid and thiobarbituric acid reacting substances values increased significantly (p < .05) throughout the storage. The results conclude that green cabbage, which is rich in antioxidant compounds, could be used in chicken meatballs to extend the storage life by retarding the lipid oxidation and microbial growth at refrigeration temperature (4 ± 1°C).
1. Introduction
Meatballs are one of the most popular meat products in India and various types (such as chicken meatball, beef meatball, fish meatball and prawn meatball) are available. However, the most accepted and usually consumed are the chicken meatballs, fish meatballs and beef meatballs. The consumption of poultry meat and poultry meat products is growing all over the world (Mielnik et al. Citation2002). Ingredients used in processing of food products are among the most important factors influencing the final product quality. Nowadays, demand for healthier meat products is increasing rapidly. Such consumer demands have stimulated the development of meat products formulations with various types of healthier bioactive compounds. In this regard, various plants such as oat, soya, wheat, sunflower and rosemary and plant-origin ingredients are being used in meat products formulations. Incorporation of these compounds improves product binding, provides beneficial components such as phytochemical, as well as decreases the cost of formulation (Pennington Citation2002). Plant-origin proteins are used as non-meat ingredients for incorporation of bioactive components into meat products (Jimenez-Colmenero Citation2007). Cabbage is a good source of the vitamins (such as K, A, C, B6, folate, thiamine and riboflavin), minerals (calcium, potassium and magnesium) and tryptophan amino acid. Green cabbage is rich in antioxidant compounds (Nilsson et al. Citation2006; Roy et al. Citation2007; Kusznierewicz et al. Citation2008) such as vitamin C (Gould et al. Citation2006), polyphenols, flavonoids and glucosinolates. Due to its anti-inflammatory and antibacterial properties (Ayaz et al. Citation2008), cabbage has extensively been used in traditional medicine, in mitigation of symptoms related with gastrointestinal disorders (gastritis, peptic and duodenal ulcers, irritable bowel syndrome) as well as in treatment of minor injury, rheumatism and sore throat. It also contains significant amounts of glutamine, an amino acid that has anti-inflammatory properties. Green cabbage has a benefit of being a low calorie food because of which it is usually included in dieting schedules. India ranks second in cabbage production after China (http://en.wikipedia.org); so the incorporation of cabbage in chicken meatballs would increase utilization of cabbage and reduced the cost of formulation of the chicken meatballs preparation. Antioxidant and antimicrobial properties of the cabbage may also increase the shelf life of the cabbage-incorporated chicken meatballs.
Table 1. Formulation of chicken meatballs prepared by incorporating green cabbage (B. olerecea).
The present study was envisaged for development of green cabbage-incorporated chicken meatballs and for reducing the cost of production by better utilization of green cabbage. The study was designed to evaluate the effect of different levels of green cabbage on physicochemical properties of chicken meatballs and to assess the storage quality of the developed products.
2. Materials and methods
2.1. Raw material
Broiler chicken (6–8 weeks, type-Vencobb-400) carcasses were purchased from local market Mathura. Trimming and deboning of the chicken meat was done at the Department of Livestock Products Technology DUVASU, Mathura. Green cabbage (Brassica olerecea), condiments, salt, refined oil, spice mix, salt, bread, sodium tri-phosphate and monosodium glutamates (MSG) were purchased from local market Mathura India. Textured Soya was procured from local market and grounded at the department by using Inalsa food mixture.
2.2. Methods of preparation of chicken meatball
Chicken meatballs were prepared using formulation as presented in . Chicken meat was cut into small chunks and minced in a meat mincer (Sciencetech® TSM#8, Factory model LW-6118A-8) with 6 mm and 4 mm plate twice. Green cabbage was also minced twice with 6 mm and 4 mm plate. The common salt, vegetable oil, textured soya flour, nitrite, MSG, spice mixture and condiment mixture were added to weighed meat according to formulation. Meat emulsion for chicken meatballs was prepared in bowl Chopper (Stadler Corporation manufacturing and supplier of food processing system Mumbai, India). Minced meat was mixed and blended with salt, water, MSG and nitrite for 1 min followed by addition of refined vegetable oil and blending for another 30 s. This was followed by addition of green cabbage in treated group only and run the bowl chopper for 1 min, addition of ground textured soya, spice mixture, condiments and chopping for another 1 min to get the desired emulsion. Adequate care was taken to keep the temperature below 18°C by addition of crushed ice. Each ball was prepared from 20 g emulsion. The moulded raw balls were deep fat fried till the temperature of the inner core of the meatball reached 80°C. The prepared chicken meatballs were packed in low-density polyethylene (LDPE) aerobically and stored for analysis at refrigerated temperature 4 ± 1°C for 9 days.
2.3. Proximate analysis
Moisture, protein, fat and ash contents were determined in accordance with standard AOAC methods of (Citation2000). Protein determination involved a Kjeldahl assay (Nx6.25). Fat was determined by extracting samples in a Soxhlet apparatus using petroleum ether as a solvent. Moisture was quantified by oven drying 10 g samples at 100°C overnight. Ash was determined after incineration in a furnace at 500°C and carbohydrate content was calculated by computing the difference.
2.3.1. Cooking yield
Cooking yield was determined by measuring the difference in the sample weight before and after cooking and was calculated according to Murphy et al. (Citation1975):
2.3.2. Moisture retention
Moisture retention value represents the amount of moisture retained in the cooked product per 100 g of sample and was determined according to the equation by El-Magoli et al. (Citation1996). Calculation of moisture retention was done by the following formula:
2.3.3. Energy and carbohydrate
Estimates of total calories in cooked chicken meatballs both in control as well as in treatment were calculated on the basis of 100 g portion using Atwater values for fat (9 kcal/g), protein (4.02 kcal/g) and carbohydrate (4 kcal/g). An analysis of the percentage of carbohydrate in samples was estimated on the basis of subtraction, that is, (carbohydrate = 100−moisture + protein + fat + ash). Therefore, the calorie values were estimates.
2.4. pH
The pH of cooked chicken meatball was measured with a glass electrode with digital pH meter (Elico make, model: L1 127) following method of Trout et al. (Citation1992).
2.5. Thiobarbituric acid reacting substances value and free fatty acid
The extraction method described by Witte et al. (Citation1970) was used with suitable modifications for determination of thiobarbituric acid reacting substances (TBARS) numbers or values in chicken meatballs. Briefly, 10 g of sample was triturated with 25 ml of precooled 20% trichloroacetic acid (TCA) in 2 M orthophosphoric acid solution for 2 min. The content was then transferred quantitatively to a beaker by rinsing with 25 ml of cold distilled water, well mixed and filtered through filter paper (Whatman filter paper No. 1). Then 3 ml of TCA extract (filtrate) was mixed with 3 ml of 2-thiobarbituric acid (TBA) reagent (0.005 M) in test tubes and placed in a dark cabinet for 16 h. A blank sample was made by mixing 3 ml of 10% TCA and 3 ml of 0.005 M TBA reagent. Absorbance (OD) was measured at a fixed wavelength of 532 nm with a scanning range of 531–533 nm using UV-VIS spectrophotometer (Elico make). The TBA value was calculated as milligram malonaldehyde per kilogram of sample by multiplying OD value with K factor of 5.2. The free fatty acid (FFA) content was determined following the method described by Koniecko (Citation1979).
2.6. Microbial analysis
Total plate count (TPC), Yeast and Mould count in the sample was determined as per the method described by American Public Health Association (Citation1984) using Plate Count Agar and Potato dextrose Agar, respectively (Hi-Media Laboratories Pvt. Ltd., Mumbai). The average number of colonies were multiplied by the reciprocal of the dilution and expressed as log10 cfu/g.
2.7. Sensory evaluation
A panel of six experienced judges consisting of teachers and postgraduate students of College of Veterinary Science and animals husbandry (DUVASU), Mathura evaluated the samples for the attributes of appearance and colour, odour, juiciness, texture, tenderness, flavour and overall acceptability using 8-point descriptive scale (Keeton Citation1983), where 8 = extremely desirable and 1 = extremely undesirable. Sensory panellists were selected on the basis of availability, sensitivity, willingness and health status. Semi-trained panellists were given trial sessions for the product and their sensory attributes prior to final sensory analysis for obtaining better result. The test samples were presented to the panellists after assigning suitable codes. Three sittings (n = 18) were conducted for each replicate and at each storage time samples were warmed in a microwave oven for 20 sec before serving. Potable water was available for rinsing the oral cavity before and after sample evaluation.
2.8. Statistical analysis
Means and standard errors were calculated for different parameters. Data obtained in the study were analysed statistically using ‘SPSS-16.0’ software package as per standard methods (Snedecor & Cochran, Citation1994). Duplicate samples were drawn for each parameter and the experiment was replicated thrice (n = 6). Sensory evaluation was performed by a panel of six member judges three times; thus, total observations were 18 (n = 18). Data were subjected to one way analysis of variance. The storage data were analysed on the basis of 3 (treatments) × 4 (storage days) × 3 (replications) with two-way analysis of variance. The statistical significance was expressed at p < .05.
3. Results and discussion
3.1. Proximate composition
Proximate composition of the green cabbage-incorporated chicken meatballs has been tabulated in . The mean values of moisture increased significantly (p < .05) while the mean values of the protein, fat and ash decreased significantly (p < .05) with increasing level of green cabbage incorporation. Carbohydrates content increased with the increase in added green cabbage; however this increase was not statistically (p > .05) significant. The increase in moisture and carbohydrates values might be due to the higher moisture and carbohydrates in green cabbage as compared to chicken meat, while decrease in protein, fat and ash might be attributed to lesser amount of these in the green cabbage as compared to chicken meat (Rumeza et al. Citation2006).
Table 2. Proximate composition, cooking yield, moisture retention, moisture–protein ratio and energy of cooked chicken meatballs incorporated with green cabbage (Mean values ± SE, n = 6).
3.1.1. Cooking yield, moisture retention, moisture protein ratio and energy
Mean values of cooking yield, moisture retention, moisture protein ratio and energy of green cabbage-incorporated chicken meatballs has been presented in the . The cooking yield, moisture retention and the moisture protein ratio values increased significantly (p < .05) as the incorporation of the green cabbage percentage increased in chicken meatball. The highest cooking yield was recorded in the T2 group, which might be due to the higher moisture content in the green cabbage as compared to the meat and also due to the more fibre content in the T2 that have water retaining properties. The values of energy in the green cabbage chicken meatball decreased significantly (p < .05) as the replacement of the meat increased, which might be due to the decrease in protein and fat content.
3.2. pH values
As shown in , the initial pH ranged from 6.14 to 6.21 among the various treatments. During storage, the pH value differed significantly (p < .05) among samples. The pH values increased significantly (p < .05) during storage in all treatments. These results were similar to the findings of Sallam et al. (Citation2004), who reported that storage time had a significant (p < .05) effect on pH values that increased with storage. Increase in pH may be attributed to the accumulation of microbial metabolites produced due to the growth of microorganisms in the chicken meatballs during storage. Similar findings have also been reported by Jay (Citation1996); the increase in pH was due to the accumulation of metabolites by bacterial action in the meat by the deamination of proteins.
Table 3. The effect of green cabbage incorporation in chicken meatballs on pH, FFA, TPC as well as Yeast and mould counts value during storage study at 4 ± 1°C(Mean values ± SE, n = 6).
3.3. Free fatty acid
FFAs are the products of enzymatic or microbial degradation of lipids. Determination of FFA gives information about stability of fat during storage. shows that the FFA content was higher in the control as compared to the treatment and differed significantly (p < .05) from them. The FFA values increased significantly (p < .05) among all treatments and control as the days of storage advanced. The treated sample had lower content of FFA as compared to the control, which might be due to the antioxidant properties of green cabbage in the treated groups. The antioxidant effect of green cabbage is due to the presence of various polyphenolic compounds, ascorbic acid, carotenoids and vitamin E (Roy et al. Citation2007). These results were in agreement with the finding of Chacko and Patterson (Citation2011) in octopus meatballs and with the work of Nagamallika et al. (Citation2006) in chicken patties.
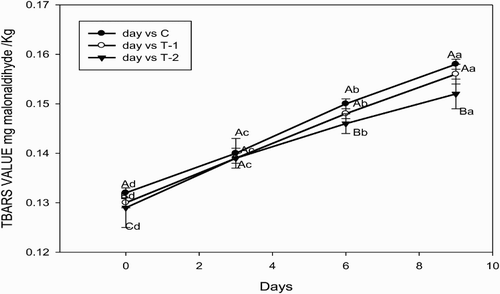
3.4. TBARS values
The TBARS values of treatments and control has been presented in . During storage, the TBARS values differed significantly (p < .05) in T2 than T1 and control at the 6th and 9th day of storage. The TBARS values increased significantly (p < .05) during storage in all treatments. The rate of malonaldehyde formation in the control was higher than that in the samples having green cabbage. This might be due to the antioxidant activity of polyphenols, total flavonoids and glucosinolates contents, which are naturally present in green cabbage. Antioxidant activity in Brassica species can be correlated with their carotenoids and total phenolic content, which have strong antioxidant properties due to the inhibition of lipid oxidation and scavenging reactive oxygen species (Sroka & Cisowski Citation2003; Hounsome et al. Citation2009). Glucosinolates present as allyl-isothiocyanate are considered as one of the most important phytochemical having the antioxidant, antimicrobial and chemopreventive anti-cancer properties (Volden et al. Citation2009). Many authors suggest that polyphenol antioxidants significantly affect the rate of the oxidation process in foodstuffs (Frankel Citation1991; Pokorny Citation1991; Lindberg et al. Citation1996).
Figure 1. Variations in TBARS values (mg malonaldehyde/kg) of green-cabbage-incorporated chicken meatballs during refrigerated storage (Mean values ±SE, n = 6). Notes: Mean values ± SE with different lower case letters and upper case letters respectively indicate day-wise and treatment-wise statistically significant difference at p < .05. C, Control (without green cabbage); T1, Treatment 1 (replacement of meat with 15% green cabbage); T2, Treatment 2 (replacement of meat with 25% green cabbage).
3.5. Microbiological analysis
TPC, yeast and mould counts evinced a non-significant (p > .05) difference among control and green-cabbage-treated samples () throughout storage. The value of TPC increased during storage study from 0 to 9 days; however, yeast and mould appeared only after 6th day of storage. TPC as well as yeast and mould values were within the acceptable limit. The microbial counts in the freshly prepared product were within the maximum permissible limits (5 × 104–1 × 106 for APC; 1 × 103 for yeast and mould count in processed meats) (Shapton & Shapton Citation1991). The increase found in counts during storage was in accordance with Chacko and Patterson (Citation2011). The lower microbial counts in T2 and T1 than in the control might be due to the presence of some antimicrobial compound in green cabbage, which retards the growth of the microorganism in the treated products. Delaquis and Mazza (Citation1995) have reported that isothiocyanate (a compound present in green cabbage) inhibits oxygen uptake, alter proteins and inactivates intracellular enzymes of microbes. Similar results have also been reported by Ikhlas et al. (Citation2012) in quail meatballs stored under refrigeration conditions. The principal antibacterial compound present in the green cabbage is methyl methanethiosulfinate (Kyung & Fleming Citation1994).
3.6. Sensory evaluation
The mean values of various sensory parameters of aerobically packaged cooked chicken meatballs containing 15% and 25% green cabbage and control are presented in . All the sensory attributes such as appearance and colour, juiciness, odour, texture, tenderness, flavour and over all acceptability decreased significantly (p < .05). Gradual decline in appearance and colour scores of meatballs stored at refrigeration conditions might be due to pigment and lipid oxidation resulting in non-enzymatic browning between lipids and amino acids. A similar result was reported by Kumar and Tanwar (Citation2011) in ground mustard incorporated chicken meat nugget. Eyas (Citation2001) indicated that diminution in juiciness occurs becasue LDPE has a high permeability to moisture. The lower flavour scores may be related to the increased malonaldehyde formation due to oxidation of fat, which has detrimental effect on the flavour and firmness of the product (Miller et al. Citation1980). Deterioration of flavour during storage might be due to microbial growth, formation of FFA and oxidative rancidity (Devatkal et al. Citation2003). Decrease in overall acceptability scores of meatball might be due to decrease in the values of other sensory attributes. Comparable results were reported by Chacko and Patterson (Citation2011) in the qualities of octopus meatballs and by Agnihotri et al. (Citation2006) in goat meatballs.
Table 4. Changes in the Sensory attributes of green cabbage-incorporated chicken meatballs storage study at 4 ± 1°C (Mean values ± SE, n = 18).
4. Conclusions
The results of the present study propose that green cabbage is a good source of bioactive components and has great prospects in the formulation of healthy and stable chicken meatballs. Incorporation of green cabbage is positively associated with cooking yield, moisture retention and moisture–protein ratio. Under aerobic refrigerated storage of chicken meatballs, addition of green cabbage in the product improved its oxidative stability (TBARS and FFA), microbiological quality (TPC and yeast and mould values) and better maintenance of sensory attributes. Thus, green cabbage, a valuable bioactive ingredient often ignored, can be utilized in the processing of chicken meatballs and other meat products. So in the present study, the replacement of chicken meat with 15% green cabbage in chicken meatballs was found more suitable as compared to other levels.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agnihotri MK, Rajkumar V, Dutta TK. 2006. Effect of feeding complete rations with variable protein and energy levels prepared using by-products of pulses and oilseeds on carcass characteristics, quality of goat meat and meat balls. Asian Austral J Anim. 19:1437–1449.
- APHA. 1984. Compendium of methods for the microbiological examination of foods, 2nd Ed. Washington, DC: M.L. Speck.
- AOAC. 2000. Official methods of analysis 17th edition. Washington, DC: Association of official Analytical chemists.
- Ayaz FA, Hayırlıoglu-Ayaz S, Alpay-Karaoglu S, Gruz J, Valentova K, Ulrichova J Strnad M. 2008. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC) extracts and their antioxidant and antibacterial activities. Food Chem. 107:19–25.
- Chacko D, Patterson J. 2011. Qualities of octopus meat balls developed using smashed potato and Bengal gram starches. World J Dairy Food Sci. 6:130–135.
- Delaquis P, Mazza G. 1995. Antimicrobial properties of isothiocyanates in food preservation. Food Technol. 49:73–84.
- Devatkal S, Mendiratta SK, Anjaneyulu ASR. 2003. Effect of calcium lactate on the quality and shelf life of restructured pork rolls. J Meat Sci. 1:1–6.
- El-Magoli S, Laroia S, Hansen PMT. 1996. Flavor and texture characteristics of low fat ground beef patties formulated with whey protein concentrate. Meat Sci. 42:179–193.
- Eyas AM. 2001. Studies on development of enrobed buffalo meat cutlets. M.V.Sc. thesis, Izatnagar, Uttar Pradesh, India: Indian Veterinary Research Institute.
- Frankel EN. 1991. Recent advances in lipid oxidation – A review. J Sci Food Agr. 54:495–511.
- Gould S, Tkesslee DK, King CG. 2006. Vitamin-C content of vegetables, V. cabbage. J Food Sci. 1:427–434.
- Hounsome N, Hounsome B, Tomos D, Edwards-Jones G. 2009. Changes in antioxidant compounds in white green cabbage during winter storage. Postharvest Biol Tec. 52:173–179. Available from: http://en.wikipedia.org/wiki/Agriculture_in_India#cite_ref-fao2009_52-1.
- Ikhlas B, Huda N, Ismail N. 2012. Effect of cosmos caudatus, polygonum minus and BHT on physical properties, oxidative process, and microbiology growth of quail meatball during refrigeration storages. J Food Process Pres. 36:55–66.
- Jay JM. 1996. Antioxidants. Modern food microbiology, 4th Ed., (J.M. Jay, editor.), New Delhi: CBS Publisher and Distributors. pp. 265–266
- Jimenez-Colmenero F. 2007. Meat based functional foods. In: Hui YH. editor. Handbook of food products manufacturing. Hoboken, NJ: John Wiley & Sons; pp: 989–1015.
- Keeton JT. 1983. Effect of fat and sodium chloride phosphate levels on the chemical and sensory properties of pork patties. J Food Sci. 36:261–76.
- Koniecko EK. 1979. Handbook for meat chemists. Wayne, NJ: Avery Publishing Group; pp 53–55.
- Kumar D, Tanwar VK. 2011. Effects of incorporation of ground mustard on quality attributes of chicken nuggets. J Food Sci Tec. 48:59–762.
- Kusznierewicz B, Bartoszek A, Wolska L, Drzewiecki J, Gorinstein S, Namiésnik J. 2008. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT – Food Sci Tec. 41:1–9.
- Kyung KH, Fleming HP. 1994. S-Methyl-L-cysteine sulfoxide as the precursor of methyl methane thiosulfinate, the principle antibacterial compound in green cabbage. J Food Sci. 59:350–355.
- Lindberg MH, Andersen L, Christiansen L, Brockhoff P, Bertelsen G. 1996. Anioxidative activity of summer savory (Satureja hortensis L.) and rosemary (Rosmarinus officinalis L.) in minced, cooked pork meat. Zeitschrift fuer Lebensmittel-Untersuchung und –Forschung. 203:333–338.
- Mielnik MB, Aaby K, Rolfsen K, Ellekjær MR, Nilsson A. 2002. Quality of comminuted sausages formulated from mechanically deboned poultry meat. Meat Sci. 61:73–84.
- Miller AJ, Ockerman SA, Palumbe SA. 1980. Effect of frozen storage on functionality of meat for processing. J Food Sci. 50:531–534.
- Murphy EW, Criner PE, Grey BC. 1975. Comparison of methods for calculating retentions of nutrients in cooked foods. J Agr Food Chem. 23:1153–1157.
- Nagamallika E, Prabhakara RK, Masthan RP. 2006. Effect of storage on physico-chemical, microbiological and sensory quality of chicken patties. Indian J Poult Sci. 41:271–274.
- Nilsson J, Olsson K, Engqvist G, Ekvall J, Olsson, M, Nyman M, Akesson B. 2006. Variation in the content of glucosinolates, hydroxycinnamic acids, carotenoids, total antioxidant capacity and low-molecular-weight carbohydrates in Brassica vegetables. J Sci Food Agr. 86:528–538.
- Pennington AT. 2002. Food composition databases for bioactive food components. J Food Comp Anal. 15:419–434.
- Pokorny J. 1991. Natural antioxidants for food use. Trend Food Sci Tech. 2:223–227.
- Roy MK, Takenaka M, Isobe S, Tsushida T. 2007. Antioxidant potential, antiproliferative activities, and phenolic content in water-soluble fractions of some commonly consumed vegetables: effects of thermal treatment. Food Chem. 103:106–114.
- Rumeza H, Zafar I, Mudassar I, Shaheena H, Masooma R. 2006. Use of vegetables as nutritional food: role in human health. J Agr Biol Sci. 1:18–22.
- Sallam KI, Ishioroshi M, Samejima K. 2004. Antioxidants and antimicrobial effects of garlic in chicken sausage. LWT – Food Sci Tech. 37:849–855.
- Shapton DA, Shapton NF. 1991. Principles and practices for the safe processing of foods. Oxford: Butterworth Heineman; pp. 377–444.
- Snedecor GW, Cochran WG. 1994. Statistical methods. New Delhi: First East West press edn.
- Sroka Z, Cisowski W. 2003. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 41:753–758.
- Trout ES, Hunt NC, Johnson DE, Claus JR, Kastner CL, Kropf DH. 1992. Chemical, physical and sensory characterization of ground beef containing 5 to 30% fat. J Food Sci. 57:25–29.
- Volden J, Bengtsson GB, Wicklund T. 2009. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L., ssp. botrytis); effects of long-term freezer storage. Food Chem. 112:967–976.
- Witte VC, Krause GF, Bailey ME. 1970. A new extraction method for determining 2 Thiobarbituric acid values of pork and beef during storage. J Food Sci. 35:582–585.
