ABSTRACT
The present feeding trial was conducted to assess the optimal level of phytase supplementation required for maximum nutrient absorption and growth performance of Labeo rohita fingerlings fed canola meal-based diet. A standard diet having 30.21% protein and an energy value of 4.26 kcalg−1 was used as reference diet. The experimental diet having similar protein and caloric density was formulated by using 70% reference diet and 30% of canola meal as test ingredient. This experimental diet was then divided into seven test diets and were supplemented by graded levels (0, 250, 500, 750, 1000, 1250 and 1500 FTU kg−1) of phytase enzyme. Chromic oxide was used in reference and test diets as an inert marker. Results showed that phytase supplementation at 750 FTU kg−1 level effectively increased apparent digestibility coefficients of crude protein (64%), crude fat (76%) and gross energy (68%) as compared to reference and other phytase-supplemented diets. The results of present study showed increased growth and feed performance of fingerlings in response to phytase supplementation. Maximum performance was obtained by the fish fed on test diet having 750 FTU kg−1 level. It was concluded that 750 FTU kg−1 level of phytase supplementation in canola meal-based diet is sufficient for increasing nutrient digestibility and growth performance of L. rohita fingerlings.
1. Introduction
The aquaculture industry is developing more efficiently than other food production industries. However, economic factors such as cost of fish feed are limiting its development (Yıldırım et al. Citation2014). Fishmeal is being widely used in aqua feeds (Davis et al. Citation2010) because it is an excellent source of fundamental nutrients such as essential amino acids, fatty acids, vitamins, minerals and growth factors (NRC Citation2011). However, increasing demand, high price and unstable supply of the fish meal with the extension of aquaculture industry makes it necessary to search for better alternative protein sources (Lech & Reigh Citation2012; Shapawi et al. Citation2013). Plant byproducts are considered as good source of protein and energy (Hardy Citation2010) for the formulation of economical and environment friendly aqua-feed (Cheng & Hardy Citation2002). However, the main problem associated with the use of these plant byproduct-based proteins in feed formulation is the presence of anti-nutritional factors such as phytate or phytic acid chemically known as myo-inositol 1, 2, 3, 4, 5, 6-hexaphosphate (Baruah et al. Citation2004). Phytate is present in large quantities in plants ingredients such as cereals, legumes, nuts and oilseeds (Rao et al. Citation2009). It makes complexes with protein and decreases its availability to fish (Helland et al. Citation2006).
Phytase is an enzyme chemically known as myo-inositol hexaphosphate phosphohydrolase (Class 3: Hydrolases) that may be either present in some plant ingredients or may be produced by microorganisms. It is very specific to hydrolyse the indigestible phytate that is present in plant protein sources. Monogastric and agastric fishes cannot produce this enzyme so cannot hydrolyse the complex phytate. Phytase-supplemented fish feeds has been generally reported to improve the bio-availability and utilization of plant phosphorus by fish (Cao et al. Citation2007). Furthermore, better use of nutrients results in less discharge of phosphorous into the aquatic environment causing less aquatic pollution (Baruah et al. Citation2004).
Agastric fish species such as carps including L. rohita have insignificant intestinal phytase activity which is due to microbiota present in the fish intestinal tract. Therefore, these fish species are unable to hydrolyse the phytate present in plant-based diet. Nevertheless, improved nutrients digestibility was reported by several researchers in plant-based diets in response to microbial phytase supplementation (Baruah et al. Citation2007; Cao et al. Citation2007). In our previous studies, L. rohita fingerlings have shown improved nutrient and minerals absorption (Hussain et al. Citation2011a, Citation2011b, Citation2011c)
The most important product of canola is its oil content, whereas canola meal (CM) is also a valuable protein source for use in animal feed. Some researchers have been intended for the assessment of the nutritional value of CM for a variety of animals (Newkirk Citation2009; Enami and Safafar Citation2010). It is being used in the feeds of various fish species such as trout, salmon, catfish, carp, tilapia, bass, perch, sea bream and turbot (Enami Citation2011). Protein content in CM is 36–39% (Newkirk Citation2009). Its amino acid profile is similar to that of herring meal protein and superior to soybean meal protein (Shafaeipour et al. Citation2008). On the other hand, CM is economical as compared to fish meal and soybean meal (Sajjadi and Carter Citation2004).
Labeo rohita (rohu) is one of the most important indigenous major carp species being cultured in Pakistan. It is usually cultivated in poly-culture system with other major and Chinese carps (Hussain et al. Citation2011b). Regional culture practices are mainly based on semi-intensive culture system. Various crude formulations in the form of mesh feeds are used whereas no cost effective and eco-friendly fish feeds are available to local fish farmers. However, less information is available for formulation of artificial feeds for commercially important stomach-less fish like L. rohita (Iqbal et al. Citation2014). A comprehensive research work is required to overcome the problems of this costly fish meal and aquatic pollution caused by phytate rich excreta. Hence, the major objective of our study was to evaluate the efficacy of phytase supplementation on nutrient digestibility of L. rohita fingerlings fed on CM-based diets and to formulate cost effective and environment friendly feed for the indigenous culturable fish species.
2. Materials and methods
2.1. Fish and experimental conditions
L. rohita fingerlings were obtained from Government Fish Seed Hatchery, Faisalabad and kept in V-shaped tanks (specially designed for the collection of faeces having 70 L water capacity) for two weeks to acclimatize them with experimental conditions. Before the start of feeding trial, L. rohita fingerlings were treated with NaCl (5g L−1) to make sure that the fingerlings are free from ectoparasites and to prevent further fungal infection (Rowland and Ingram Citation1991). During this acclimatization period the L. rohita fingerlings were given reference diet once daily to apparent satiation (Allan and Rowland Citation1992). Water quality parameters such as water temperature, pH and dissolved oxygen were monitored through pH meter (Jenway 3510), DO meter (Jenway 970) and electrical conductivity (EC) meter (HANNA: HI. 8633) on daily basis. Aeration (24 h) was provided to all the experimental tanks through capillary system. The range of water quality parameters was noted such as pH 7.4–8.6, dissolved oxygen 5.8–7.3 mgL−1, electrical conductivity 1.30–1.52 dSm−1 and temperature 24.9–28.7°C.
2.2. Experimental design
CM was used as test ingredient to formulate experimental diet. Experimental diet comprised 70% reference diet and 30% of test ingredient. Experimental diet was then divided into seven test diets and supplemented with graded (0, 250, 500, 750, 1000, 1250 and 1500 FTU kg−1) levels of phytase. One reference diet and seven CM-based phytase-supplemented test diets were fed to eight fish groups stocked in water tanks having 70 L water capacity. Three replicates each having 15 fingerlings were used for each treatment. Tanks were specially designed for the collection of faecal material from water media. Fingerlings were provided with 12 h dark and night period throughout the trial. Total duration of experiment was 10 weeks during which 4–5 g faeces were collected from each replicate for proximate analysis. CM-based phytase-supplemented diets were compared with each other and with reference diet in relation to determine nutrient digestibility parameters by using Completely Randomized Design. The optimum level of phytase supplementation for different nutrient digestibility parameters was determined by using quadratic regression analysis. The trial consisted of assessing the apparent nutrient digestibility coefficients (ADCs) of crude protein, crude fat and gross energy by using chromic oxide as inert marker.
2.3. Feed ingredients and formulation of experimental diets
The feed ingredients were purchased from a commercial feed mill and were analysed for chemical composition following AOAC (Citation1995) prior to the formulation of the experimental diet (). The reference diet was prepared to supply sufficient levels of required nutrients for normal fish growth (). The feed ingredients were finely grinded to pass through .5 mm sieve size. All ingredients were mixed in an electric mixer for 10 minutes and fish oil was gradually added during mixing of diet. During mixing of ingredients 10–15% water was also added to prepare suitable texture (Lovell Citation1989). Then these mixed feed ingredients were extruded to form floating pellets (3 mm) through Lab Extruder (SYSLG30-IV Experimental Extruder). The temperatures of first, second, third and fourth heaters of the extruder were kept 60°C, 90°C, 120°C and 150°C, respectively. All diets were equally treated in the given extruder to formulate one reference diet and seven CM-based test diets. The required concentrations (0, 250, 500, 750, 1000, 1250 and 1500 FTU kg−1) of phytase (Phyzyme® XP 10000 FTU g−1; Danisco Animal Nutrition, Fin-65101 Vaasa, Finland) were prepared in 25 mL distilled water and sprayed on 1 kg of test diets (Robinson et al. Citation2002). The 0 FTU kg−1 (control diet) was sprayed with a similar amount of distilled water to maintain an equal level of moisture contents. All the prepared diets were dried and stored at 4°C until use ().
Table 1. Proximate analysis (%) of feed ingredients (dry matter basis).
Table 2. Ingredients (%) of reference and canola meal-based test diets (as fed basis).
Table 3. Analysed composition of nutrients (%) in reference and canola meal-based test diets.
2.4. Growth study
Fish in each tank were bulk weighed every 15th day during experiment to assess the growth performance of L. rohita fingerlings. Weight gain (%), feed conversion ratio (FCR) and specific growth rate (SGR) of fingerlings was evaluated based on standard formulae.
2.5. Chemical analysis of feed and faeces
The samples of fish feed ingredients, experimental diets and faeces were homogenized by using mortar and pestle. These were analysed by standard methods (AOAC Citation1995). Moisture contents were determined by oven-drying at 105°C for 12 hours whereas crude protein (CP) (N × 6.25) by micro kjeldahl apparatus. Ether extract (EE) was extracted by petroleum ether extraction method through Soxtec HT2 1045 system; crude fibre (CF) as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH, whereas ash by ignition at 650°C for 12 hours in electric furnace (Eyela-TMF 3100) to constant weight. Total carbohydrates (N-free extract) were calculated by difference i.e. . Gross energy digestibility was determined with the help of adiabatic oxygen bomb calorimeter (Parr Instrument Co., Moline, USA).
2.6. Digestibility studies
Chromic oxide was used as an inert marker at 1% inclusion level in reference diet and .7% in test diets, assuming that the amount of the marker in the feed and faeces remains constant throughout the experimental period and that all of the ingested marker will appear in the faeces.
After the feeding session of two hours the water tanks were washed completely to remove the particles of diets and refilled with ground water stored in holding tanks at room temperature. It was changed manually daily. Then faeces were collected from the faecal collection tube of each tank by opening the valves subsequently. Faecal material of each replicated treatment was dried in oven, grinded and stored for chemical analysis. Chromic oxide content in diets and faeces was estimated after oxidation with molybdate reagent using UV-VIS 2001 Spectrophotometer at 370 nm absorbance (Divakaran et al. Citation2002). The ADCs (%) of crude protein, crude fat and apparent gross energy for fish were calculated indirectly at the end of the experiment using chromic oxide as inert marker.
ADC (%) of diets was calculated by the following formula (NRC Citation1993):
2.7. Statistical analysis
Data of nutrient (crude protein, crude fat), apparent gross energy digestibility and growth performance of reference diet and test diets were subjected to one-way analysis of variance (ANOVA) (Steel et al. Citation1996). The differences among means were compared by Tukey's Honestly Significant Difference Test and considered significant at p < .05 (Snedecor and Cochran Citation1991). The CoStat computer software (Version 6.303, PMB 320, Monterey, CA, USA) was used for statistical analysis.
3. Results
The data presented in make it clear that phytase enzyme supplementation played a significant role in increasing nutrient digestibility and minimum amount of nutrients was discharged through faeces at 750 and 1000 FTU kg−1 phytase levels. It is also obvious from the results that in comparison with reference diet and phytase-supplemented CM-based diets, 750 FTU kg−1 level showed maximum values of crude protein (64%), crude fat (76%) and gross energy (68%) digestibility (). Whereas quadratic regression analysis indicated that optimum values were observed for crude protein digestibility at 776 FTU kg−1, fat digestibility at 716 FTU kg−1 and gross energy digestibility at 768 FTU kg−1 phytase level (). It was observed that gross energy digestibility value at 750 FTU kg−1 level did not differ significantly from phytase level of 1000 FTU kg−1 but varied significantly (p < .05) from the reference diet and remaining phytase-supplemented diets. Interestingly, it was also observed that except 750 FTU kg−1 phytase level, remaining levels of phytase supplementation could not show higher crude protein and crude fat digestibility as compared to the reference diet (). Feeds supplemented with further higher (1250 and 1500 FTU kg−1) levels of phytase enzyme surprisingly resulted in a decreased nutrient digestibility. In general, the results showed that nutrient digestibility started increasing from 250 FTU kg−1 level of phytase supplementation and reached to its maximum at 750 FTU kg−1 level. Similarly, P and Ca showed similar trend as in other nutrients regarding their bio-availability. Their digestibility ratio (P:Ca) was also enhanced in phytase-treated diets (up to 1.09 in test diet-III) as compared to reference diet (.92). This showed more P liberation as compared to Ca in phytase-treated diets which represent a direct affect of phytase on P availability (data not shown).
Figure 1 . The quadratic relationship between apparent nutrient digestibility (%) of reference, canola meal-based test diets and phytase levels (FTU kg−1)
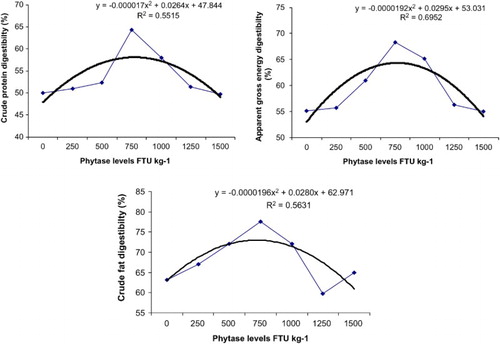
Table 4. Analysed composition of nutrients (%) in faeces of L. rohita fingerlings fed on reference and canola meal-based test diets.
Table 5. Apparent nutrient digestibility (%) of reference and canola meal-based test diets.
The maximum weight gain (4.81g) of L. rohita fingerlings fed on CM-based test diets was again recorded at 750 FTU kg−1 level. It was found significantly different (p < .05) from the weight gain value recorded for reference diet and remaining CM-based test diets supplemented with graded levels of phytase. The results elucidated that weight gain in reference diet, 0, 250, 1250 and 1500 FTU kg−1 phytase diets were statistically similar. The feed intake of reference diet, 0, 1250 and 1500 FTU kg−1 diets were significantly lower as compared to the value recorded for 750 FTU kg−1 diet. However, the second highest value was observed at 1000 FTU kg−1. The best FCR value (1.30) was also found at 750 FTU kg−1 level which was significantly different (p < .05) from FCR values of other diets except reference and 1000 FTU kg−1 level diets ().
Table 6. Growth performance of L. rohita fingerlings fed on reference and canola meal-based test diets.
4. Discussion
In aquaculture industry, the fishmeal is being replaced with plant byproduct-based feed ingredients but use of these byproducts is nutritionally restrained because of phytate contents. This phytate chelates the nutrients resulting in reduced bio-availability of these nutrients to fish. Phytase supplementation in plant ingredient-based diet may result in release of these chelated nutrients by hydrolysing the phytate and makes them available to the fish (Liu et al. Citation2013). Use of phytase dose is usually considered sufficient in many fish species ranging from 250 to 1500 FTU kg−1 level. However, optimum level of its supplementation varies depending upon its production source, fish species; feed processing technology and phytate concentration in the diet. Therefore, phytase supplementary level in feed should be adjusted by considering all of these factors (Cao et al. Citation2007). In the present study, the highest ADC values of crude protein (64%), crude fat (76%) and gross energy (68%) were observed at 750 FTU kg−1 phytase level. However, at higher levels of phytase supplementation (1000, 1250 and 1500 FTU kg−1), a significant decrease in their digestibility was recorded. In our previous studies, a similar trend and optimum level (750 FTU kg−1) of phytase supplementation for L. rohita fingerlings was also observed in corn gluten (Hussain et al. Citation2011b) and sunflower (Hussain et al. Citation2011a) meal-based diets. Likewise, a similar trend of crude protein digestibility against phytase supplementation was also reported by Baruah et al. (Citation2007) in L. rohita juveniles fed soybean meal-based diet. Its maximum digestibility was recorded at the 750 FTU kg−1 phytase level, while at a higher concentration (1000 FTU kg−1) of phytase its digestibility was decreased.
Other workers such as Vielma et al. (Citation2004) and Liebert and Portz (Citation2005) also concluded that phytase-supplemented plant-based test diets increases the protein digestibility in fish but contrary to it, Cheng and Hardy (Citation2002); Yan and Reigh (Citation2002) and Dalsgaard et al. (Citation2009) observed no significant effects of phytase supplementation on protein digestibility of plant-based diets. This inconsistency for nutrient digestibility was associated to the variation in different factors such as protein quality of feed ingredients, pH of fish stomach, feed processing and drying procedures (Wang et al. Citation2009). This higher ADC% of protein observed in the present research work showed the higher acceptability of the alternative plant ingredients protein-based test diets supplemented with phytase enzyme (Nwanna et al. Citation2008).
In this study, fingerlings fed on 250 and 500 FTU kg−1 phytase levels showed slight increase in ADCs of nutrients. The possible reason for this insignificant increase in nutrient digestibility is the insufficient dose of phytase supplementation, which results in the inadequate degradation of phytate contents (Liu et al. Citation2013). It is interesting to note that further higher levels (1250 and 1500 FTU kg−1) of phytase supplementation resulted in reduced ADCs of nutrients. The reasons for this decline are difficult to access but it is suggested that use of phytase should be done carefully within certain limits. On the other hand, Ashraf and Goda (Citation2007) found that optimum dose of phytase supplementation for increasing ADCs of nutrients (crude protein 90%, crude lipid 88% and gross energy 73%) is 1000 FTU kg−1 and at further higher (1500 and 2000 FTU kg−1) levels, the ADCs of nutrients started deceasing. They argued that this reduced nutrient (crude protein, crude fat and gross energy) digestibility from test diets having 1500 and 2000 FTU kg−1 levels of phytase supplementation in Nile tilapia was due to its feeding behaviour (Ashraf & Goda Citation2007).
In Nile tilapia, Portz and Liebert (Citation2004) observed significantly improved crude fat digestibility for test diets supplemented with 1000 and 2000 FTU kg−1 levels of phytase, whereas Dalsgaard et al. (Citation2009) found no significant effect on fat digestibility in rainbow trout (Oncorhynchus mykiss) fed with phytase-supplemented plant-based diets. On contrary, Wang et al. (Citation2009) found a decreased lipid digestibility by phytase supplementation. The possible reason for this decrease may be that phytase supplementation inhibited lipase activity which leads to reduced lipase hydrolysis efficiency for lipid, resulting in reduced lipid digestibility. Moreira et al. (Citation2007) found that phytase supplementation increased gross energy digestibility in diets of tambaqui (Colossoma macropomum). In another study, Cheng and Hardy (Citation2002) also reported that phytase supplementation of diets having canola protein concentrate improved apparent gross energy digestibility for rainbow trout, whereas Lanari et al. (Citation1998) did not find any positive response for rainbow trout fed on soybean meal-based test diets. The effects of phytase on nutrient digestibility may be dependent on different nutritional factors, i.e. concentration and source of phytic acid/phytate in the fish diet, amount and source of protein in fish diet (Selle et al. Citation2000) and digestibility levels of protein sources in fish feed (Sugiura et al. Citation2001).
Phytase supplementation played an important role in minimizing the amount of nutrients discharge through faeces at 750 and 1000 FTU kg−1 phytase levels. It indicates that at these levels more nutrients were utilized by L. rohita fingerlings as compared to the reference diet and remaining levels of phytase-supplemented diets. This reduced nutrient excretion through fish faeces was due to the hydrolysis of phytate contents by the phytase resulting in the utilization of more nutrients by L. rohita fingerlings (Hussain et al. Citation2011a, Citation2011b). Our results showed that phytase supplementation in diets resulted in decreased excretion of nutrients into the aquatic environment which invariably meant more nutrient deposition in the fish. The reduction of nutrients effluent into the aquatic environment would certainly minimize aquatic pollution problem associated with fish culture effluents. The present observations are supported by Storebakken et al. (Citation1998), who found that fish fed with diet consisting of phytase-supplemented soybean meal discharged significantly lower amount of faecal nutrients into the aquatic environment than those fish groups fed either with fish meal or soy concentrate diets. Sajjadi and Carter (Citation2004) also reported that phytase incorporation in diets having high levels of plant protein significantly increased nutrient digestibility and decreased nutrient excretion through faeces. The use of phytase to fish feed may be very helpful in reducing the nutrient discharge into aquatic environment through faeces which ultimately results in less aquatic pollution (Wang et al. Citation2009; Hussain et al. Citation2011c).
The growth and feed performance of L. rohita fingerlings in terms of weight gain and FCR was significantly improved by CM-based diets supplemented with phytase. The maximum weight gain and best FCR were observed at 750 FTU kg−1 phytase supplementation level. A linear increase in growth performance was observed with the increase in phytase supplementation dose upto 750 FTU kg−1, however interestingly, higher doses caused decrease in growth performance resulting in overall quadratic trend. The findings of the present study provide evidence that phytase supplementation at 750 FTU kg−1 level is sufficient to minimize the effect of phytic acid while using CM as major feed ingredient in the diet of L. rohita. The present results of growth performance of L. rohita fingerlings fed on phytase-supplemented diets are in agreement with the findings of Baruah et al. (Citation2007) and Hussain et al. (Citation2011a). Liebert and Portz (Citation2007) have concluded that supplementation of phytase at 750 FTU kg−1 is sufficient for maximum degradation of phytate in plant-based diet resulting in higher growth performance of Nile tilapia (Oreochromis nilotics). In the present study, maximum weight gain % (70%) was observed at 750 FTU kg−1 level of phytase supplementation, whereas the literature revealed more increase for the similar time duration in fish feeding trials with juvenile fish (Debnath et al. Citation2005; Baruah et al. Citation2007; Sardar et al. Citation2007; Hussain et al. Citation2014). It indicates that fish did not respond well to the experimental feed. The poor growth performance might be partially due to less intake of feed as the fish could not be domesticated properly, partially because of the experimental water salinity, which was higher as compared to its natural riverine habitats. It is also evident that pond fisheries has also been affected alarmingly, probably due to the rapidly deteriorating quality of ground waters of this region as the same was used in the present study.
5. Conclusion
The present study confirmed that CM-based diet supplemented with 750 FTU kg−1 level of phytase significantly released chelated nutrients resulting in higher growth performance of L. rohita fingerlings. It was also found that phytase supplementation played a significant role in developing environment friendly and economical feed for indigenous culturable major carp L. rohita. It is recommended that further studies should be conducted to investigate the most suitable dose of phytase supplementation near 750 and 1000 FTU kg−1 levels under different dietary and rearing conditions.
References
- Allan GL, Rowland SJ. 1992. Development of an experimental diet for silver perch (Bidyanus bidyanus). Austasia Aqua. 6:39–40.
- Ashraf M, Goda AS. 2007. Effect of dietary soybean meal and phytase levels on growth, feed utilization and phosphorus discharge for Nile tilapia (Oreochromis niloticus L.). J Fish Aquat Sci. 2:248–263. doi: 10.3923/jfas.2007.248.263
- Association of Official Analytical Chemists (AOAC). 1995. Official methods of analysis. 15th ed. Washington, DC: Association of Official Analytical Chemists, p. 1094.
- Baruah K, Pal KAK, Narottam PS, Debnath D. 2007. Microbial phytase supplementation in rohu, Labeo rohita, diets enhances growth performance and nutrient digestibility. J World Aqua Soc. 38:129–137. doi: 10.1111/j.1749-7345.2006.00081.x
- Baruah, K, Sahu NP, Pal AK, Debnath D. 2004. Dietary phytase: an ideal approach for cost effective and low-polluting aquafeed. NAGA, World Fish Center Quar. 27:15–19.
- Cao L, Wang W, Yang C, Yang Y, Diana J, Yakupitiyage A, Luo Z, Li D. 2007. Application of microbial phytase in fish feed. Enz Microb Technol. 14:342–362.
- Cheng ZJ, Hardy RW. 2002. Effect of microbial phytase on apparent nutrient digestibility of barley, canola meal, wheat and wheat middlings, measured in vivo using rainbow trout (Oncorhynchus mykiss). Aqua Nutr. 8:271–277. doi: 10.1046/j.1365-2095.2002.00219.x
- Dalsgaard J, Ekmann KS, Pedersen PB, Verlhac V. 2009. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus-depleted juvenile rainbow trout (Oncorhynchus mykiss) and on the magnitude and composition of phosphorus waste output. Aquaculture. 286:105–112. doi: 10.1016/j.aquaculture.2008.09.007
- Davis LK, Fox BK, Lim C, Lerner DT, Hirano T, Grau EG. 2010. Effects of 11-ketotestosterone and fishmeal in the feed on growth of juvenile tilapia (Oreochromis mossambicus). Aquaculture. 305:143–149. doi: 10.1016/j.aquaculture.2010.04.006
- Debnath D, Pal AK, Narottam PS, Jain KK, Yengkokpam S, Mukherjee SC. 2005. Effect of dietary microbial phytase supplementation on growth and nutrient digestibility of Pangasius pangasius (Hamilton) fingerlings. Aquacult Res. 36:180–187. doi: 10.1111/j.1365-2109.2004.01203.x
- Divakaran S, Leonard GO, Ian PF. 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J Agric Food Chem. 50:464–467. doi: 10.1021/jf011112s
- Enami HR. 2011. A review of using canola/rapseed meal in aquaculture feeding. J Fish Aquatic Sci. 1:22–36.
- Enami HR, Safafar H. 2010. Evaluation of adding canola meal to diet on growth performance of male wistar rats. Asian J Animal Vet Sci. 5:478–483. doi: 10.3923/ajava.2010.478.483
- Hardy RW. 2010. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquacult Res. 41:770–776. doi: 10.1111/j.1365-2109.2009.02349.x
- Helland S, Denstadli V, Witten PE, Hjelde K, Strobakken T, Skrede A, Asgard T, Baeverfjord G. 2006. Hyper dense vertebrae and mineral content in Atlantic salmon (Salmo salar L) fed diets with graded levels of phytic acid. Aquaculture. 261:603–614. doi: 10.1016/j.aquaculture.2006.08.027
- Hussain SM, Afzal M, Rana SA, Javid A, Hussain M. 2011a. Impact of phytase supplementation on nutrient digestibility for Labeo rohita fingerlings fed on sunflower meal based diets. Pak J Life Soc Sci. 9:85–90.
- Hussain SM, Afzal M, Rana SA, Javid A, Iqbal M. 2011b. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int J Agric Biol. 13:916–922.
- Hussain SM, Rana SA, Afzal M, Shahid M. 2011c. Efficacy of phytase supplementation on mineral digestibility in Labeo rohita fingerlings fed on corn gluten meal (30%) based diets. Pak J Agric Sci. 48:237–241.
- Hussain SM, Shahzad MM, Afzal M, Jabeen F, Nasir S, Javid A, Chaudhary MA, Ahmad S, Arsalan MZH, Riaz D, et al. 2014. Growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed on soybean meal-based diet supplemented by phytase. Int J Biosci. 5:212–221. doi: 10.12692/ijb/5.12.212-221
- Iqbal KJ, Ashraf M, Abbas F, Javid A, Hafeez-ur-Rehman M, Abbas S, Rasool F, Khan N, Khan SA, Altaf M. 2014. Effect of Plant-Fishmeal and Plant By-Product Based Feed on Growth, Body Composition and Organoleptic Flesh Qualities of Labeo rohita. Pak J Zool. 46:253–260.
- Lanari D, Agaro ED, Turri C. 1998. Use of nonlinear regression to evaluate the effects of phytase enzyme treatment of plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture. 161:345–356. doi: 10.1016/S0044-8486(97)00282-2
- Lech GP, Reigh RC. 2012. Plant products affect growth and digestive efficiency of cultured Florida pompano (Trachinotus carolinus) fed compounded diets. PLoS ONE. 7:e34981. doi:10.1371/journal.pone.0034981).
- Liebert F, Portz L. 2005. Nutrient utilization of Nile tilapia Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture. 248:111–119. doi: 10.1016/j.aquaculture.2005.04.009
- Liebert F, Portz L. 2007. Different sources of microbial phytase in plant based low phosphorus diets for Nile tilapia Oreochromi sniloticus may provide different effects on phytate degradation. Aquaculture. 267:292–299. doi: 10.1016/j.aquaculture.2007.01.023
- Liu LW, Su JM, Zhang T, Liang XF, Luo YL. 2013. Apparent digestibility of nutrients in grass carp (Ctenopharyngodon idellus) diet supplemented with graded levels of neutral phytase using pretreatment and spraying methods. Aquacult Nutr. 19:91–99. doi: 10.1111/j.1365-2095.2012.00942.x
- Lovell RT. 1989. Fish Nutrition and Feeding. New York: Van Nostrand Reinhold Co.
- Moreira-da-Silva JA, Pereira-Filho M, Cavero BAS, de-Oliveira-Pereira MI. 2007. Apparent digestibility of nutrients and crude energy in diets with addition of exogenous digestive enzymes in tambaqui juveniles (Colossoma macropomum Cuvier,1818). Acta Amazon. 37:157–164.
- National Research Council (NRC). 1993. Nutrient requirements of fish. Washington, DC: National Academy Press, p. 114.
- National Research Council (NRC). 2011. Nutrient requirement of fish. Washington, DC, USA: National Research Council, National Academy Press.
- Newkirk RW. 2009. Canola meal: feed industry guide. 4th ed. Winnipeg, MB: Canadian International Grains Institute, pp. 18–38.
- Nwanna LC, Oishi CA, Filho MP. 2008. Use of phytase to improve the digestibility of alternative feed ingredients by Amazon tambaqui, Colossoma macropomum. Sci Asia. 34:353–360. doi: 10.2306/scienceasia1513-1874.2008.34.353
- Portz L, Liebert F. 2004. Growth, nutrient utilization and parameters of mineral metabolism in Nile tilapia Oreochromis niloticus (Linnaeus, 1758) fed plant based diets with graded levels of microbial phytase. J Anim Physiol Anim Nutr. 88:311–320. doi: 10.1111/j.1439-0396.2004.00486.x
- Rao DE, Rao KV, Reddy TP, Reddy VD. 2009. Molecular characterization, physicochemical properties, known and potential applications of phytases: an overview. Crit Rev Biotechnol. 29:182–198. doi: 10.1080/07388550902919571
- Robinson EH, Li MH, Manning BB. 2002. Comparison of microbial phytase and dicalcium phosphate or growth and bone mineralization of pond-raised channel catfish, Ictalurus punctatus. J Appl Aquacult. 12:81–88. doi: 10.1300/J028v12n03_08
- Rowland SJ, Ingram BA. 1991. Diseases of Australian native freshwater fishes with particular emphasis on the ectoparasite and fungal diseases of Murray cod (Maccullochella peeli), golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus). NSW Fisheries Bulletin Number 4, Sydney.
- Sajjadi M, Carter CG. 2004. Effect of phytic acid and phytase on feed intake, growth, digestibility and trypsin activity in Atlantic salmon (Salmo salar L.). Aqua Nutr. 10:135–142. doi: 10.1111/j.1365-2095.2003.00290.x
- Sardar PH, Randhawa S, Abid M, Prabhakar SK. 2007. Effect of dietary microbial phytase supplementation on growth performance, nutrient utilization, body compositions and haemato-biochemical profiles of Cyprinus carpio (L.) fingerlings fed soy protein-based diet. Aquacult Nutr. 13:444–456. doi: 10.1111/j.1365-2095.2007.00497.x
- Selle PH, Ravindran V, Caldwell RA, Bryden WL. 2000. Phytate and phytase: consequences for protein utilization. Nutr Res Rev. 13:255–278. doi: 10.1079/095442200108729098
- Shafaeipour A, Yavari V, Falahatkar B, Maremmazi JG, Gorjipour E. 2008. Effects of canola meal on physiological and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquacult Nutr. 14:110–119. doi: 10.1111/j.1365-2095.2007.00509.x
- Shapawi R, Ebi I, Yong A. 2013. Soybean meal as a source of protein in formulated diets for tiger grouper, Epinephelus fuscoguttatus juvenile. Part I: effects on growth, survival, feed utilization and body compositions. Agricult Sci. 4:317–323.
- Snedecor GW, Cochran WG. 1991. Statistical Methods. 8th ed. Ames: Iowa State Univ. Press, p. 503.
- Steel RGD, Torrie JH, Dickey DA. 1996. Principles and procedures of statistics. 3rd ed. New York: McGraw Hill International Book Co. Inc., p. 336–352.
- Storebakken T, Shearer KD, Roem AJ. 1998. Availability of protein, phosphorus and other elements in fishmeal, soy-protein concentrate and phytase-treated soy-protein concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture. 161:365–379. doi: 10.1016/S0044-8486(97)00284-6
- Sugiura SH, Gabaudan J, Dong FM, Hardy RW. 2001. Dietary microbial phytase supplementation and the utilization of phosphorus, trace minerals and protein by rainbow trout [Oncorhynchus mykiss (Walbaum)] fed soybean meal-based diets. Aqua Res. 32:583–592. doi: 10.1046/j.1365-2109.2001.00581.x
- Vielma J, Ruohonen K, Gabaudan J, Vogel K. 2004. Top-spraying soybean meal-based diets with phytase improves protein and mineral digestibilities but not lysine utilization in rainbow trout, Oncorhynchus mykiss (Walbaum). Aqua Res. 35:955–964. doi: 10.1111/j.1365-2109.2004.01106.x
- Wang F, Yang YH, Han ZZ, Dong HW, Yang CH, Zou ZY. 2009. Effects of phytase pretreatment of soybean meal and phytase-sprayed in diets on growth, apparent digestibility coefficient and nutrient excretion of rainbow trout (Oncorhynchus mykiss Walbaum). Aqua Int. 17:143–157. doi: 10.1007/s10499-008-9187-5
- Yan W, Reigh RC. 2002. Effects of fungal phytase on utilization of dietary protein, minerals and dephosphorylation of phytic acid in the alimentary tract of channel catfish Zctulurus punctutus fed an all-plant-protein diet. J World Aqua Soci. 33:10–22. doi: 10.1111/j.1749-7345.2002.tb00473.x
- Yildirim O, Acar U, Türker A, Sunar MC, Kesbiç OS. 2014. Effects of replacing fish meal with peanut meal (Arachis hypogaea) on growth, feed utilization and body composition of mozambique tilapia fries (Oreochromis mossambicus). Pak J Zool. 46:497–502.
