ABSTRACT
In the present study, polymorphisms at two single nucleotide polymorphisms (SNPs) and one microsatellite locus of SLC11A1 gene were investigated for finding their association with susceptibility to bovine tuberculosis trait (tuberculin reaction) in Indian cattle. A total of 245 animals were tested with single intradermal tuberculin test for screening positive animals for tuberculin reaction. At rs109915208 locus very low polymorphism information content of 0.0454 was observed, whereas it was moderate (0.352) at rs109453173 locus of SLC11A1. At rs109915208 locus the genotypic as well as allelic frequencies were differing significantly (p-value < .05) in case–control animals where the odds ratio (OR) of ‘CC’ verses ‘CT’ genotype and the OR of ‘C’ verses ‘T’ allele were approaching towards infinity, suggesting that animals having ‘CT’ genotype and ‘T’ allele were less susceptible for tuberculin reaction as compared to their contemporary genotype/allele. The significantly associated SNP was non-synonymous causing an amino acid change.
1. Introduction
The Mycobacterium tuberculosis comprises Mycobacterium tuberculosis, Mycobacterium africanum, Mycobacterium canettii, Mycobacterium pinnipedii, Mycobacterium microti, Mycobacterium bovis and Mycobacterium bovis subspecies caprae responsible for causing chronic disease tuberculosis. However, M. bovis is the most universal pathogen among mycobacteria and affects many vertebrate animals of all age groups. In 46% of African, 44% of Asian and 35% of the South American and the Caribbean countries, sporadic and enzootic occurrences of bovine tuberculosis (bTB) have been reported (Cosivi et al. Citation1998). The bTB has significant public health importance, apart from being the most important disease with serious effects on animal production (O'Reilly & Daborn Citation1995). Generally treatment for bTB is not recommended in animals since there is no cost-effective treatment. The socio-economic condition of people in India and prevailing religious customs prevent the slaughtering of affected cows making bTB much difficult to eradicate. Therefore, there is an urgent need to develop some alternative strategies to combat infectious diseases.
An ideal approach to the control of the infectious diseases in animals is the development of genetic resistance. One of the candidate genes having role in resistance/susceptibility to infectious diseases is solute-like carrier family 11 A1 (SLC11A1) also known as NRAMP1 (Natural resistance-associated macrophage protein 1) (Ganguly et al. Citation2008; Prakash et al. Citation2014). NRAMP1 is a member of the solute carrier (SLC11A1) family of ion transporter (Horin & Matiasovic Citation2000), which is an integral trans-membrane protein and expresses particularly on phagosome of macrophage (Gruenheid et al. Citation1997). Genetic studies in mice have demonstrated SLC11A1 controls' innate resistance and susceptibility for M. bovis (Gros et al. Citation1981). The NRAMP1 gene mediates activity of macrophages against intracellular parasites during the early stages of infection (Blackwell et al. Citation1994). NRAMP1 affects the intraphagosomal microbial replication primarily by eliminating Mn2+ (Supek et al. Citation1996) or other divalent cations (Fe2, Mn2, Co2, etc.) from phagosomal interior (Gruenheid & Gros Citation2000; Forbes & Gross Citation2003), which serve as essential cofactors for their survival by helping in many microbial metabolic processes. There are reports about association of SNPs in SLC11A1 to resistance/susceptibility to tuberculin reaction in humans as well as in animals (Puzyrev et al. Citation2002; Selvaraj et al. Citation2002; Qu et al. Citation2006). In the present study, an association of two polymorphic SNPs and one microsatellite marker pertaining to SLC11A1 genes with tuberculin reaction was observed.
2. Materials and method
A cattle population (N = 245) comprising Indigenous (Koshi, Sahiwal, Gir)/Nondescript and crossbred (two groups Indigenous/Nondescript verses crossbred) from Shri Mataji Gaushala, Barsana, Mathura, India, was selected for case and control study for bTB trait in cattle. All animals were kept in same herd had an equal opportunity of infection. Cattle of both sexes were aged from 2 to 8 years (two groups < 3 years verses ≥ 3 years) and kept under similar managemental and feeding practices. The tuberculin test was used for diagnosis of mycobacterium infection in herd, which is globally one of the most accepted tests for diagnosis of bTB. Single intradermal tuberculin test was performed and increase in thickness of skin after 72 h of intradermal injection of tuberculin antigen was noted to develop Case (tuberculin positive) and Control (tuberculin negative) resource panel as per the standard protocol. Bovine Purified Protein derivative (PPD) 0.1 ml was injected in cervical region and measurement of skin was taken before injecting PPD and after 72 h of Bovine PPD injection. Animals were divided into three groups: those showed marked swelling and skin thickness more than 4 mm (Positive), skin thickness < 4 mm and >2 mm (inconclusive) and no reaction > 2 mm (negative). The inconclusive animals were not included in the present investigation. A total of 35 tuberculin positive and 49 negative animals were included in the present study.
Approximately 6 ml of venous blood from Jugular vein was collected in an ethylenediaminetetraacetic acid coating tube (2 mg/ml of blood) and stored at −20°C till further use. DNA was extracted by using Promega Wizard® Genomic DNA Purification Kit as per recommended protocols. The concentration of DNA was determined using Qubit Flurometer. Primers for the 2 SNPs (rs109915208 and rs109453173) were designed using Oligoanalyser while reported primer for SLC11A1 microsatellite marker was used for amplification of the loci. The details of primers and restriction enzymes are tabulated in . Concerned amplicons were amplified under the optimized PCR condition. The polymerase chain reaction (PCR) product are resolved in 1.5% agarose gel and visualized under UV light after staining with ethidium bromide. The Restriction enzyme digestion was made at the optimized conditions and the restriction digested products were resolved in 3– 5% agarose gel and visualized under UV light after staining with ethidium bromide. Mass genotyping of all case–control resource population was done by using PCR-RFLP (restriction fragment length polymorphism). The PROC ALLELE procedure of the SAS 9.3 used for the estimation of polymorphism information content (PIC), Hardy Weinberg Equilibrium (HWE) and heterozygosity. Initially in univariate logistic regression analysis, the non-genetic factors like age (two levels), sex (two levels) and breed (two levels) were fitted and found that none of these effects were significantly affecting the tuberculin test reaction. The PROC LOGISTIC procedure of SAS 9.3 was used to find association of allelic and genotypic frequencies with tuberculin reaction and to find out the overall association of the microsatellite loci with the tuberculin reaction status of the animal.
Table 1. Details of SNPs and microsatellite marker from Slc11A1 gene.
3. Results and discussion
Out of total screened 245 animals only 35 cattle (14.28%) were found to be positive for the tuberculin test. All non-genetic factors (breed, age and sex) had non-significant (p < .05) effect the tuberculin test reaction.
3.1. PCR-RFLP assay
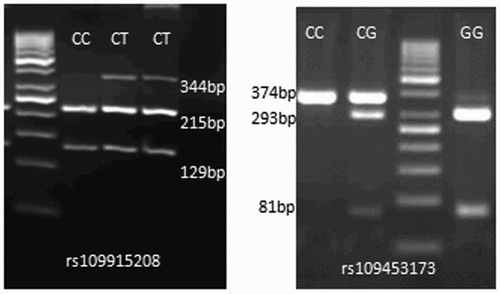
The case–control population was genotyped by using PCR-RFLP for two SNPs from SlC11A1 gene and these SNPs were found to be polymorphic in our resource population. At rs109915208 locus, two genotypes, ‘CC’ (215 and 129 bp) and ‘CT’ (344, 215 and 129 bp) were found, while three genotypes ‘GG’ (293 and 81 bp), ‘CC’ (374 bp) and ‘CG’ (374, 293 and 81 bp) were observed at rs109453173 locus (). A moderate heterozygocity (0.488) was observed at rs109453173 locus and it was very low (0.047) at rs109915208 locus. At rs109915208 locus very low PIC of 0.0454 was observed, whereas moderate PIC of 0.352 was estimated at rs109453173 single nucleotide polymorphism (SNP) locus. The population was in HWE for the SNPs pertaining to SLC11A1 gene.
The chi-square probabilities revealed that out of two SNPs from SLC11A1 gene, one had the significant association with the susceptibility to tuberculin reaction. At rs109915208 locus, the frequencies of ‘CC’ and ‘CT’ genotypes were 0.918 and 0.082, respectively, in control population, whereas only one genotype (CC) was present in case population (). The differences for the genotypic frequencies between case and control population were found to be statistically significant (p = .027). While the ‘T’ and ‘C’ alleles had the frequencies of 0.041 and 0.959, respectively, in the control population, whereas in the tuberculin test positive animals only ‘C’ allele was observed. The difference in allelic frequencies between case and control population was found significant (p = .03) (). At SNP locus (rs109453173), the genotypic as well as allelic frequencies did not differ significantly in case and control populations.
Table 2. Allelic frequencies and their association with susceptibility to the tuberculin test in case:control population.
Table 3. Genotypic frequencies and their association with susceptibility to the tuberculin test in case:control population.
The genotypes observed at rs109915208 locus showed significant association with the susceptibility to bovine tuberculin reaction in cattle. At rs109915208, the OR of ‘CC’ verses ‘CT’ genotype was approaching towards infinity, suggesting that animals having ‘CT’ genotype were less susceptible for tuberculin positive reaction as compared to ‘CC’ genotype. The OR of ‘C’ allele verses ‘T’ allele was approaching towards infinity, suggesting the association of ‘T’ allele with negative tuberculin test as compared to ‘C’ allele. The SNP (rs109915208) was non-synonymous which was causing an amino acid change of Alanine to Valine due to mutation of ‘C’ to ‘T’. The change of amino acids may have some role in susceptibility/resistance of animals for bovine tuberculin reaction.
NRAMP1 gene polymorphisms at 823 C/T (exon 8) are associated with resistance to human tuberculosis (Selvaraj et al. Citation2002). In a study in human, the G > C mutation of intron 4 of NRAMP1 gene was reported as a susceptible factor to Paratuberculosis (Qu et al. Citation2006). Studies at 469 + 14 G/C (INT4), 1465–85 G/A and C274T polymorphisms of NRAMP1 in ethnic Russians revealed that none of the polymorphisms was associated with tuberculosis (Puzyrev et al. Citation2002).
3.2. Microsatellite analysis
One microsatellite marker from intronic region of SLC11A1 was studied for its association with the tuberculin test. The locus was found to be highly polymorphic. A total number of six alleles and six genotypes were identified at this microsatellite locus (). Estimates of 0.61 for PIC and 0.68 for allelic diversity also revealed high polymorphism at this locus, however, the heterozygocity was moderate (0.50) at the locus. A non-significant association (p = .37) with the tuberculin test was observed for the microsatellite locus present inside gene SLC11A1 by the univariate logistic regression analysis, however one allele of 233 bp was differing significantly (p = .04) with the odds ratio towards infinity in tuberculin positive cases as compared to control population. However, all the observed genotypes differed non-significantly (p ≤ .05) in case versus control animals.
Table 4. Allelic and genotypic frequencies with their χ2 probabilities at SLCLLA1-I locus in case verses control animals.
The microsatellite locus investigated in the present study was reported to be significantly associated with occurrence of tuberculosis (Ali et al. Citation2009). One microsatellite marker from the 3′UTR region of SLC11A1 (NRAMP) gene was reported to be significantly (p < .001) associated with the incidence of bTB (Kadarmideen et al. Citation2011). Ali et al. (Citation2013) reported a significant association of ILSTS005 and ILSTS006 microsatellite loci with Single Intradermal Comparative Cervical Tuberculin Test positive animals.
In summary, it could be concluded that SNPs of SLC11A1 gene had a significant role for the tuberculin reaction test, a key trait to bTB. But these findings need further validation of markers on larger population by including few more confirmatory diagnostic tests.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ali A, Kadarmideen HN, Thomson PC, Flury C, Müller B, Zinsstag J. 2009. Association of microsatellite makers and Nramp1gene with bovine tuberculosis traits in Zebu Cattle. Proc Advance Anim Breed Genet. 18:468–471.
- Ali AA, Thomson PC, Kadarmideen HN. 2013. Association between microsatellite markers and bovine tuberculosis in Chadian Zebu cattle. Open J Anim Sci. 3(1):27–35.
- Blackwell JM, Barton CH, White JK, Roach TIA, Shaw MA, Whittehead SH, Mock BA, Searle S, Williams H, Baker AM. 1994. Genetic regulation of Leishmanial and Mycobactrial infections;the Lsh/Ity/Bcg gene story continues. Immunol Lett. 43:99–107.
- Cosivi O, Grange JM, Dabron CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeye HF, Kantor DI, Meslin FX. 1998. Zoonotic tuberculosis due to mycobacterium bovis in developing countries. Emerg Infect Dis. 4:59–70.
- Forbes JR, Gross P. 2003. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) andNramp2 (Slc11a2) expressed at the plasma membrane. Blood. 102:1884–1892.
- Ganguly I, Sharma A, Singh R, Deb SM, Singh DK, Mitra A. 2008. Association of microsatellite (GT)n polymorphism at 3´UTR of NRAMP1with the macrophage function following challenge with Brucella LPS in buffalo (Bubalus bubalis). Vet Microbiol. 129:188–196.
- Gros P, Skamene E, Forget A. 1981. Genetic control of natural resistance to mycobacterium bovis (BCG) in mice. J Immunol. 127:2417–2421.
- Gruenheid S, Gros P. 2000. Genetic susceptibility to intracellular infections: Nramp1, macrophage function and divalent cations transport. Curr Opin Microbiol. 3:43–48.
- Gruenheid S, Pinner E, Desjardins M, Gros P. 1997. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J Exp Med. 185:717–730.
- Horin P, Matiasovic J. 2000. Two polymorphic markers for the horse SLC11A1 (RNAMP1) gene. Anim Genet. 31:152.
- Kadarmideen HN, Ali AA, Thomson PC, Muller B, Zinsstag J. 2011. Polymorphisms of the SLC11A1 gene and resistance to bovine tuberculosis in African Zebu cattle. Anim Genet. 42:656–658.
- O'Reilly LM, Daborn CJ. 1995. The epidemiology of mycobacterium bovis infections in animals and man – a review. Tubercle Lung Dis. 76:1–46.
- Prakash O, Kumar A, Sonwane A, Rathore R, Singh RV, Chauhan A, Kumar P, Renjith R, Yadav R, Bhaladhare A, Baqir M, Sharma D. 2014. Polymorphism of cytokine and innate immunity genes associated with bovine brucellosis in cattle. Mol Biol Rep. 41:2815–2825.
- Puzyrev VP, Freĭdin MB, Rudko AA, Strelis AK, Kolokolova OV. 2002. Polymorphisms of the candidate genes for genetic susceptibility to tuberculosis in the Slavic population of Siberia: a pilot study. Mol Biol+. 36:788–791.
- Qu YB, Tang YX, Zhang ZB, Zhu R, Liu J, Gu SY, Lu GL, Xia ZL. 2006. Relationship between single nucleotide polymorphisms of NRAMP1 gene and susceptibility to pulmonary tuberculosis in workers exposed to silica dusts. Chin J Ind Hygi Occup Dis. 24:531–533.
- Selvaraj P, Chandra G, Kurian SM, Reetha AM, Charles N, Narayanan PR. 2002. NRAMP1 gene polymorphism in pulmonary and spinal tuberculosis. Curr Sci. 82:451–454.
- Supek F, Supekova L, Nelson H, Nelson N. 1996. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci. 93:5105–5110.