ABSTRACT
The objective of this study was to study the electrophoretic properties of Large White Yorkshire boar seminal plasma and sperm proteins. Semen was collected from eight sexually mature breeding boars by the gloved hand method; seminal plasma proteins were precipitated by the ice-cold ethanol method while sperm proteins were isolated through the Triton X detergent extraction method. Discontinuous sodium dodecyl sulphate polyacrylamide gel electrophoresis was performed to assess the molecular weight of seminal proteins. A total of 11 protein bands with the molecular weight ranging from 14 to 200 kDa were observed in the porcine seminal plasma. Protein bands of 116–200 kDa, 66–97 kDa, 55–66 kDa and 45–55 kDa were present in all the 8 boars (100%) while protein bands of molecular weight 97–116 kDa, 36–45 kDa, 29–36 kDa, 20–29 kDa and 14–20 kDa were observed only in 50%, 12.5%, 62.5%, 25% and 25% of the boars, respectively. In the case of sperm proteins, a total of 7 bands with molecular weight ranging from 29 to 200 kDa were detected. Protein bands of molecular weight 97–116 kDa, 36–45 kDa and 29–36 kDa were observed only in 62.5%, 25% and 62.5% of the boars, respectively. Seminal plasma protein fractions such as 20–29 kDa and 14–20 kDa were not detected in sperm. In conclusion, some of the seminal protein fractions in porcine semen are similar to those reported in other animal species and their role in boar fertility needs to be studied to use them as a marker for selection of breeding boars.
1. Introduction
Artificial insemination (AI) is the reproductive biotechnology that has made possible the safe use of semen from selected males in a breeding female population. Application of AI using semen from superior males has contributed to the improvement of the genetic quality of breeding herds. Utilization of preserved semen for AI in pigs has increased approximately threefold in the past 15 years.
The variations in the fertility rate among the breeding males were not addressed by the routine semen evaluation parameters, such as sperm cell concentration, motility, viability and morphology (Larson & Miller Citation2000). Subsequently, attention is now being directed towards the assessment of other aspects of semen quality as predictors of fertility. Proteins present in the seminal plasma and sperm have been reported as markers of fertility. It has been suggested that seminal proteins mediate the binding of sperm cells to oviductal epithelium and preserve membrane integrity by exerting inhibiting effects on the mitochondrial activity and metabolism to conserve energy needed until fertilization as well as to minimize the production of reactive oxygen species and lipid peroxidation of sperm membrane (Schoneck et al. Citation1996, Karunakaran et al. Citation2012a, Citation2013). Proteins would also have a role in anti-apoptosis and cell survival (Rangaswami et al. Citation2006). It has been suggested that proteins would promote capacitation of sperm cells by increasing the number of heparin-binding sites on the sperm surface and stimulating cholesterol release (Therien et al. Citation1998). Seminal proteins would mediate sperm–oocyte interaction and fertilization (Moura et al. Citation2006). Proteins such as osteopontin, prostaglandin D synthase, bovine seminal plasma proteins (BSP A1, A2, A3) and heparin-binding proteins have been reported as indicators of bull fertility (Moura et al. Citation2006). Reports on seminal proteins in bovine (Karunakaran et al. Citation2012b), buffalo bull (Asadpour et al. Citation2007), ram, equine and Yak are available. However, there is little information exists regarding the boar seminal plasma and sperm proteins. This study was carried out to assess the protein profile of boar seminal plasma and sperm by using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE).
2. Materials and methods
2.1. Experimental animals
The experiment was carried out at Research Farm, ICAR-Central Coastal Agricultural Research Institute, Old Goa, Goa, India. The experiment was conducted in accordance with the approval of Institute Research Council. Experimental animals included eight sexually matured Large White Yorkshire boars maintained for breeding. The mean age and body weight of boars were 23.33 ± 5.14 months and 195.66 ± 7.08 kg, respectively.
2.2. Management of animals
Breeding boars were housed individually in well-ventilated pens with an average temperature range of 25–30°C. Animals were fed with the standard breeder mixture containing maize, rice polish, soybean meal, mineral mixture and common salt as ingredients to fulfil the nutrient requirements (Nutrient Requirements of Swine Citation1998). Feeding was done by offering the ration twice daily in equally divided portions after moistening with water in semi-solid form, while clean drinking water was made available throughout the day.
2.3. Semen collection
Ejaculates were collected with the ‘gloved hand' method (Hancock & Hovell Citation1959) using dummy sow (IMV, France). Sperm-rich fractions were used for the semen analysis. Three ejaculates from each boar and a total of 24 ejaculates were used for the study. The seminal plasma and sperm cells were separated immediately after collection by centrifugation (560g for 10 min at 5°C). The sperm cells were washed with 2 ml of Tris Calcium chloride (TC) buffer (40 mM Tris, 2 mM CaCl2 and 0.01% sodium azide, pH 7.3) by centrifugation (560g for 5 min at 5°C) to remove the left over seminal plasma, if any. The sperm cells were re-suspended with 1 ml of TC buffer containing protease inhibitor (1 mM phenyl methyl sulphonyl fluoride) and washed thrice by centrifugation (560g for 10 min at 5°C). The sperm pellet and the seminal plasma were transported in ice to the laboratory and stored at −80°C until extraction of protein.
2.4. Extraction of seminal plasma and sperm proteins
Proteins in the seminal plasma were precipitated by adding ice-cold ethanol nine times the volume of seminal plasma and incubating at refrigeration temperature overnight (Asadpour et al. Citation2007). Protein precipitates were separated by centrifugation (8950g for 10 min at 5°C), air-dried and re-suspended in milli-Q water. Protein concentration was estimated using a spectrophotometer (Nanodrop, ND-1000, USA) and stored at −80°C.
Sperm proteins were extracted as per the method described by Nass et al. (Citation1990) with slight modifications. The sperm pellets were thawed to room temperature and washed twice with 1 ml of TC buffer at 5°C. Washed pellets were re-suspended in 1 ml of TC buffer containing Triton X-100 (0.1% v/v) and incubated for 2 h at 5°C with vortexing at 15 min interval. After completion of 2 h incubation, the suspension was centrifuged (8950g for 10 min at 5°C) to remove cellular debris. The supernatant containing sperm protein was recovered and the proteins were precipitated by adding ice-cold ethanol nine times the volume of supernatant and incubating at refrigeration temperature overnight with intermittent vortexing for initial 2 h. Protein precipitates were separated by centrifugation (8950g for 10 min at 5°C), air-dried and re-suspended in milli-Q water. Protein concentration was estimated using a spectrophotometer and stored at −80°C.
2.5. Characterization of seminal plasma and sperm proteins by electrophoresis
Discontinuous SDS-PAGE was performed according to Laemmli (Citation1970). Twelve per cent resolving gel solution and 5% stacking gel were used. Stored protein samples (seminal plasma proteins and sperm proteins) were thawed to room temperature. One hundred microgram of sample protein from each boar and standard marker proteins (broad range marker 3–205 kDa, Bangalore Genei, India) were prepared by mixing the protein solution with sample buffer (4:1) and boiling in water bath at 60–70°C for 10 min. Then the samples were loaded into each well in the stacking gel with the help of a Hamilton syringe. Three seminal plasma protein and three sperm protein samples for each boar were used for characterization.
The electrophoresis was carried out at room temperature in the constant voltage mode at 70 V till the dye front entered the resolving gel, thereafter electrophoresis was carried out at 85 V. The gel was stained with coomassie brilliant blue R-250 (0.15%) including 50% methanol and 10% acetic acid for 10–12 h and de-stained in a mixture of methanol (25%) and acetic acid (10%) in distilled water until no background was detectable. The apparent molecular mass was determined by using molecular weight markers and Gel Documentation and Analysis System (Gel-Doc. Bio-Rad, UK) and the gels were stored in acetic acid (7%).
3. Results and discussion
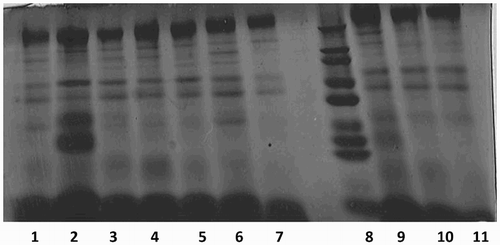
In the present study, electrophoretic profile of porcine seminal proteins was carried out by SDS-PAGE. Protein bands in the molecular weight ranging from 14 to 200 kDa were observed in the porcine seminal plasma (). A total of 11 protein bands with different molecular weight were observed. Protein bands of 116–200 kDa, 66–97 kDa, 55–66 kDa and 45–55 kDa were present in all the eight boars (100%). Protein bands with high intensity were observed at 116–200 kDa. Protein bands of molecular weight 97–116 kDa, 36–45 kDa, 29–36 kDa, 20–29 kDa and 14–20 kDa were observed only in 50%, 12.5%, 62.5%, 25% and 25% of the boars, respectively. In case of porcine sperm, protein bands with molecular weight ranging from 29 to 200 kDa were observed. A total of seven bands with different molecular weight were identified. Some of the protein fractions found in seminal plasma such as 20–29 kDa and 14–20 kDa proteins were not detectable in sperm. Protein bands of 116–200 kDa, 66–97 kDa, 55–66 kDa and 45–55 kDa were present in all the eight boars (100%). Protein bands of molecular weight 97–116 kDa, 36–45 kDa and 29–36 kDa were observed only in 62.5%, 25% and 62.5% of the boars, respectively.
Figure 1. Comassie Brillian Blue-stained protein bands in porcine seminal plasma separated by SDS-PAGE. Notes: Lane 8 molecular weight marker. Other lanes (1–7, 9–11) seminal plasma protein.
Arangasamy et al. (Citation2005) and Harshan et al. (Citation2006) reported a total of 18 and 19 protein bands, respectively, in buffalo seminal plasma with molecular weight ranging from 3 to 205 kDa. Karunakaran et al. (Citation2012b) reported protein bands with molecular weight ranging from 3 to 205 kDa in the bovine sperm membrane proteins. The comparative sequence analysis revealed strong similarities between certain seminal plasma proteins identified in several species. The sequence of the ram seminal plasma proteins RSVP14 fragment determined by N-terminal automatic sequencing (Barrios et al. Citation2005) showed a high homology with several seminal plasma proteins of other species, particularly bovine PDC-109 (Esch et al. Citation1983) and goat GSP-14/15 kDa (Villemure et al. Citation2003). Seminal protein osteopontin was detected in semen of several species, including bovine, equine and porcine. Attempts have been made to analyse the roles of different fractions on the fertility of semen samples (Yue et al. Citation2009). Using two-dimensional gel electrophoresis, Killian et al. (Citation1993) identified 2 seminal plasma proteins with high fertility in bulls (26 and 55 kDa) and 2 proteins that were correlated with low fertility (16 and 16 kDa). The 55-kDa fertility-associated protein has been identified as osteopontin (Cancel et al. Citation1999), and the 26-kDa fertility-associated protein has been identified as lipocalin-type prostaglandin D synthase (Gerena et al. Citation1998). Using the Western blotting technique, Novak et al. (Citation2010) identified porcine seminal proteins, namely AWN-1 with molecular weight of 14 kDa and osteopontin with molecular weight of 70 kDa (OPN-70), 12 kDa (OPN-12) and 9 kDa (OPN-9). In pig, the addition of osteopontin during in vitro fertilization reduced polyspermy rates (Hao et al. Citation2006) and improved embryo development after fertilization. Twenty-six kilo Dalton protein, identified as glutathione peroxidase in porcine semen had positive correlation with pregnancy rate, farrowing rate and fertility index. The 60 kDa protein had negative correlation with farrowing rate and fertility index while the 22 kDa protein had a strong negative relationship with sperm motility and total piglets born. The 22 kDa protein had been identified as PSP-I by mass spectrometry. Information on the proteins that determine the rate of capacitation and effective sperm binding to the zona pellucida and to the oocyte will undoubtedly help to improve the selection of such high-impact boars (Novak et al. Citation2010). As like that of in bovine species, identification of fertility-associated proteins in the porcine semen will be of much useful for selection of breeding boars for AI purpose.
Acknowledgement
Authors are thankful to the Director, Central Coastal Agricultural Research Institute, Old Goa for providing necessary support to carry out the experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arangasamy A, Singh LP, Ahmed N, Ansari MR, Ram, GC. 2005. Isolation and characterization of heparin and gelatin binding buffalo seminal plasma proteins and their effect on cauda epididymal spermatozoa. Anim Reprod Sci. 90:243–254. doi: 10.1016/j.anireprosci.2004.12.014
- Asadpour R, Alavi-Shoushtari SM, Asri Rezaii S, Khan MH, Ansari KM. 2007. SDS-polyacrylamide gel electrophoresis of buffalo bulls seminal plasma proteins and their relation with semen freezability. Anim Reprod Sci. 102:308–313. doi: 10.1016/j.anireprosci.2007.03.003
- Barrios B, Fernandez-Juan M, Muino-Balanco T, Cebrian-Perez JA. 2005. Immunocytochemical localization and biochemical characterization of two seminal plasma proteins which protect ram spermatozoa against cold-shock. J Androl. 26:539–549. doi: 10.2164/jandrol.04172
- Cancel AM, Chapman DA, Killian GJ. 1999. Osteopontin localization in the Holstein bull reproductive tract. Biol Reprod. 60:454–460. doi: 10.1095/biolreprod60.2.454
- Esch FS, Ling NC, Bohlen P, Ying SY, Guillemin R. 1983. Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem Bioph Res Co. 113:861–867. doi: 10.1016/0006-291X(83)91078-1
- Gerena RL, Irikura D, Urade Y, Eguchi N, Chapman DA, Killian GJ. 1998. Identification of a fetility-associated protein in bull seminal plasma as lipocalin-type prostaglandin D synthase. Biol Reprod. 58:826–833. doi: 10.1095/biolreprod58.3.826
- Hancock J, Hovell G. 1959. The collection of boar semen. Vet Rec. 71:664–665.
- Harshan HM, Singh LP, Arangasamy A, Ansari MR, Kumar S. 2006. Effect of buffalo seminal plasma heparin binding protein (HBP) on freezability and in vitro fertility of buffalo cauda spermatozoa. Anim Reprod Sci. 93:124–133. doi: 10.1016/j.anireprosci.2005.07.010
- Hao Y, Mathialagan N, Walters E, Mao J, Lai L, Becker D, Li W, Critser J, Prather RS. 2006. Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol Reprod. 75:726–733. doi: 10.1095/biolreprod.106.052589
- Karunakaran M, Devanathan TG, Kulasekar K, Sridevi P, Tilak Pon Jawahar, Loganatahsamy K, Dhali A, Selvaraju S. 2012a. Effect of heparin binding protein and hydrogen peroxide on lipid peroxidation status of bovine sperm cells. Indian J Anim Sci. 82(9):976–978.
- Karunakaran M, Devanathan TG, Tilak Pon Jawahar, Manimaran K, Anand Chitra, Dhali A, Selvaraju S. 2012b. Electrophoretic profile of bull sperm membrane proteins as a tool for selection of breeding bull. Indian J Anim Sci. 82(11):48–50.
- Karunakaran M, Devanathan TG, Tilak Pon Jawahar, Manimaran K, Anand Chitra, Dhali A, Selvaraju S. 2013. Effect of heparin binding proteins on the in vitro sperm characters and lipid peroxidation status of frozen thawed bull semen. Indian J Anim Sci. 83(8):788–790.
- Killian GJ, Chapman DA, Rogowski LA. 1993. Fertility associated proteins in Holstein bull seminal plasma. Biol Reprod. 49:1202–1207. doi: 10.1095/biolreprod49.6.1202
- Laemmli VK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. doi: 10.1038/227680a0
- Larson JL, Miller DJ. 2000. Can relative spermatozoal galactosyl transferase activity be predictive of dairy bull fertility? J Dairy Sci. 83:2473–2479. doi: 10.3168/jds.S0022-0302(00)75139-3
- Moura AA, Chapman DA, Koc H, Killian GJ. 2006. A comprehensive proteomic analysis of cauda epididymal fluid and identification of proteins associated with fertility scores of mature dairy bulls. J Androl. 98(5):71–77.
- Nass SJ, Miller DJ, Winner MA, Ax RL. 1990. Male accessory sex glands produce heparin-binding proteins that bind to cauda epididymal spermatozoa and are testosterone dependant. Mol Reprod Dev. 25:237–246. doi: 10.1002/mrd.1080250305
- Novak S, Ruiz-Sánchez A, Dixon WT, Foxcroft GR, Dyck MK. 2010. Seminal plasma proteins as potential markers of relative fertility in boars. J Androl. 31(2):188–200. doi: 10.2164/jandrol.109.007583
- Nutrient Requirements of Swine : 10th Revised Edition. 1998. The National Academies Press.
- Rangaswami H, Bulbule A, Kundu GC. 2006. Osteopontin: role in cell signalling and cancer progression. Trend Cell Biol. 16:79–87. doi: 10.1016/j.tcb.2005.12.005
- Schoneck C, Braun J., Einspanier R. 1996. Sperm viability is influenced in vitro by the bovine seminal protein aSFP: effects on motility, mitochondrial activity and lipid peroxidation. Theriogenology. 45:633–642. doi: 10.1016/0093-691X(95)00409-2
- Therien I, Moreau R, Manjunath P. 1998. Major proteins of bovine seminal plasma and high-density lipoproteins induce cholesterol efflux from epididymal sperm. Biol Reprod. 59:768–776. doi: 10.1095/biolreprod59.4.768
- Villemure M, Lazure C, Manjunath P. 2003. Isolation and characterization of gelatin-binding proteins from goat seminal plasma. Reprod Biol Endocrin, 1: 39–45. doi: 10.1186/1477-7827-1-39
- Yue W, Shia L, Bai Z, Rena Y, Zhao Y. 2009. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of ram seminal plasma proteins and their correlation with semen characteristics. Anim Reprod Sci. 116:386–391. doi: 10.1016/j.anireprosci.2009.02.014
