ABSTRACT
An eight-month-old Holstein bullock with a history of recumbency and swelling of the proximal right extremity was necropsied. Gross lesions included a complete fracture in the proximal third of the right femur and multifocal haemorrhages of the surrounding soft tissues. Histologically, there was massive bone marrow emboli of varying sizes located in the pulmonary arterial branches. The emboli were osmium tetroxide, factor VIII-related antigen and myeloid-histiocyte antigen positives, consistent with fat and haematopoietic cells of bone marrow origin. No gross or microscopic abnormalities were detected elsewhere.
1. Introduction
Bone fracture may give rise to emboli of fat that lodge in blood vessels, especially in the lung and central nervous system of man (Pollack and Mayers Citation1978; Davison and Cohle Citation1987). Embolized fat within capillary beds cause direct tissue damage as well as induce a systemic inflammatory response, known as fat embolism syndrome (Ten Duis Citation1997; Landolfi et al. Citation2004; Fernández et al. Citation2005). Syndrome is commonly seen following orthopaedic trauma, but bone marrow transplant, pancreatitis, and liposuction are also patients in risk. Yet despite all that has been written about this disease in human beings, fat embolism is infrequently described in the Veterinary literature (Caswell and Williams Citation1997; Fernández et al. Citation2005; Sierra et al. Citation2007), and the author did not find a report of bone marrow emboli apart from an experimental research using sheep as animal model (Oberst et al. Citation2009). The following is a case report of bone marrow pulmonary emboli associated with bone fracture in a bovine.
2. Material and methods
An eight-month-old Holstein calf with a history of recumbency for 40 days was submitted to Las Palmas de Gran Canaria University-Veterinary School, once a diagnosis of fracture of the right femur was achieved. Due to the poor prognosis, the animal was euthanized and a complete necropsy procedure was carried out immediately afterwards. At necropsy, the right femur showed a complete fracture affecting the proximal third with visualization of the medullary cavity and oedema and multifocal haemorrhage in the surrounding soft tissues. The right hock (tibial-tarsal) joint showed degeneration of the articular cartilage and haemorrhages in the surface of the ligaments.
Tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin wax, sectioned (3 µm) and stained with haematoxylin and eosin. Osmium tetroxide (OsO4) postfixation and paraffin embedding was also used to detect fat emboli (Davison and Cohle Citation1987; Fernández et al. Citation2005; Sierra et al. Citation2007). Formol-fixed tissues were cut into sections no thicker than 3 mm to ensure adequate reagent penetration. After washing in distilled H2O for 30 min, they were placed in a closed bottle with 1% aqueous solution of OsO4 for 2 h, with continuous agitation. The samples were then rinsed under running tap water for 30 min, immersed in 0.5% periodic acid until the dark, osmicated tissues were uniformly cleared for 30 min, and then rinsed again under running tap water. Finally, the samples were placed in distilled H2O and then processed routinely. Immunohistochemistry was performed by using a polyclonal (anti-factor VIII-related antigen, diluted 1 in 800) and a monoclonal (anti-myeloid-histiocyte antigen, MAC387, diluted 1 in 50) antibody (Dakopatts, Glostrop, Denmark) in an avidin-biotin-peroxidase complex (ABC) technique (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA), in order to demonstrate in the emboli megakaryocytes and myelomonocytic cells, respectively.
3. Results and discussion
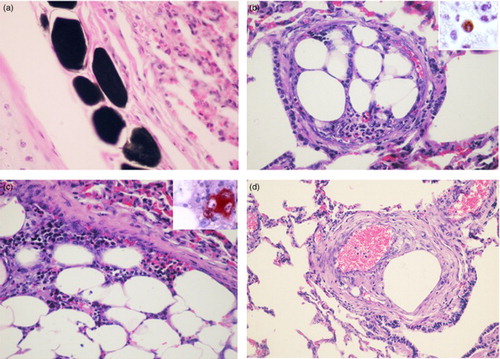
Histologically, sections of affected tissues showed oedema and haemorrhage and locally extensive necrosis and degeneration affecting the fat and muscular tissues surrounding the area of bone fracture. In the lung a large amount of fat emboli, revealed by the OsO4, was the main finding ((a)). The emboli varied in size, most were small, 50–150 µm in diameter, composed of fat and found mainly within terminal branches of the pulmonary and bronchial arteries. Largest ones, 200–1000 µm in diameter, were partly attached to the arterial walls and were covered by endothelial cells in their free surfaces ((b)). They usually appeared as fat emboli containing numerous bone marrow cells ((c)). Counts of the number of emboli observed at 100X magnification in 20 randomly selected fields revealed an average of 22 ± 7. Intimal thickening of some arteries with embolic material in the lumen and moderate proliferation of fusiform cells was a significant finding ((d)). L1 antigen was demonstrated with MAC387 antibody in the cytoplasm of granulocytes and numerous cells of the monocyte-macrophage lineage ((b), inset), and megakaryocytes were immunolabelled for FVIII-related antigen ((c), inset). Regardless of their size, none of the emboli was accompanied by tisular response or haemodynamic disturbances in the surrounding pulmonary tissue. Morphological findings were consistent with a diagnosis of bone marrow embolization associated with bone fracture.
Figure 1. Lung; 8-month-old Holstein calf. (a) Dark-staining in small fat emboli within peribronchial vessels. OsO4 postfixation. Haematoxylin and eosin (HE). 400X. (b) Bone marrow embolus within a pulmonary artery. HE. 200X. Inset: Immunoreaction to anti-MAC387 in the cytoplasm of a macrophage within a pulmonary embolus. ABC method, 3-amino-9-ethylcarbazole (AEC) chromogen. 400X. (c) Detail of the fat and haematopoietic cells components of the emboli. HE. 400X Inset: Anti-FVIII-related antigen in a megakaryocyte. ABC method, chromogen. 400X. (d) Pulmonary artery with intimal fibromuscular hyperplasia associated with the presence of an embolus. HE. 100X
Although an incidental microscopic finding of no direct clinical significance in this case, these pulmonary emboli of bone marrow were an indicator of bone fracture. The clinical manifestations of fat emboli depend on the volume of fat reaching the lungs and other affected tissues (Caswell and Williams Citation1997; Fernández et al. Citation2005; Sierra et al. Citation2007). Forensic pathologists associate fat emboli with bone fractures, diabetes mellitus, burns, acute pancreatitis, fat and soft tissue injury, and decompression sickness (Pollack and Mayers Citation1978; Ten Duis Citation1997).
The pathogenesis of fat embolism is not fully understood, and it is likely multifactorial. Direct (mechanical theory) release of bone marrow into the bloodstream after trauma following elevated intramedullar pressure and the release of free fatty acids (biochemical theory) following the inflammatory response after trauma, have been proposed as mechanisms for the development of fat emboli (Ten Duis Citation1997; Landolfi et al. Citation2004; Fernández et al. Citation2005). Pulmonary and systemic fat emboli that develop after traumatic injury are typically considered a consequence of injury to fat deposits, bone fracture and increased bone pressure during orthopaedic procedures (Ten Duis Citation1997; Landolfi et al. Citation2004; Oberst et al. Citation2009). The presence in the current case of fat in small emboli and fat with haematopoietic cells in larger emboli support the hypothesis that both mechanical and biochemical mechanisms can be simultaneously involved. Emboli were not found within the arteries of the central nervous system in spite that they are found during human trauma. In contrast to fibrocartilaginous material associated to ischemic myelopathy in humans and numerous animal species (Landolfi et al. Citation2004), fat embolization occurs most frequently in the lungs and rarely in organs belonging to the area supplied by the systemic blood circulation, which may be due to the composition and the deformability of embolic material that influences the pulmonary filtering capacity (Byrick et al. Citation1994), or due to that embolism in general affecting the cerebrospinal arterioles is very seldom observed in animals (Caswell and Williams Citation1997; Sierra et al. Citation2007).
An interesting finding was the intimal hyperplasia due to proliferative changes affecting some arteries with lodged emboli. Toxic and/or mechanical injury by the material of the emboli may have increased the expression of a number of mediators, such as matrix metalloproteinases and endothelin-1, described as inductors of myofibroblast proliferation and ground substances and fibres deposits which produces intimal thickening (Matsui et al. Citation2002). Additionally, fat emboli can cause nervous, cardiovascular and respiratory dysfunctions, pain, and disorientation in human beings (Ten Duis Citation1997; Fernández et al. Citation2005). Toxic injury in the lung and subsequent respiratory insufficiency when free fatty acids are released are described as important pathogenic mechanisms (Ten Duis Citation1997). The evolution and/or the severity of the lesions may explain the lack of respiratory signs in the current case.
This case report provides the first description of bone marrow emboli in bovine. The findings indicated that the most plausible mechanism of pulmonary embolization was associated with bone marrow passage into the vasculature due to bone fracture. It would be worth in cases of bone fracture to carry out procedures, such as radiograph or lung biopsy, to rule out respiratory complications.
ORCID
Francisco Rodríguez http://orcid.org/0000-0002-4968-5333
Acknowledgements
The author is grateful to the personnel of the laboratory of the Institute for Animal Health for the excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Byrick RJ, Mullen JB, Mazer CD, Guest CB. 1994. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Res Crit Care Med. 150:1416–1422. doi: 10.1164/ajrccm.150.5.7952570
- Caswell JL, Williams KJ. 1997. Respiratory system. In: Maxie MG editor. Jubb, Kennnedy, and Palmer's pathology of domestic animals. 5th ed. Philadelphia (PA): Elsevier Saunders; p. 523–653.
- Davison PR, Cohle SD. 1987. Histologic detection of fat emboli. J Forensic Sci. 32:1426–1430. doi: 10.1520/JFS11190J
- Fernández A, Edwards JF, Rodríguez F, Espinosa de los Monteros A, Herráez P, Castro P, Jaber JR, Martín V, Arbelo M. 2005. ‘Gas and fat embolic syndrome’ involving a mass stranding of beaked whales (family Ziphiidae) exposed to anthropogenic sonar signals. Vet Pathol. 42:446–457. doi: 10.1354/vp.42-4-446
- Landolfi JA, Saunders GK, Swecker WS. 2004. Fibrocartilaginous embolic myelopathy in a calf. J Vet Diag Invest. 16:360–362. doi: 10.1177/104063870401600421
- Matsui K, Takano Y, Yu ZX, Hi JE, Stetler-Stevenson WG, Travis WD, Ferrans VJ. 2002. Immunohistochemical study of endothelin-1 and matrix metalloproteinases in plexogenic pulmonary arteriopathy. Path Res Pract. 198:403–412. doi: 10.1078/0344-0338-00273
- Oberst M, Herget G, Riede U, Kreim, SY, Konrad G, Suedkamp NP, Haberstroh J. 2009. Fat marrow embolism during intramedullary bone endoscopy: an experimental study in sheep. J Orthop Res. 27:1060–1066. doi: 10.1002/jor.20841
- Pollack R, Mayers RA. 1978. Early diagnosis of the fat embolism syndrome. J Trauma Acute Care. 18:121–123. doi: 10.1097/00005373-197802000-00008
- Sierra E, Rodríguez F, Herráez P, Fernández A, Espinosa de los Monteros A. 2007. Post traumatic fat embolism causing haemothorax in a cat. Vet Rec. 161:170–172. doi: 10.1136/vr.161.5.170
- Ten Duis HJ. 1997. The fat embolism syndrome: review. Injury. 28:77–85. doi: 10.1016/S0020-1383(96)00085-X
