ABSTRACT
Flow cytometry (FC) is a straightforward, highly specific and sensitive procedure, which provides objective and quantitative recording of fluorescent signals from individual cells. FC has been used widely in the area of aquaculture research due mainly to the development of monoclonal antibodies (mabs). Phagocyte activity of head–kidney macrophages of several fish species, antibody production against specific antigens or detection of viruses inside immune cells are some of the several possible analysis approached by this assay. The aim of this work is to analyse and to compare kinetics of IgM-positive cells in the gill and spleen of sea bream juveniles after bath immunization against Photobacterium damselae subsp. piscicida (Phdp) using a direct staining method. Fish were immunized with three different inactivated vaccines prepared with 94/99 antigen and a commercial vaccine was used as a positive control. The effect on IgM-positive cells post-vaccination was compared among groups. Results show that the direct staining method with mabs is an effective and repeatable method for staining sea bream IgM-positive cells.
1. Introduction
Vaccination is an alternative to chemotherapeutic treatment that can help to protect fish against infection and has the advantage that no chemical residues remain (FAO/NACA Citation1995; Sanmartin et al. Citation2008). Recently, the interest in developing standard methods for measuring antibody titers in fish has increased. This is due to the insertion of monitoring programmes in aquaculture, for example, detection of serum antibody against nodavirus could be used in screening of sea bass hatcheries. Serum antibody titers have also been used as an indicator of vaccine efficacy in different fish species (Fraser et al. Citation2012). In some cases, there is a positive correlation between antibody and protection but this is not always the case, particularly with the administration of Photobacterium damselae subsp. piscicida (Phdp) vaccine by long bath to sea bream larvae. Mulero et al. (Citation2008) found that there was an increased susceptibility to the infection in vaccinated fish, and they detected IgM in vaccinated fish by ELISA. Immersion vaccination is a very common practice in aquaculture, for example, with commercial vaccines for pathogens such as Vibrio spp. or Photobacterium. Immunizating fish at early stage by this method is a good strategy since it is easier to manipulate (short bath vs. intraperitoneal injection) and requires fewer amounts of vaccine and time. An additional advantage of immersion is that vaccine delivery is through the same route that utilized by many fish pathogens, generating topologically specific mucosal immunity. A good vaccine should present several characteristics such as being easy to supply, it should produce a quick and long-term protection against pathogens and eventually to be cost-effective. In this experiment, we chose to bath-vaccinate fish. The aim of this work is to develop an effective flow cytometry (FC)-based method for staining IgM-positive cells of sea bream, and to analyse, at the same time, the production and kinetics of this globulin along time after immunization with Phdp bacterin.
2. Materials and methods
2.1. Experimental animals
A total of 1800 sea bream juveniles with an average body weight of 3 g were kindly provided by a local farm (ADSA, Alevines y Doradas S.A.) and held at the Instituto Canario de Ciencias Marinas (ICCM, Canary Islands, Spain) facilities. Fish were randomly distributed into 10 fiberglass tanks of 500 l (150 fish/tank triplicate for each treatment). All tanks were supplied with an open water system, continuous aeration (flow-through) and exposed to natural photoperiod (around 12h: 12h L: D). Fish were fed to satiation twice a day for 7 days a week. Temperature of the water was kept at 22°C ± 0.5 throughout all the experimental period.
2.2. Bacteria and vaccines
Bacterium used in this work was isolated from a natural outbreak in the Canary Islands (94/99 strain). This bacterium was identified using biochemical characterization by API 20E (Biomerieux®, Spain) and PCR analysis, after characterization it was stored by lyophilization method.
Lyophilized bacteria were rehydrated and initially grown in blood agar plate enriched with 2% of sodium chloride and 1% glucose in order to stimulate cells to produce a capsule (Acosta et al. Citation2006). The following inactivated vaccines were prepared in our laboratory using the 94/99 strain: (1) a formalin-killed vaccine: bacteria was incubated with 5‰ concentration of formalin overnight with continuous shaking, (2) a heat-shock vaccine: inactivation was reached by exposing bacteria to a temperature of 80°C for 10 min, as described by López-Dóriga et al. (Citation2000) and (3) a UV light-killed vaccine: bacteria was exposed to UV rays for 2 h. After inactivation, bacteria were washed by centrifugation in order to remove the residual culture medium. Final concentration of each of the three prepared bacterins was adjusted to an equivalent of 108 cfu/ml using a spectrophotometer (optical density = 600 nm). A fourth, commercial bath vaccine, containing Phdp antigens, was also used as a control (Ictiovac PD® , Hipra, Spain).
2.3. Immunization and sampling of fish
Once reached 5 g of body weight, fish were immunized by direct immersion in a 10-fold dilution of each bacterin (9 liter seawater: 1 liter vaccine) during 60 seconds. After being rinsed in clean water, they were replaced into their respective tanks. Control fish had the same treatment but received a 60-second bath in PBS (9 liter seawater: 1 liter Phosphate Buffered Saline). Eight fish per tank, at each sampling point, were anesthetized with 2-phenoxy ethanol, euthanized and kept on ice until necropsy; samples of spleen and gill leucocytes were isolated as described elsewhere (Dos Santos et al. Citation2001) and stored at −80°C in dMEM (Sigma) with 10% dimethyl sulfoxide (DMSO). Previous experiments showed that leucocytes could be stored at –80°C without losing their viability. Samples were taken at days 4, 9, 11, 14, 18 and 23 post-vaccination as described by Dos Santos et al. (Citation2001). At day 31 post-immunization fish received a booster and samples of spleen and gill leucocytes were taken following the same sequence as after the primary immunization.
2.4. Flow cytometry
For staining of leucocytes, an anti-seabream IgM monoclonal antibody (Aquatic Diagnostics Ltd., Stirling, UK) was conjugated with using the FluoroTag™ FITC Conjugation Kit (Sigma, USA) according to the recommendations of the manufacturer.
Leucocyte concentration of the samples was adjusted to 106 cells ml–1 in a final volume of 250 ml, and the cells were incubated with 6 µl conjugate anti-IgM-FITC at room temperature (25°C), in a dark environment for 30 min. Samples were analysed in a Coulter Epics XL flow cytometer (Coulter, Miami, FL, USA) with an air-cooled 488 nm argon-ion laser. Each cell was characterized by three optical parameters: forward-angle-scatter (FSC), side-angle-scatter (SSC) and green fluorescence (525 nm, FL1 detector) for FITC, acquired in a four-decade logarithmic scale. Green fluorescence from FITC was read combining a 550-dichroic long filter and a 525-band pass filter. Optical alignment was based on an optimized signal from 10 nm fluorescent beads (Flow-Check; Beckman-Coulter Inc., Fullerton, CA, USA). The readings were analysed with the SYSTEM II software (Coulter) and the WinMDI software version 2.8 (Joseph Trotter; the Scripps Research Institute, La Jolla, CA, USA).
3. Results
As a preliminary result, we identified three different cell subpopulations in unprocessed gill and spleen of sea bream juveniles. By using gradient separation with Lymphoprep® centrifugation, we were able to separate leucocytes from the rest of cells. The preliminary assay showed that after incubation of leucocytes with the stained antibody there were no differences in fluorescence patterns between cells washed with PBS and cells without washing (), consequently we decided not to wash cells after incubation.
Figure 1. (A) Histogram of non-washed spleen leucocytes from commercial group after incubation. We observe two picks of cells. Stained cells (H) and not-stained cells (on the left, not-gated). (B) Histogram of washed spleen leucocytes from commercial group after incubation. We observe two picks of cells. Stained cells (H) and not-stained cells (on the left). No differences between (A) and (B) are observed.
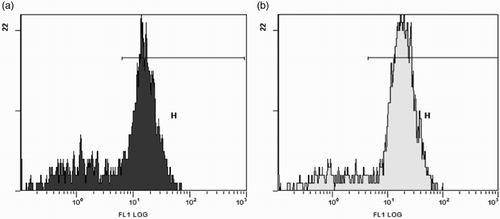
Fish which received a bath in PBS (control group) showed the lowest percentages of stained cells (); however, a pick of fluorescence is also present in this group. The percentage of IgM-positive cells was lower after the first immunization ((A) and 2(B)) in comparison with the percentage of IgM-positive cells after booster ((C) and (D)), and, in particularly, was considerably lower in the spleen than in the gills of vaccinated fish.
Figure 2. Percentages of IgM-positive cells in the gill and spleen of vaccinated fish. Values expressed as increment above control.
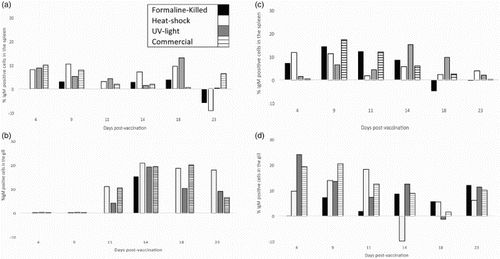
Kinetics of response was not homogeneous among treatments since some vaccines reached a pick of IgM-positive cells earlier than others. For example, after the first immunization in the gill, IgM-positive cells started to increase at day 11, while in the spleen IgM started to increase at day 4 but with considerably lower percentages of stained cells. UV-light vaccine produced a pick at day 4 after booster (week 6 after the first immunization) while commercial vaccine reached the highest percentage of IgM-positive cells at day 9 and heat-shock vaccine at day 11 after booster. Formalin-killed vaccine showed the lowest levels of IgM-positive cells and the pick was later at day 23. In the spleen, IgM-positive cells percentage was noticeably lower than the gill. In this organ, UV-light vaccine showed a pick at day 14, formalin-killed and commercial vaccine at day 9 and heat-shock remained constant from day 4 to 9. IgM-positive cells in the formalin-vaccinated fish were down-regulated twice: 23 days after the first immunization and 18 days after the booster. The same down-regulation occurred in the heat-shock-vaccinated fish at day 23 in the spleen after the first immunization and at day 14 in the gill after booster.
4. Discussion
FC is a very sensitive method that allows multiparameter analysis and which represents a cell by cell qualitative and quantitative measurement technique (Chilmonczyk & Monge Citation1999). No studies on the kinetics of sea bream IgM-positive cells after bath vaccination using FC have been carried out so far. Here we report the development of an effective FC-based method for measuring the IgM-positive cells response in gilthead sea bream spleen and gill after bath immunization. In our experiments, immersion immunization resulted in an increased number of IgM-positive cells in vaccinated fish. Our results (picks of fluorescence) were repetitive among groups and days. FC histograms showed a considerable high pick of fluorescence () and stained cells were always the gated cells. In order to reach 106 cells ml–1 in a final volume of 250 ml, a pool of organs from 8 fish/tank was analysed at each sample point, which justifies why no statistical analysis was carried out. The kinetics of response shows that there is a tendency of a higher proportion of IgM-positive cells presence in the gill than in the spleen. These data are in accordance with those from Dos Santos et al. (Citation2001) in which immersion vaccinated sea bass showed higher abundance of IgM-positive cells in the gill than in other organs. This result indicates the importance of the gill in immersion vaccination. Other authors (Nakanishi & Ototake Citation1997) postulated that the gill and the skin are the major sites of antigen uptake after immersion vaccination and that only small amount of antigen are transported to the kidney and the spleen. This may explain the lower response in the spleen of analysed fish. These authors also found that the amount of antigen taken up is correlated with the length of immersion time in dilute vaccine solutions. In this experiment, we decided to give a short bath in order to not to produce stress to the fish. In the study of Raida et al. (Citation2011), rainbow trout vaccinated by bath also showed an increased IgM response; specific antibody titres were measured by ELISA and the increased levels were found at week 8 post-immunization.
FC is likely to be considered a trustable method for measuring IgM-positive cells of fish. Up to date this technique has been used by other authors to study the immune response of sea bream phagocytes against some bacteria such as Vibrio anguillarum (Esteban et al. Citation1998) or against whole yeast cells (Cuesta et al. Citation2007) or to analyse the leucocytes production and leucocytes function in other fish species such as Atlantic salmon (Pettersen et al. Citation2000), carp (Koumans-van Diepen et al. Citation1994), European flat oyster (Renault et al. Citation2001), oyster (Goedken & De Guise Citation2004), silver perch, golden perch and crimson-spotted rainbow fish (Hardford et al. Citation2006). The increased existence of IgM appeared almost quick in the gill and spleen of vaccinated fish, especially after booster. Although no statistical analysis could be done, we found a different tendency in IgM expression among vaccines. The advantages that this method offers regarding the other known methods are the specificity, showing specifically antibody secreting cells and sensitivity, due to the fact that it permits studing these cells using several parameters such as size and complexity. In conclusion and thus all these considerations, it is still necessary to improve knowledge about specific and non-specific IgM production after bath immunization in order to know the exact moment in which booster is required. We suggest that this method is a trustable and quick tool that can be used as a complementary technique to measure sea bream IgM-positive cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acosta F, Ellis AE, Vivas J, Padilla D, Acosta B, Déniz S, Bravo J, Real F. 2006. Complement consumption by Photobacterium damselae subsp. piscicida in sea bream, red porgy and seabass normal and immune serum. Effect of the capsule on the bactericidal effect. Fish Shellfish Immunol. 20:709–717. doi: 10.1016/j.fsi.2005.08.011
- Chilmonczyk S, Monge D. 1999. Flow cytometry as a tool for assessment of the fishcellular immune response to pathogens. Fish & Shellfish Immunol. 9:319–333. doi: 10.1006/fsim.1998.0188
- Cuesta A, Rodríguez A, Salinas I, Meseguer J, Esteban MA. 2007. Early local and systemic innate immune responses in the teleost gilthead seabream after intraperitoneal injection of whole yeast cells. Fish Shellfish Immunol. 22:242–251. doi: 10.1016/j.fsi.2006.05.005
- Dos Santos NMS, Taverne-Thiele JJ, Barnes AC, Ellis AE, Rombout JHWM. 2001. The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax L.) in a Photobacterium damselaessp. Piscicida bacterin: an ontogenetic study. Fish Shellfish Immunol. 11(1):65–74. doi: 10.1006/fsim.2000.0295
- Esteban MA, Mulero V, Muñoz J, Mesenguer J. 1998. Methodological aspects of assessing phagocytosis of Vibrio anguillarum by leucocytes of gilthead seabream (Sparus aurata L.) by flow cytometry and electron microscopy. Cell Tissue Res. 293:133–141. doi: 10.1007/s004410051105
- FAO/NACA. 1995. Regional study and workshop of the environmental assessment and management of aquaculture development. FAO and Network of Aquaculture Centre in Asia-Pacific, Bangkok Tahiland. NACA Environ Aquacult Dev Ser. 1:492.
- Fraser T, Rønneseth A, Haugland Gyri T, Fjelldal P, Mayer I, Wergeland H. 2012. The effect of triploidy and vaccination on neutrophils and B-cells in the peripheral blood and head kidney of 0 + and 1 + Atlantic salmon (Salmo salar L.) post-smolts. Fish Shellfish Immunol. 33:60–66. doi: 10.1016/j.fsi.2012.04.001
- Goedken M, De Guise S. 2004. Flow cytometry as a tool to quantify oyster defence mechanisms. Fish Shellfish Immunol. 16:539–552. doi: 10.1016/j.fsi.2003.09.009
- Hardford A, O'Halloran K, Wright PFA. 2006. Flow cytometry analysis and optimization for measuring phagocytosis in three Australian freshwater fish. Fish Shellfish Immunol. 20:562–573. doi: 10.1016/j.fsi.2005.07.005
- Koumans-van Diepen JCE, van de Lisdonk MHM, Taverne-Thiele AJ. 1994. Characterisation of immunoglubulin-binding leucocytes in carp (Cyprinus carpio L). Devlop Comp Immunol. 18:45–56. doi: 10.1016/0145-305X(94)90251-8
- López-Dóriga MV, Barnes AC, dos Santos NM, Ellis AE. 2000. Invasion of fish epithelial cells by Photobacterium damselae subsp. piscicida: evidence for receptor specificity, and effect of capsule and serum. Microbiology. 146:21–30. doi: 10.1099/00221287-146-1-21
- Mulero I, Sepulcre MP, Fuentes I, García-Alcázar A, Meseguer J, García-Ayala A, Mulero V. 2008. Vaccination of larvae of the bony fish gilthead sea bream reveals a lack of correlation between lymphocyte development and adaptive immunocompetence. Mol Immunol. 45(10):2981–2989. doi: 10.1016/j.molimm.2008.01.017
- Nakanishi T, Ototake M. 1997. Antigen uptake and immune responses after immersion vaccination. Dev Specific Biotechnol Pharm Prod. 90:59–68.
- Pettersen EF, Bjerknes R, Wergeland HI. 2000. Studies of Atlantic salmon (Salmo salar L.) blood, spleen and head kidney leucocytes using specific monoclonal antibodies, immunohistochemistry and flow cytometry. Fish Shellfish Immunol. 10:695–710. doi: 10.1006/fsim.2000.0284
- Raida MK, Nylén J, Holten-Andersen L, Buchmann K. 2011. Association between Plasma Antibody Response and Protection in Rainbow Trout Oncorhynchus mykiss Immersion Vaccinated against Yersinia ruckeri. PLoS One. 6(6):e18832. doi:10.1371/journal.pone.0018832
- Renault T, Xue QG, Chilmonczyk S. 2001. Flow cytometric analysis of European flat oyster, Ostrea edulis, haemocytes using a monoclonal antibody specific for granulocytes. Fish Shellfish Immunol. 11:269–274. doi: 10.1006/fsim.2000.0312
- Sanmartin ML, Paramá A, Castro R, Cabaleiro S, Leiro J, Lamas J, Barja JL. 2008. Vaccination of turbot; Psetta maxima, against the protozoan parasite Philasterides dicentrachi: effects on antibody production and protection. J Fish Dis. 31:135–140. doi: 10.1111/j.1365-2761.2007.00876.x
