ABSTRACT
There is the need to investigate the toxicological potential of increasing doses of amitraz on the haematology as recent trend in development of resistance by pests may lead to increasing the quantity of amitraz required to control them. Eighty male rats, 180 ± 20 g assigned into 4 groups were used for the study. Groups A, B and C were treated daily with an oral dose of 10.0, 2.0 and 0.4 mg/kg body weight of amitraz, while group D was treated with 10 mL/kg water. Parameters evaluated were packed cell volume (PCV), red blood cells (RBCs) counts, reticulocyte counts (RTC), haemoglobin (Hb) concentration, white blood cell (WBC) count and differential white blood cell (DWBC) counts. There was reduction (p < .05) in the mean PCV, RBC, TWBC and Hb of rats in group A four weeks from the start of administration of the amitraz when compared to groups B, C and D. There was dose dependent increase (p < .05) in the mean percentage RTC of rats among groups A, B, C and D between days 56 and 84 of the experiment with group A producing the highest percentage counts. It was concluded that sub-chronic administration of sub-lethal doses of amitraz could lead to regenerative anaemia.
1. Introduction
Amitraz is a synthetic formamidine acaricide that is labelled for topical use to control ticks, mites and lice on cattle, pigs, poultry, dogs and to control Psylla infestation of pears (Jones Citation1990). For livestock use amitraz is available as a pour-on product and as a dip. For dogs amitraz formulations include dips, shampoos and tick collars. Amitraz is also used as an agricultural pesticide for crops, particularly fruit trees. Commercial formulations of amitraz such as Zamitraz® incorporate organic solvents such as xylene up to 75% or other solvents to the active agent in order to stabilize the agent and potentiate its ability to eradicate pests (Gupta Citation2004; Gwaltney-Brant Citation2004). Amitraz exerts toxic effects on ectoparasites by interaction with the octopamine receptors of arthropods (Evans & Gee Citation1980). Although mammals do not have octopamine receptors, amitraz exerts side effects in mammals through activation of α2-adrenoceptors (Hsu Citation1996). Amitraz poisoning is frequently encountered in dogs and cats (Grossman et al. Citation1993; Hugnet et al. Citation1996). This is partly due to their grooming habits and accidental ingestion of amitraz-containing tick collars and other products. Amitraz is well absorbed when administered orally or applied as a bath on the skin; therefore, there is high risk of toxicity (Folz et al. Citation1984). Most of the time, poisoning in animals (especially dogs and cats) with amitraz is acute (Gupta Citation2004). In children, poisoning occur due to widespread use of amitraz veterinary product (Ertekin et al. Citation2002). There is no specific antidote to amitraz poisoning and treatment is still supportive (Gwaltney-Brant Citation2004). Excessive reliance on chemical pesticides to reduce or eliminate populations of pests has been accompanied by development of insect resistance and environmental pollution. Until recently there was very little resistance to the product and it was the mainstay of tick control in many countries because of its high efficacy and relative low cost. Resistance to amitraz by ticks and other pests is now a problem to livestock and pet producers around the world and as these pests develop resistance to this pesticide, so is the need to increase the quantity of this chemical used to eliminate them thereby exposing pets and livestock to the toxicological effects of amitraz. There is information on the toxicological effects of amitraz and its combination on the central nervous system including its effects on the eye, cardiovascular, respiratory, gastrointestinal, hyperglycemic as well as its anti-inflammatory effects. Amitraz has been shown to induce cytochrome P450-dependent monooxygenases in the liver of treated rats (Ueng et al. Citation2004). Free oxygen radicals are produced during amitraz oxidation (Kruk & Bounias Citation1992). However, limited information is available on the effects of amitraz and its commercial preparation on the haematology of animals they are used on. This research therefore tends to study the toxicological effects of sub-chronic administration of sub-lethal doses of Zamitraz® (12.5% amitraz commercial preparation) on the haematology of albino wister rats.
2. Materials and methods
2.1. Amitraz
The amitraz used was Zamitraz® a product of Zampharm limited London – England containing 12.5% solution of amitraz (125 mg/mL) with batch number 120406; manufacturing date of 12/04/2012 and expiration date of 11/04/2015.
2.2. Experimental animals
Albino Wister Rats: Live animal procedures used in this study were approved by the University Institutional Animal Care and Use Committee. Eighty adult albinos wister rats (180 ± 20 g) were obtained from a stock bred and maintained at the Department of Veterinary Physiology and Pharmacology, University of Nigeria, Nsukka. The rats were kept in aluminium cages fed standard commercial rat pellets (Vital feed) prepared by Grand Cereal and Oil Meal (GCOM) Nigeria Ltd and were provided with portable water ad libitum. The rats were kept in the experimental cages and allowed to acclimatize for two weeks before the start of the experiment.
The animals were randomly assigned into four groups: Groups A, B and C were treated with 10.0, 2.0 and 0.4 mg/kg body weight of amitraz, while group D (control group) was treated with 10 mL/kg water. The amitraz and water were given by oral route daily for 12 weeks. Parameters evaluated were packed cell volume (PCV), red blood cells (RBCs) counts, reticulocyte counts (RTC), haemoglobin (Hb) concentration, total white blood cell (TWBC) counts and differential white blood cell (DWBC) counts.
Blood samples were collected at day zero and every four weeks (four observations were made) through the median canthus of the eye of the rats. PCV, RBC, TWBC, DWBC and Hb concentration were determined according to the methods of Coles (Citation1986), Schalm et al. (Citation1975) and Kachmar (Citation1970), respectively. RTC were done by the method described by Anosa (Citation1977).
2.3. Statistical analyses
Data obtained from the study were analysed using SPSS package (version 20). One way analysis of variance was used. The differences between the means were separated using post hoc, least significant difference and values of p < .05 were considered statistically significant.
3. Results and discussion
The mean PCV, Hb, RBC and TLC of rats did not vary among the groups at day zero of the experiment. The mean PCV, Hb, RBC and TLC of rats in group A was lower (p < .05) than that of groups B, C and D at day 28 of the experiment. There was no difference (p < .05) in the mean PCV, Hb, RBC and TLC of rats in groups B, C and D at day 28 of the experiment. There was dose dependent reduction (p < .05) among the mean PCV, Hb, RBC and TLC of rats in groups B, C and D from day 56 to the end of the experiment. The mean PCV, Hb, RBC and TLC of rats in group D was higher (p < .05) than that of group C from day 56 to the end of the experiment. The mean PCV, Hb, RBC and TLC of rats in group C was higher (p < .05) than that of group B from day 56 to the end of the experiment. The mean PCV, Hb, RBC and TLC of rats of group B were higher than that of rats in group A from day 28 to the end of the experiment (– and ). The dose dependent decrease in the mean PCV, RBC, Hb and TLC of the amitraz-treated groups from day 56 of the experiment revealed that sub-chronic exposure of rats to sub-lethal dose of amitraz could lead to anaemia. This anaemia observed may have been produced by the amitraz and the xylene since the preparation used contain up to 75% xylene. The anaemia observed in this research is of clinical implication for experts that increase the dose of amitraz because of development of resistance by pests as the values obtained are lower than the reference values documented for rats (Mitruka Citation1977). The anaemia developed because the rate of marrow red cell production fails to keep pace with destruction or loss of cells (MacSween & Whaley Citation1995). The results of the RTC of rats given oral sub-chronic amitraz administration expressed as the percentage of the RBC counts are presented in . The results revealed that there was no difference (p > .05) in the mean RTC of the rats at day zero of the experiment. The mean RTC of rats in group A were higher (p < .05) than those of rats in groups B and C at day 28 of the experiment. The mean RTC of rats in group B did not vary (p > .05) from that of group C at day 28 of the experiment. The mean RTC of rats in group D were lower (p < .05) than those of rats in groups A, B and C at day 28 of the experiment. There was dose dependent increase (p < .05) in the mean RTC of rats among groups A, B, C and D between days 56 and 84 of the experiment with group A producing the highest counts. The anaemia observed in this study could be said to be haemolytic and regenerative because there is effective erythropoesis as observed by the increased percentage of reticulocytes in the amitraz-treated groups. The reticulocyte is the immature red cells following extrusion of the nucleus. Maturation of reticulocyte to erythrocyte takes 48–72 hours, the final 24 hours being in the peripherial circulation. The reticulocytes, containing polyribosomes, RNA and mitochondria, can synthesize haemoglobin and their oxygen carrying capacity is limited compared to mature RBC with more haemoglobin mass. The reduction in the haemoglobin mass of the reticulocytes when compared to that of the mature RBC is probably the reason why the Hb counts of group A was lower than that of other amitraz-treated groups and the control group. The dose dependent increase in the percentage RTC of groups A, B and C could be said to be physiologic, a condition that attempts to ameliorate the anoxia associated with anaemia. It could be speculated that the anaemia observed in the amitraz-treated groups especially group A is because the dose of amitraz administered release free oxygen radicals during oxidation (Kruk & Bounais Citation1992) indicating amitraz potential to be cytotoxic to red cells. This anaemia is probably responsible for the decrease number of lymphocytes, neutrophil, eosinophil and monocytes counts of the amitraz-treated group when compared to the control group (). The basophilia observed in group A when compared to other treatment groups and the control could be as a result of the dose of amitraz used in group A being able to precipitate reticulocyte RNA. Lead also precipitate reticulocytes RNA producing punctate basophilia (MacSween & Whaley Citation1995).
Figure 1. The PCV values of rats given oral sub-chronic amitraz administration (range = 33–55%). Group A treated with amitraz at the dosage of 10.0 mg/kg body weight. Group B treated with amitraz at the dosage of 2.0 mg/kg body weight. Group C treated with amitraz at the dosage of 0.4 mg/kg body weight. Group D treated with water at the dosage of 10.0 mL/kg. Treatment was done daily using the oral route and a total of four different observations were made.
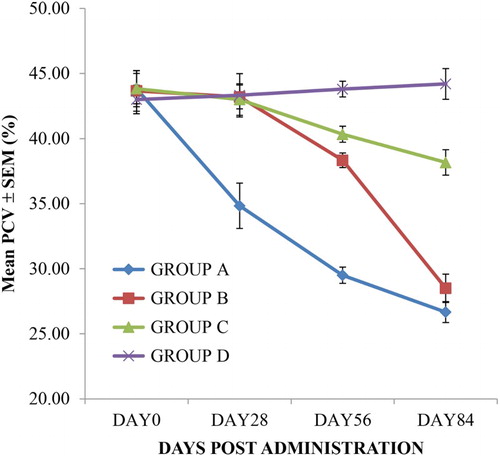
Figure 2. The haemoglobin values of rats given oral sub-chronic amitraz administration (range = 10.7–16 mg/dL). Group A treated with amitraz at the dosage of 10.0 mg/kg body weight. Group B treated with amitraz at the dosage of 2.0 mg/kg body weight. Group C treated with amitraz at the dosage of 0.4 mg/kg body weight. Group D treated with water at the dosage of 10.0 mL/kg. Treatment was done daily using the oral route and a total of four different observations were made.
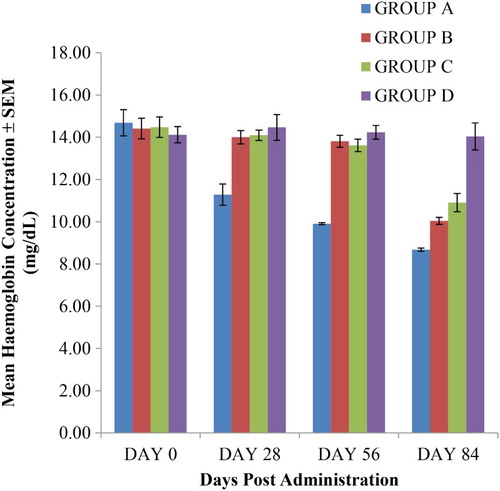
Figure 3. The RBC values of rats given oral sub-chronic amitraz administration (range = 8.86–11.3 × 106 per mL of blood). Group A treated with amitraz at the dosage of 10.0 mg/kg body weight. Group B treated with amitraz at the dosage of 2.0 mg/kg body weight. Group C treated with amitraz at the dosage of 0.4 mg/kg body weight. Group D treated with water at the dosage of 10.0 mL/kg. Treatment was done daily using the oral route and a total of four different observations were made.
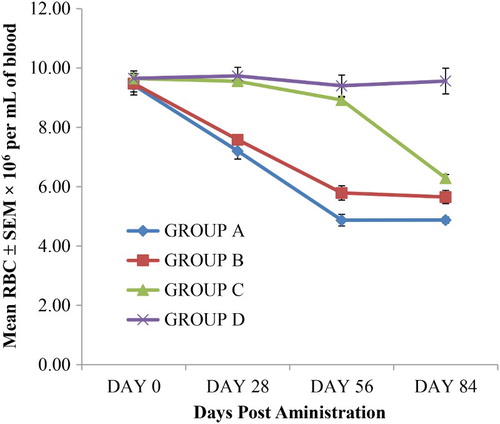
Figure 4. The RTC of rats given oral sub-chronic amitraz administration (range = 0–2% of RBC). Group A treated with amitraz at the dosage of 10.0 mg/kg body weight. Group B treated with amitraz at the dosage of 2.0 mg/kg body weight. Group C treated with amitraz at the dosage of 0.4 mg/kg body weight. Group D treated with water at the dosage of 10.0 mL/kg. Treatment was done daily using the oral route and a total of four different observations were made.
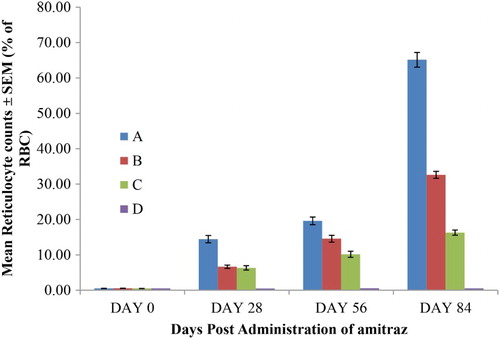
Table 1. Total leucocytes and lymphocytes counts of rats treated with amitraz daily for 84 days.
Table 2. The neutrophils and basophils counts of rats treated with amitraz daily for 84 days.
4. Conclusion
It could be concluded from the findings of this research that sub-chronic administration of sub-lethal dose of amitraz could lead to dose dependent and regenerative anaemia. Further research work need to be performed to determine whether the anaemia was produced by the amitraz, xylene or both since the amitraz preparation used (Zamitraz®) contain up to 75% xylene.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anosa VO. 1977. Haematological observations on helminthoses caused by Haemonchus contortus in Nigeria dwarf sheep. Tropical Animal Health Prod. 9:11–17. doi:10.1007/BF02297382
- Coles EH. 1986. Determination of packed cell volume. In: Coles EH editor. Veterinary clinical pathology. Philadelphia: W.B. Saunders Co, p. 17–19.
- Ertekin V, Alp H, Selimoglu M, Karacan M. 2002. Amitraz poisoning in children: retrospective analysis of 21 cases. J Intl Med Res. 30:203–205. doi:10.1177/147323000203000215
- Evans PD, Gee JD. 1980. Action of formamidine pesticides on octopamine receptors. Nature (Lond.). 28:60–62. doi:10.1038/287060a0
- Folz SD, Kakuk TJ, Henke CL, Rector DL, Tesar FB. 1984. Clinical evaluation of amitraz as a treatment for canine demodicosis. Vet. Parasitol. 16:335–341. doi:10.1016/0304-4017(84)90051-7
- Grossman MR, Garvey MS, Murphy MJ. 1993. Amitraz toxicosis associated with ingestion of acariside collar in a dog. J Am Vet Med Assoc. 203:55–57.
- Gupta CR. 2004. Amitraz. In: Plumlee KH, editor. Veterinary toxicology. Missouri: Mosby Inc; p. 514–517.
- Gwaltney-Brant S. 2004. Insecticides and molluscicides. In: Plumlee K, editor, Clinical veterinary toxicology. Missouri: Mosby Inc; p. 177–178.
- Hsu WH. 1996. Antiparasitic agents. In: Ahrens FA, editor. Famacology. Baltimore: Williams & Wilkins; p. 243–260.
- Hugnet C, Buronfusse F, Pineau X, Cadore J-L, Lorgue G, Berney PJ. 1996. Toxicity and kinetics of amitraz in dogs. Am J Vet Res. 57:1506–1510.
- Jones RD. 1990. Xylene/Amitraz: Pharmacological review and profile. Vet Hum Toxicol. 32:446–448.
- Kachmar JF. 1970. Determination of blood haemoglobinby the cyanomethaemoglobin procedure. In: Tietz NW, editor, Fundamentals of clinical chemistry. Philadelphia: W. B. Saunders Company; p. 268–269.
- Kruk I, Bounias M. 1992. Chemiluminescence from oxidation of formamidine amitraz. The generation of cytotoxic oxygen species and electronically excited compounds. Sci Total Environ. 123–124: 195–203. doi: 10.1016/0048-9697(92)90145-I
- MacSween RMN, Whaley K. 1995. The blood and bone marrow. In: David L, Robin R, Stewart F, David H, Alastair B, editors, Muir's Textbook of pathology. New Delhi: Thirteenth edition Thomson Press (India) Ltd; p. 589.
- Mitruka BM. 1977. Clinical biochemical and haematological reference values in normal experimental animals. New York: Masson publishing USA, Inc. p 76.
- Schalm OW, Jain NC, Carroll EJ. 1975. Veterinary haematology. 3rd ed. Philadelphia: 522 Lea & Febiger; p. 17–25, p. 129–125 and 268–269.
- Ueng TH, Hung CC, Wang HW, Chan PK. 2004. Effects of amitraz on cytochrome P450-dependent monooxygenase and estrogenic activity in MCF-7 human breast cancer cells and immature female rats. Food Chem Toxicol. 42:1785–1794. doi:10.1016/j.fct.2004.06.010
