ABSTRACT
The effect of combining probiotics (Lactobacillus plantarum and Lactobacillus fermentum) with flaxseed (a source of n-3 PUFAs) on the lipid metabolism and long-chain fatty acid profile of conventional piglets after weaning was studied. The levels of total lipids and high-density lipoproteins cholesterol decreased from Day 7 post-weaning, whereas levels of low-density lipoproteins cholesterol, total cholesterol and triglycerides did not change significantly in piglets with supplemented diet. The levels of alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) increased seven days post-weaning; however, the levels of dihomogamma-linolenic acid and arachidonic acid (AA) were lower and linoleic acid (LA) higher in synbioticsfed piglets compared with controls. This study demonstrates the efficacy of conversion of ALA to EPA and DHA, where delta-6-desaturase was predominantly used for n-3 polyunsaturated fatty acid synthesis from ALA at the expense of n-6 PUFAs from LA, which caused rapid increase in EPA/AA ratio on Day 14 after weaning. Combination of probiotic cheese and flaxseed is a good dietary supplement for piglets before weaning, helping them to adapt to change in diet more easily by regulating changes in lipid metabolism and vitality, and reducing the likelihood of chronic diseases.
1. Introduction
Nutritional, environmental and immune challenges associated with weaning may lead to considerable economic losses to pork producers. Weaning is generally characterized by decreased voluntary feed intake, altered gut integrity and increased concentrations of inflammatory cytokines in blood. Such nutritional and physiological abnormalities often result in diarrhoea and depression of growth in newly weaned piglets.
One option to eliminate the negative impact of the transition to solid feed in the weaning piglets is the use of probiotics, regulating intestinal microbial balance of young animals and thus maximally reinforcing the prevention of diseases (Bomba et al. Citation1999).
The mechanism of the inhibitory effect of probiotic microorganisms against pathogens has been studied by many authors (Mead Citation2000; Chaucheyras-Durand & Durand Citation2010; Cho et al. Citation2011; Heo et al. Citation2013).
Lactobacilli possess properties that make them a promising candidate for probiotics (Zhou et al. Citation2012). Lactobacillus plantarum is a plant-associated lactic acid bacterium that has also been found in human, murine, chicken and porcine gastrointestinal tract (Khonyoung & Yamauchi Citation2012; Brajdes & Vizireanu Citation2013) and shows antagonistic properties against potential intestinal pathogens (De Vries et al. Citation2006; Loh et al. Citation2013).
The current application forms of probiotics for pigs are prepared for the use just in loose forages or water. The tablet and other forms of individual applications for pigs are impractical in general. Probiotics were previously stabilized by encapsulation or lyophilization, but had high demands on the storage and activation. Fresh inoculants are unstable and therefore difficult to be used in practice (Kailasapathy Citation2002).
Hard rippened cheese of Cheddar type as the preferred vehiculum for probiotic strains of genus Lactobacillus sp., which potentiates their survival and metabolic activity has been studied by many authors (Stanton et al. Citation2005; Phillips et al. Citation2006; Mäkeläinen et al. Citation2009; Borovská et al. Citation2013).
The efficacy of probiotics may be potentiated by synergistic components of natural origin (so-called synbiotics). Recently great attention has been paid to essential polyunsaturated fatty acids (PUFAs) and their effects on piglets nutrition (Tanghe & De Smet Citation2013; Tanghe et al. Citation2014), which may help the piglets to adapt quickly to the rapidly changed diet during weaning (Li et al. Citation2014). It is widely known that flaxseed is an abundant alternative plant source of n-3 (though less of n-6) PUFAs, and one of the richest plant sources of alpha-linolenic acid (ALA), which is readily absorbed in the digestive system (Rodriguez-Leyva et al. Citation2010). Due to the metabolism of n-6 and n-3 PUFAs and their final products their ratio should be as close as possible to the value of 4–1:1 for maintaining good health (Tribole Citation2007). Through the effect on metabolism of oestrogens, PUFAs play a positive role in reducing serum cholesterol levels (Prasad Citation2001).
They could significantly affect the metabolism and function of oestrogens and thus may play a positive role in reducing serum cholesterol levels (Prasad Citation2001).
The effect of synbiotics supplementation on lipid metabolism in pigs is still little known; thus the aim of our study was to investigate the effect of L. plantarum – BiocenolTM LP96 (CCM 7512) and Lactobacillus fermentum-Biocenol™ LF99 (CCM 7514) in combination with flaxseed as a source of n-3 PUFAs on the lipid metabolism and fatty acid profile of conventional piglets originating from problem breed.
2. Material and methods
2.1. Probiotic bacteria
The Lactobacillus probiotic strains were isolated in the laboratory of the Institute of Microbiology and Gnotobiology, University of Veterinary Medicine and Pharmacy (UVMP) in Košice, Slovak Republic. The L. plantarum – Biocenol™ LP96 (CCM 7512) strain was isolated from the gut contents of healthy suckling piglets. The strain was characterized by strong adherence to the epithelial cells from the porcine intestine, by inhibitory activity against Escherichia coli O8:K88ab:H9 under in vitro conditions, and by production of hydrogen peroxide (Nemcová et al. Citation1997). The L. fermentum – Biocenol™ LF99 (CCM 7514) was isolated from the gastrointestinal tract of adult chickens. The strain was characterized by growth in the presence of bile acids and gastric juice, sensitivity to antibiotics, inhibitory activity against Salmonella enterica serovar Enteritidis and S. enterica serovar Düsseldorf (Nemcová et al. Citation2003).
2.2. Animals, housing and diets
The experiments with piglets (weanlings) were performed at the Institute of Microbiology and Gnotobiology, UVMP in Košice, Slovak Republic. The experiments were approved by the State Veterinary and Food Administration of the Slovak Republic (Approval No. 2519/10–221) and the animals were handled in accordance with the guidelines established by the relevant commission. The experiment was carried out on 36 piglets at the age of 28 days of Slovak white × Landrace cross-breed (KOAN s.r.o., Krásnovce, Slovak Republic) divided into two groups after being transported to the experimental housing in the Institute of Microbiology and Gnotobiology, UVMP in Košice, Slovak Republic, where the trial continued, differing in the diet supplementation. The experimental animals were housed (Institute of Microbiology and Gnotobiology) in stainless steel cages fitted with a slatted floor strewn with 3/4 insulating rubber and an ambient temperature of 20–22°C.
The animals were divided into two groups: Control C (n = 18, control cheese) and the E group (n = 18, probiotic cheeses + crushed flax seed). Throughout the study, the animals were fed diets mixed for early weaning of piglets OŠ-02 (Spišské Vlachy, Slovak Republic; ) and had ad libitum access to water. The feed mixture was supplemented with crushed flax seed (cultivar Flanders, Agritec, Czech Republic) as a source of PUFAs at a concentration of 10% (continuously mixed in the feed; ). In the period starting 10 days before weaning and lasting up to 14 days post-weaning, the experimental piglets in group E were supplied probiotic cheeses at a dose of 4 g/animal/day for each cheese, and in the same period the feed of group E was supplemented with crushed flax seed. Piglets in the control group C were supplied control cheese at a dose of 8 g/animal/day. Both kinds of cheeses were sprinkled on the surface of the feed.
Table 1. Ingredients (%) and chemical composition (g/kg DM) of the basal diet for early weaned pigs.
Table 2. Fatty acid composition (%) of flaxseed and feed mixtures.
Cheddar cheese (chemical composition/1 kg/: proteins 23.8%, sugars 2.8%, lipids 30.1%, metabolizable energy 1.62 MJ) was used as a vehiculum for probiotic strains. The cheeses containing probiotic strains (each cheese contained one strain) at 1 × 109 CFU/g of cheese were referred to as probiotic cheeses. The probiotic bacteria were added to the cheese milk together with 2% starter culture (Lactococcus lactis subsp. lactis, L. lactis subsp. cremoris) during typical Cheddar cheese production. The cheese used as the control one was similar to Cheddar cheese, but without probiotic strains (referred to as control cheese).
2.3. Sampling
Blood samples from piglets were taken from the plexus venosus suborbitalis on Day 0 (day of weaning; n = 6) and Days 7 (n = 6) and 14 (n = 6) after weaning. Blood serum was separated from blood samples by centrifugation at 4°C and 958×g for 10 min. All samples were analysed using gas chromatography (determination of PUFAs), spectroscopy (determination of total cholesterol (TC), total lipids (TL), triglycerides (TG), total bilirubin, alanine aminotranspherase (ALT), aspartate aminotranspherase (AST), alkaline phosphatase (ALP), gamma-glutamyltranspherase (GMT), total protein (TP)) and electrophoresis (determination of high-density lipoproteins (HDL) and low-density lipoproteins (LDL) cholesterol).
2.4. Determination of plasma fatty acids using gas chromatography
The samples of blood serum were stored in 1.5 mL aliquots in sterile tubes at −70°C until analysis. Plasma fatty acid extraction was performed using the Folch method (Folch et al. Citation1957) using dichloromethane instead of chloroform (Carlson Citation1963) according to the study of Tvrzická et al. (Citation2002). A Clarity Chromatography Station data integration system was used to integrate peak areas. The fatty acid content of pig serum was calculated as the concentration (mg/g) = peak area of a given fatty acid × concentration of internal standard (mg/mL)/peak area of internal standard/sample (mL).
2.5. Determination of TC, ALT, AST, ALP, GMT, TL, protein and bilirubin
In blood serum the concentration of TC and TG, λ 500 nm, ALT and AST, λ 340 nm, ALP and GMT, λ 405 nm were determined using commercial diagnostic kits (Randox, United Kingdom) with an ALIZÉ automatic biochemical analyser (LISABIO, France). TL, λ 520 nm, TP and total bilirubin were determined using SPECORD 210 PLUS (Analytic Jena, Germany).
2.6. Lipoprotein determination using electrophoretic assay
The HDL and LDL lipoproteins (HYDRAGEL 7 Lipoproteine, Ecomed, Žilina, Slovak Republic) were measured by HYDRASYS device (SEBIA, France) according to manufacturer's instructions. For electrophoretic study, 10 µL of serum was used for each separation. The electrophoreograms were created by means of EPSON PERFECTION V 700 PHOTO densitometer scanning at λ 570 nm.
2.7. Statistical analyses
Results are expressed as mean ± standard error of mean (SEM). Significant differences between groups were determined using t-test and differences between days using Repeated Measures Anova (GraphPad Prism 5.0 for Windows, GraphPad Software, San Diego, CA, USA).
3. Results and discussion
3.1. Health status of animals
Before the experiment diagnostic analysis was carried out in the herd of the farmer, the samples of biological material were collected for laboratory sampling (bacteriological examination of rectal swabs, virological and parasitological examination of faeces, haematological and biochemical examination of blood. These findings allowed us to diagnose hypoproteinaemia and lymphocytic leukocytosis with increased activity of hepatic enzymes (ALT, GMT) and bilirubinaemia (), and the presence of enterotoxigenic E. coli (Institute of Microbiology and Immunology, UVMP in Košice, Slovak Republic) and Coronavirus (Vetservis, s.r.o., Nitra, Slovak Republic).
Table 3. The values of selected biochemical parameters measured in the herd of farmer (Mean = 7 sucklings).
3.2. Plasma lipid level
In order to understand the modulatory effect of dietary synbiotics on plasma lipids after weaning, the lipid profile of conventional piglets during the 14-day period was studied. The supplementation of feed with a mixture of probiotic cheese and flax seed decreased the concentration of TL and HDL cholesterol on Days 7 and 14 after weaning (p < .05); however a significant decrease in HDL cholesterol (p < .05) was observed in the control group only on Day 7 (). The level of HDL cholesterol was higher in the experimental group by 40% (p < .01) on the weaning day compared to the control group, which could be affected by the combination of high lipid content in the sow's milk (Eliasson & Isberg Citation2011) with the diet (probiotic cheese with flax seed for 10 days before weaning) also rich in lipids (). During the application of synbiotics after weaning the levels of HDL cholesterol decreased on Days 7 and 14 after weaning (p < .05; by 29% and 31%, respectively) in the experimental group compared to control. Other plasma lipid parameters were not changed significantly. Many studies are focused on the effect of either probiotics (reduction in TG, TC, LDL and HDL cholesterol levels; Jin et al. Citation1998; Wang et al. Citation2009) or flaxseed (no effect; Lemay et al. Citation2002; Lucas et al. Citation2002) on lipid metabolism in animals. Several authors discussed the effect of synbiotics on cholesterol levels (Liong et al. Citation2007), who observed decrease in HDL cholesterol, but in hypercholesterolaemic pigs. In the current study, differences in HDL cholesterol levels might have been influenced by: (a) a higher intake of ALA in the form of flax seed (De Lorgeril et al. Citation1994); (b) impaired function of the liver and small intestine observed during infection, thus suppressed biosynthesis of HDL cholesterol (Češka Citation2012); (c) diversity in size and density of HDL particles (HDL 1–3) (Dobiášová et al. Citation2011), which could be examined in subsequent experiments.
Table 4. The effect of cheese supplemented with L. plantarum and L. fermentum in combination with flaxseed on lipid parameters in control (C; n = 18) and experimental (E; n = 18) groups of piglets.
3.3. Plasma fatty acids
Maternal nutrition provides all important components, including PUFAs, for young piglets (Eliasson & Isberg Citation2011). Weanlings are highly sensitive to changes that occur in the transition to solid food (Tanghe et al. Citation2014), which may lead to a variety of diseases and decrease in vitality of piglets. The addition of flax seed seems to be a suitable supplement for weanlings for high content of mainly n-3 PUFAs and fibre, thus for a smoother transition to solid feed.
As flax seed contains mostly n-3 PUFAs, the effect of their feeding in combination with probiotic on n-3 and n-6 PUFA levels was studied in conventional piglets. The concentration of ALA (a) in the blood serum increased on Days 7 and 14 (p < .05) post-weaning in experimental piglets, and was higher than in the control group (Day 7 – p < .05; Day 14 – p < .001).
Figure 1. The effect of probiotics and flax seed on the level of ALA (a), EPA (b), DHA (c), and the ratio of EPA/AA (d) in the blood serum of piglets. Days 0, 7, and 14, study days of the experiment; ALA, alpha-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; C, control group (n = 18); E, experimental group (n = 18); Data are means (mol%) ± SEM; a,x,y,z Mean values within columns with the same superscript letters differ significantly (a,x=p < .05;y=p < .01; z=p < .001). Superscript letters a show differences on Days 7 and 14 compared to Day 0 and x,y,z show differences between the groups.
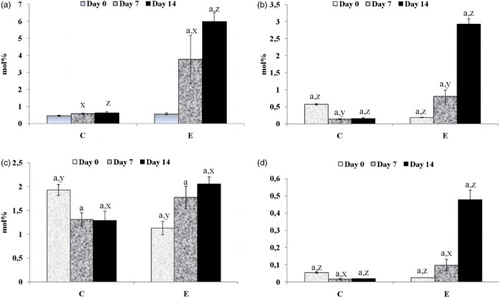
The probiotics with flax seed significantly increased the level of eicosapentaenoic acid (EPA) (b) in the blood serum of weanlings on Days 7 and 14 (p < .05), but in the control group these levels decreased (p < .05). Comparing the differences between the groups, the level of EPA in the experimental group was higher on Days 7 (p < .01) and 14 (p < .001).
Similarly, an increasing tendency of the docosahexaenoic acid (c) level was observed on Day 14 (p < .05) in piglets with supplemented diets, but a decreasing tendency in the control group on the 7th day (p < .05) post-weaning. Fourteen-day supplementation period with synbiotics markedly influenced the level of DHA (p < .05) in comparison with control animals.
The changes in concentration of n-6 PUFAs (linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid; ) were not as evident as those in n-3 PUFAs. A fourteen-day supplementation period with synbiotics increased the concentration of LA (p < .001), but significantly decreased the concentration of DGLA (p < .001) and AA (p < .05) in comparison with the control group. Such changes could be caused by the ALA-rich diet, thus the conversion of n-3 PUFAs (EPA, DHA) was predominant (Gunstone Citation2012) due to higher uptake of ALA by delta-6-desaturase, the common enzyme catalysing the conversion of n-6 and n-3 PUFAs (Stoffel et al. Citation2008).
Table 5. The effect of cheese supplemented with L. plantarum and L. fermentum in combination with flaxseed on n-6 PUFAs in control (C; n = 18) and experimental (E; n = 18) groups of piglets.
In order to determine the differences between the concentrations of n-3 and n-6 PUFAs, the EPA to AA ratio was calculated (d). The conversion of EPA from ALA (n-3) or AA from LA (n-6) depends on the amount of substrate for the delta-6-desaturase enzyme (Zhou et al. Citation2006). For this reason, the ratio of n-6/n-3 in the substrate determines the enzyme competition. The EPA/AA ratio decreased in the control piglets fed on basal diet 7 days after weaning and remained low also after 14 days (p < .05), but in the piglets fed on synbiotics it rapidly increased (p < .05) on Days 7 and 14 post-weaning. At the end of the supplementation period, significantly higher values of EPA/AA ratio were noticed in the experimental group (p < .001).
Kašteľ et al. (Citation2007) confirmed that the supplementation of feed with flax seed (elevated content of n-3 PUFAs) increased the concentration of ALA, EPA and DHA at the expense of AA concentration in the blood of experimental conventional and germ-free piglets, as was observed in our study with addition of synbiotics. Das (Citation2002) found that the decrease in the concentration of AA in experimental animals was caused by the production of prostaglandins. ALA, but especially EPA and DHA, delimits desaturation of LA and GLA, thus limiting the synthesis of AA (Grofová Citation2010). EPA and other n-3 PUFAs displace AA from phospholipid membranes and thus affect the metabolism of lipids in cats (Pawlosky et al. Citation1994), dogs (Heinemann et al. Citation2005), rats (Aïd et al. Citation2003), pigs (Huang & Craig-Schmidt Citation1996) and humans (Brenna et al. Citation2009).
Our results showed that the addition of probiotics with crushed flax seed containing ALA positively affected the composition of fatty acids in the blood plasma. Increase in n-3 PUFAs improved the ratio of n-3/n-6, which might have had an impact on the adhesion of lactobacilli in the intestine (Kankaanpää et al. Citation2001) and thus on the overall vitality and health of conventional piglets.
4. Conclusion
The synbiotics (10% flax seed + probiotics) had positive effects on the level of TL and the ratio of n-3/n-6 PUFAs. Probably the addition of flax seed rich in ALA increased the efficiency of conversion of ALA to EPA at the expense of the conversion of LA to AA throughout GLA and DGLA. PUFAs (n-3) in flax seed could potentiate the probiotics and therefore had a positive effect not only on the lipid profile of piglets, but also on their gut health and adaptation process after weaning.
Acknowledgements
Analysis of fatty acids was carried out at the 4th Centre for Research on atherosclerosis, Department of Internal Medicine, Faculty of Medicine, Charles University, Prague. We would like to thank Dr J. Konvicna for spectroscopy analysis, Ing. M. Kozackova for the technical assistence and Mr A. Billingham for the English correction of the manuscript.
Additional information
Funding
References
- Aïd S, Vancassel S, Poumès-Ballihaut C, Chalon S, Guesnet P, Lavialle M. 2003. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J Lipid Res. 44:1545–1551. doi:10.1194/jlr.M300079-JLR200
- Bomba A, Nemcová R, Gancarčíková S, Herich R, Kašteľ R. 1999. Potentiation of the effectiveness of Lactobacillus caseian the prevention of E. Coli induced diarrhea in conventional and gnotobiotic pigs. Adv Exp Med Biol. 473:185–190. doi: 10.1007/978-1-4615-4143-1_18
- Borovská D, Nemcová R, Gancarčíková S, Koščová J. 2013. The synbiotic effect of lactobacilli and flaxseed on selected intestinal microflora and organic acid levels in weaned piglets. Microbiology. 2:82–86.
- Brajdes C, Vizireanu C. 2013. Stability of Lactobacillus plantarum from functional beverage based sprouted buckwheat in the conditions simulating in the upper gastrointestinal tract. Glob Res Anal. 2:7–8.
- Brenna JT, Salem Jr N, Sinclair AJ, Cunnane SC, for the International Society for the Study of Fatty Acids and Lipids, ISSFAL. 2009. a-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostagl Leukotr Essential Fatty Acids. 80:85–91. doi:10.1016/j.plefa.2009.01.004
- Carlson LA. 1963. Determination of serum triglycerids. J Atheroscler Res. 3:334–336. doi:10.1016/S0368-1319(63)80012-5
- Češka R. 2012. Cholesterol a ateroskleróza, léčba dyslipidémií. Czech Republic: Prague, Triton (In Czech).
- Chaucheyras-Durand F, Durand H. 2010. Probiotics in animal nutrition and health. Benef Microb. 1:3–9. doi:10.3920/BM2008.1002
- Cho JH, Zhao PY, Kim IH. 2011. Probiotics as a dietary additive for pigs: a review. J Anim Vet Adv. 10:2127–2134. doi:10.3923/javaa.2011.2127.2134
- Das UN. 2002. Essential fatty acids as possible enhancers of the beneficial actions of probiotics. Nutrition. 18:786–789. doi:10.1016/S0899-9007(02)00840-7
- De Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. 1994. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 343:1454–1459. doi:10.1016/S0140-6736(94)92580-1
- De Vries MC, Vaughan EE, Kleerebezem M, de Vos WM. 2006. Lactobacillus plantarum-survival, functional and potential probiotic properties in the human intestinal tract. Int Dairy J. 16:1018–1028. doi:10.1016/j.idairyj.2005.09.003
- Dobiášová M, Frohlich J, Šedová M, Cheung MC, Brown BG. 2011. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J Lip Res. 52:566–571. doi:10.1194/jlr.P011668
- Eliasson CH, Isberg S. 2011. Production and composition of sow milk. Uppsala: Swedish University of Agricultural Sciences. Literature Review, Second cycle, A1N.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Grofová Z. 2010. Mastné kyseliny. Medicína pro praxi. 7:388–390. (In Slovak).
- Gunstone FD. 2012. Fatty acids and lipid chemistry. London: Springer, UK.
- Heinemann KM, Waldron MK, Bigley KE, Lees GE, Bauer JE. 2005. Long-chain (n-3) polyunsaturated fatty acids are more efficient than alpha-linolenic acid in improving electroretinogram responses of puppies exposed during gestation, lactation, and weaning. J Nutr. 135:1960–1966.
- Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 97:207–237. doi:10.1111/j.1439-0396.2012.01284.x
- Huang MC, Craig-Schmidt MC. 1996. Arachidonate and docosahexaenoate added to infant formula influence fatty acid composition and subsequent eicosanoid production in neonatal pigs. J Nutr. 126:2199–2208.
- Jin LZ, Ho YW, Abdullah N, Jalaludin S. 1998. Growth performance, intestinal microbial populations and serum cholesterol of broiler on diets containing Lactobacillus culture. Poultry Sci. 77:1259–1265. doi:10.1093/ps/77.9.1259
- Kailasapathy K. 2002. Microencapsulation of probiotic bacteria: technology and potential applications. Curr Iss Intest Microbiol. 3:39–48.
- Kašteľ R, Bomba A, Vaško L, Trebunová A, Mach P. 2007. The effect of probiotics potentiated with polyunsaturated fatty acids on the digestive tract of germ-free piglets. Veter Med. 52:63–68.
- Kankaanpää PE, Salminen SJ, Isolauri E, Lee YK. 2001. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett. 194:149–153. doi:10.1111/j.1574-6968.2001.tb09460.x
- Khonyoung D, Yamauchi K. 2012. Effects of heat-killed Lactobacillus plantarum L-137 on morphology of intestinal villi and epithelial cells in broiler chickens. J Appl Anim Res. 40:140–147. doi:10.1080/09712119.2011.640208
- Lemay A, Dodin S, Kadri N, Jacques H, Forest JC. 2002. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstetr Gynecol. 100:495–504. doi:10.1016/S0029-7844(02)02123-3
- Li Q, Brendemuhl JH, Jeong KC, Badinga L. 2014. Effects of dietary omega- polyunsaturated fatty acids on growth and immune response of weanling pigs. J Anim Sci. 56:1–7.
- Liong M-T, Dunshea FR, Shah NP. 2007. Effects of synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br J Nutr. 98:736–744. doi: 10.1017/S0007114507747803
- Loh TCH, Thu TV, Foo HL, Bejo MH. 2013. Effects of different levels of metabolite combination produced by Lactobacillus plantarum on growth performance, diarrhoea, gut environment and digestibility of postweaning piglets. J Appl Anim Res. 41:200–207. doi:10.1080/09712119.2012.741046
- Lucas EA, Wild RD, Hammond LJ, Khalil DA, Juma S, Gaggy BP, Stoecker BBJ, Arjmandi BH. 2002. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metabol. 87:1527–1532. doi:10.1210/jcem.87.4.8374
- Mäkeläinen H, Forssten S, Olli K, Granlunb L, Rautonen N, Ouwehand AC. 2009. Probiotic lactobacilli in a semi-soft cheese survive in the simulated human gastrointestinal tract. Int Dairy J. 19:675–683. doi:10.1016/j.idairyj.2009.06.005
- Mead GC. 2000. Prospects for ‘competitive exclusion’ treatment to control salmonellas and other foodborne pathogens in poultry. Vet J. 159:111–123. doi: 10.1053/tvjl.1999.0423
- Nemcová R, Guba P, Gancarčíková S, Bomba A, Lauková A. 2003. Štúdium probiotických vlastností laktobacilov u hydiny (In Slovak). Agriculture. 49:75–80.
- Nemcová R, Lauková A, Gancarčíková S, Kašteľ R. 1997. In vitro studies of porcine lactobacilli for possible probiotic use. Berliner Munchener tierarztliche Wochenschrift. 110:413–417.
- Pawlosky R, Barnes A, Salem JRN. 1994. Essential fatty acid metabolism in the feline: relationship between liver and brain production of long-chain polyunsaturated fatty acids. J Lipid Res. 35:2032–2040.
- Phillips M, Kailasapathy K, Tran L. 2006. Viability of commercial probiotic cultures (L. acidophilus, Bifidobacterium sp., L. casei, L. paracasei and L. rhamnosus) in cheddar cheese. Int J Food Microbiol. 108:276–280. doi:10.1016/j.ijfoodmicro.2005.12.009
- Prasad K. 2001. Antioxidants and diabetes. Review of the book “antioxidants in diabetes”. Review of the book “antioxidants in diabetes managements”. In: Packer L, Rosen P, Tritschler HJ, King B L, Azzi, A, editors. Trends in endocrinology and metabolism. 12:179–180. doi:10.1016/S1043-2760(00)00348-9
- Rodriguez-Leyva D, Mc Bassett C, Mc Cullough R, Pierce GN. 2010. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alphalinolenic acid. Can J Cardiol. 26:489–496. doi:10.1016/S0828-282X(10)70455-4
- Stanton C, Ross RP, Fitzgerald GF, Van Sinderen D. 2005. Fermented functional foods based on probiotics and their biogenic metabolites. Curr Opin Biotechnol. 16:198–203. doi:10.1016/j.copbio.2005.02.008
- Stoffel W, Holz B, Jenke B, Binczek E, Gunter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K. 2008. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 27:2281–2292. doi:10.1038/emboj.2008.156
- Tanghe S, Cox E, Melkebeek V, De Smet S, Millet S. 2014. Effect of fatty acid composition of the sow diet on the innate and adaptive immunity of the piglets after weaning. Vet J. 200:287–293. doi:10.1016/j.tvjl.2014.02.025
- Tanghe S, De Smet S. 2013. Does sow reproduction and piglet performance benefit from the addition of n-3 polyunsaturated fatty acids to the maternal diet? Vet J. 197:560–569. doi:10.1016/j.tvjl.2013.03.051
- Tribole E. 2007. The ultimate omega-3 diet: maximize the power of omega-3s to supercharge your health, battle inflammation, and keep your mind sharp. New York: McGraw-Hill Professional.
- Tvrzická E, Vecka M, Staňková B, Žák A. 2002. Analysis of fatty acids in plasma lipoproteins by gas chromatography-flame ionization detection: quantitative aspects. Anal Chim Acta. 465:337–350. doi:10.1016/S0003-2670(02)00396-3
- Wang Y, Xu N, Xi A, Ahmed Z, Zhang B, Bai X. 2009. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl Microbiol Biotechnol. 84:341–347. doi:10.1007/s00253-009-2012-x
- Zhou D, Ghebremeskel K, Crawford MA, Reifen R. 2006. Vitamin A deficiency enhances docosahexaenoic and osbond acids in liver of rats fed an alpha linolenic acid-adequate diet. Lipids. 41:213–219. doi:10.1007/s11745-006-5090-x
- Zhou YK, Qin HL, Zhang M, Shen TY, Chen HQ, Ma YL, Chu ZX, Zhang P, Liu ZH. 2012. Effects of Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. World J Gastroenterol. 18:3977–3991. doi:10.3748/wjg.v18.i30.3977
