ABSTRACT
The aim of the study was to assess the occurrence of bovine respiratory syncytial virus (BRSV) and bovine herpesvirus 1 (BHV-1) in herds of European (Polish) bison in the Białowieża National Park. The materials consisted of nasal swabs and sera (n = 35) obtained from male and female Polish bison ranging in age from 1 to 3 years. Anti-BRSV and BHV-1 antibodies were detected using commercially available indirect Enzyme-linked immunosorbent assay (ELISA) kits. The occurrence of BHV-1 and BRSV was confirmed using polymerase chain reaction (PCR) and reverse transcription (RT-PCR) methods, respectively. In the examined nasal samples and sera only 3 of 35 were BRSV positive using the ELISA kit and in RT-PCR. In the case of BHV-1, 6 of the 35 samples were positive in ELISA and PCR methods. Of the six individuals that tested positive for BHV-1, three were also seropositive for BRSV. Individuals reacting positively in the ELISA assay were confirmed using PCR for BHV-1 and in RT-PCR for BRSV. In summary, these studies confirm the presence of BRSV and BHV-1 viruses in European Bison in Poland, and should be continued in more herds of bison in Poland.
1. Introduction
Infections induced by the viruses, bovine herpesvirus 1 (BHV-1) and bovine respiratory syncytial virus (BRSV) are a significant health problem in the aetiopathogenesis of respiratory syndrome in cattle. Numerous epidemiological studies have confirmed that apart from direct transmission of pathogenic agents within cattle herds, a significant role in the transmission of pathogens can be played by wild ruminants, which in many cases are characterized as reservoirs of pathogens involved in the aetiopathogenesis of numerous cattle diseases. These include tuberculosis, which has been confirmed in the wild ruminants population in Poland, including red deer and European bison (Salwa et al. Citation2011). Moreover, individuals seropositive for BVDV among red deer and roe deer were found to constitute about 10% of the animal population tested. Other reports (Haigh et al. Citation2002) have confirmed the occurrence of various aetiological infectious agents for cattle in the wild ruminants population (European bison, American bison, red deer, chamois, etc.) in the United States, Canada, Australia, New Zealand and European countries. These agents include viruses of the herpes family (BHV-1 and 2), parapoxviruses, picornaviruses (Food and Mouth Disease – FMD), paramyxoviruses (PI-3), adenoviruses, coronaviruses, rotaviruses and flaviviruses (bovine viral diarrhoea virus – BVDV), as well as parasites, such as Dermacentor sp., Dictyocaulus sp., Ostertagia sp., Fasciola sp. and others (Haigh et al. Citation2002).
The occurrence of individuals seropositive for BHV-1 has not yet been confirmed in wild ruminants (Rypuła et al. Citation2011; Salwa et al. Citation2011). In the case of infections induced by BRSV, there are no data at all concerning its occurrence among wild ruminants. A significant problem is the lack of effective control and the limited possibilities for eliminating persistently infected (PI) individuals and asymptomatic carriers, due to the migration of animals within the country, as well as into other EU countries – the Czech Republic, Slovakia and Lithuania, and countries outside of the EU – Ukraine, Russia and Belarus. The problem is exacerbated by the fact that, as reported by Hofman-Kamińska and Kowalczyk (Citation2012), the number of European (Polish) bison is still growing, which increases the risk of uncontrolled transmission of pathogens to cattle herds.
In view of the above, the aim of the study was to assess the occurrence of the viruses BRSV and BHV-1 in herds of Polish bison in the Białowieża National Park.
2. Material and methods
2.1 Material collection
Nasal swabs (n = 35) suspended in 1 ml of RPMI1640 medium (Sigma, Germany) and sera (n = 35) were obtained from Polish bison – males and females ranging in age from 1 to 3 years. Because especially the occurrence of the virus BRSV is seasonal (fall, winter and early spring), the material from animals was collected in autumn in late October and early November (Van der Poel et al. Citation1994). The study included 5 herds of bison ranging in size from 8 to 12 animals. The material from the animals was collected during a routine veterinary health inspection of herds moving through the Polish regions, after asphyxiation with a shot of anaesthesia. It should be emphasized that in Poland bisons are protected and are subject to periodic monitoring at an angle size and structure of the herd. The animals showed no clinical signs of respiratory syndrome. Samples were stored at −80°C until analysis.
2.2. ELISA assays
Anti-BRSV antibodies were detected in serum using a commercially available indirect Enzyme-linked immunosorbent assay (ELISA) kit (VB005, Cypress Diagnostics, Belgium) according to the manufacturer's recommendations. Absorbance was measured using a Bio-Rad 680 ELISA reader at a wavelength of 450 nm.
The ELISA kit BIO K 028/2 was used for BHV-1 antibody detection (Bio-X Diagnostics, Belgium). Sample preparation, the test procedure and calculation of the results were performed according to the manufacturer's instructions. Absorbance was measured using a Bio-Rad 680 ELISA reader at a wavelength of 450 nm.
2.3. Reverse transcription-polymerase chain reaction (RT-PCR) protocol
RNA was extracted from supernatants containing secretions from the nasal cavity with an RNeasy Mini kit 250 (Qiagen, NL). Polymerase chain reaction (PCR) analysis was carried out using a Titan One-Tube RT-PCR System (Roche, Ge). The primers used to detect the G protein were B7A: 5′-CAT CAA TCC AAA GCA CCA CAC TGT C-3′ and B8: 5′-GCT AGT TCT GTG GTG GAT TGT TGT C-3′, yielding products of 371 bp (Vilček et al. Citation1994). RT-PCR was conducted for 1 h at 48°C, followed by 2 min denaturation at 94°C. Amplification was carried out in 40 cycles in the following conditions: denaturation for 45 s at 94°C, hybridization for 45 s at 51°C, and elongation for 1.5 min at 72°C, followed by a final elongation for 7 min at 72°C. PCR products were evaluated in 1.5% agarose gel stained with ethidium bromide and analysed with Quantity One software (Bio-Rad, USA).
2.4. PCR protocol
DNA was extracted from secretions from the nasal cavity with a Qiaamp® DNA Mini kit 50 (Qiagen, NL). The PCR mix consisted of 2.5 μl of 10× DNA polymerase buffer, 1 μl of 10 mM dNTP nucleotide mix (Fermentas, Lithuania), 1 μl of a 5 mM solution of each primer (gDp1, gDp2), 1 μl of thermo-stabile RED TaqTM DNA polymerase (Fermentas, Lithuania) and 16.5 μl DNA-RNA free water (Sigma). The mixture was supplemented with sterile water to a final volume of 25 μl. The primers used to detect the gene encoding the protein BHV-1 were p1 5′-GCT GTG GGA AGC GGT ACT-3′ and p2 5′-GTC GAC TAT GGC CTT GTG TGC-3′, yielding products of 468 bp, according to Rola et al. (Citation2005). For the reaction, denaturation was conducted for 5 min at 94°C and 35 cycles were carried out in the following conditions: denaturation for 1 min at 94°C, hybridization for 45 s at 62°C and elongation for 1 min at 72°C. The last cycle was performed in total and the elongation step after the last cycle was prolonged to 8 min. PCR products were evaluated in 1.5% agarose gel stained with ethidium bromide. The products obtained were analysed using Quantity One software (Bio-Rad, USA).
3. Results and discussion
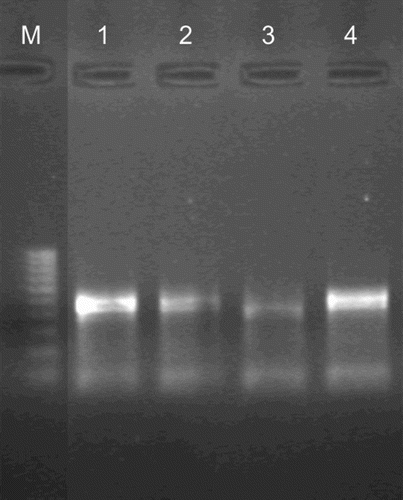
In the nasal samples and sera examined only 3 of 35 were BRSV positive in the ELISA kit and in RT-PCR BRSV detection (). It should be noted that the positive results obtained in RT-PCR exclusively concerned with nasal swabs obtained from bison at the age of two years life. The virus antibodies anti-BRSV and viral antigens were found in the same animals.
Figure 1. Detection of BRSV by the RT-PCR assay in nasal swabs.
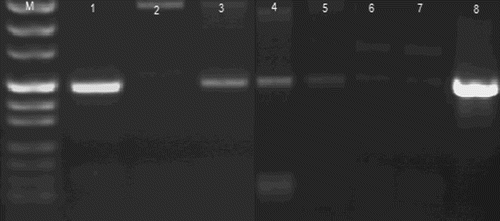
In the case of BHV-1 detection, 6 of the 35 samples were positive in PCR and 3 were positive the ELISA methods. Of the six individuals that tested positive for BHV-1, three of them were also seropositive for BRSV. All BHV-1 positive animals were older than three years. The results for the individuals reacting positively in the ELISA assay were confirmed in PCR for BHV-1 ().
Figure 2. Detection of BHV-1 in the nasal swabs by the PCR assay.
The results obtained in present study indicate a low level of occurrence of BRSV in the European bison herds examined. It can be presumed, however, that the lack of seropositive individuals is linked to the age of the animals examined. In domestic cattle the occurrence of BRSV has been shown mainly in individuals aged up to 12 months, which is linked to the greater susceptibility of young animals to infections (Antonis et al. Citation2010; Raaperi et al. Citation2012).
A small percentage of seropositive individuals, confirmed by the PCR technique was also found for BHV-1. In an earlier study, however, Rypuła et al. (Citation2011) did not obtain positive results in ELISA detection of antibodies against BHV-1. Our present study shows that the epidemiological situation in the population of European bison in Poland has not significantly increased, however three BHV-1 seropositive (ELISA) and six antigen positive (PCR) animals confirm the presence of the virus among bison.
The results obtained confirm the occurrence of the viruses BRSV and BHV-1 in five herds of bison in Poland. It cannot be definitively stated whether the individuals seropositive for BRSV and BHV-1 will pose a threat to domestic cattle in pastures in the vicinity of the bison herds. Nevertheless, the confirmation of the presence of BHV-1 and BRSV in several individuals in such a small number of herds, where the size of the herds ranges from 8 to 12 animals, may indicate the prevalence of these viruses and the possibility of PI individuals, especially since a characteristic trait of the natural behaviour of the European bison, typical for the herd instinct, is migration associated with the search for food, which makes effective and more precise monitoring of the transmission of infections more difficult. As reported by other authors (Haigh et al. Citation2002, Salwa et al. Citation2011), wild ruminants (mule deer, elk and bison) may harbour and transmit the virus without exhibiting significant disease symptoms.
4. Conclusion
To sum up, it should be emphasized that this is a preliminary study that will be continued in more herds of bison in Poland, however this study confirms the presence of BRSV and BHV-1 viruses in European Bison in Poland. Taking into account the natural behaviour of the animals associated with their migration, possible transmission of these viruses also in the neighbouring countries of Poland can be assumed where there is occurrence of bison.
Acknowledgements
The authors would like to acknowledge Michał Krzysiak, DVM, of the European Bison Breeding Center in Białowieża National Park, Poland, for technical support in blood collection and for assistance in handling the bison.
References
- Antonis AF, de Jong MC, van der Poel WHM, van der Most RG, Stockhofe-Zurwieden N, Kimman T, Schrijver RS. 2010. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J Gen Virol. 91: 2497–2506. doi:10.1099/vir.0.020842-0
- Haigh JC, Mackintosh C, Griffin F. 2002. Viral, parasitic and prion diseases of farmed deer and bison. Rev Sci Tech. 21: 219–248.
- Hofman-Kamińska E, Kowalczyk R. 2012. Farm crops depredation by European bison (Bison bonasus) in the vicinity of forest habitats in northeastern Poland. Environ Manage. 50: 530–541. doi:10.1007/s00267-012-9913-7
- Raaperi K, Bougeard S, Aleksejev A, Orro T, Viltrop A. 2012. Association of herd BRSV and BHV-1 seroprevalence with respiratory disease and reproductive performance in adult dairy cattle. Acta Vet Scand. 54: 4–13. doi:10.1186/1751-0147-54-4
- Rola J, Larska M, Polak MP. 2005. Detection of bovine herpesvirus 1 from an outbreak of infectious bovine rhinotracheitis. Bull Vet Inst Pulawy. 49: 267–271.
- Rypuła K, Krasińska M, Kita J. 2011. The occurrence of alpha-herpesvirus and pestivirus infections in European bison (Bison bonasus) in the Bialowieza Primeval Forest. European Bison Conservation Newsletter. 4: 89–94.
- Salwa A, Anusz K, Welz M, Wozikowski R, Zaleska M, Kita J. 2011. Statistical analysis of epizootical situation in farm and wild animals living in Bieszczady region in relation to the occurrence of bovine tuberculosis in bison (Bison bonasus). European Bison Conservation Newsletter. 4: 71–80.
- Van der Poel WH, Brand A, Kramps JA, Van Oirschot JT. 1994. Respiratory syncytial virus infections in human beings and in cattle. J Infect. 29: 215–228. doi:10.1016/S0163-4453(94)90866-4
- Vilček S, Elvander M, Ballagi-Pordány A, Belál S. 1994. Development of nested PCR assays for detection of bovine respiratory syncytial virus in clinical samples. J Clin Microbiol. 32: 2225–2231.
