ABSTRACT
This study was carried out in the Qassim region, central of Saudi Arabia to determine the clinical, haematological and therapeutic impact of babesiosis caused by Babesia caballi in Arabian horses. The clinical signs in infected horses were pyrexia (40.5–41.5°C), depression, pale or icteric mucous membranes and emaciation. Haematological examination revealed significant decrease in erythrocyte, thrombocyte counts and haemoglobin concentration in infected horses compared with controls. Treatment with imidocarb dipropionate was 100% effective in eliminating the parasites from the blood and led to an improvement in the clinical state. In conclusion, babesiosis should be suspected in any horses showing signs of anaemia or jaundice and severe body weight loss in addition to the presence of ticks. Bloody urine which is a characteristic feature of babesiosis in other animals is not common in Arabian horses. Moreover, treatment using imidocarb dipropionate in conjunction with hematinics and fluid therapy should be applied for treatment of equine babesiosis.
1. Introduction
Equine babesiosis is an infectious disease of horses, donkeys, zebra and mules caused by intra-erythrocytic, protozoa of the genus babesia; Babesia caballi (large babesia) and transmitted by ticks (Russell et al. Citation2005; Sakha Citation2007; Knowles Citation2010). These parasites are widely distributed in the world, including Asian continent, Europe, Africa, and South America, and the prevalence corresponds to the presence of the tick-vectors (Kumar & Kumar Citation2007; Motloang et al. Citation2008).
The clinical picture of the disease in horses is variable and may be manifested by fever, oedema of the ventral abdomen, icterus and haemoglobinuria (Radostits et al. Citation2007). However, it has been thought that carriers are generally responsible for the propagation and maintenance of the infection (Radostits et al. Citation2007).
The disease and its severity not only depend on the virulence of the causative agent, but also to a large extent on the degree of host susceptibility where the disease is more severe in those horses moved from tick-free areas to tick infested areas or in animals suffering from stress (Mehlhorn & Schein Citation1998).
The diagnosis of babesiosis in acute cases is mainly based on clinical findings and microscopic examination of 10% Giemsa-stained thin blood smears. However, expertise in piroplasm microscopy is required in unapparent carrier horses or chronic infections because parasitaemias are often extremely low and may otherwise be missed (Friedhoff & Soule Citation1996; Bashiruddin et al. Citation1999; Hodgson Citation2002).
Economic losses associated with equine babesiosis include cost of treatment, abortion, loss of performance, restrictions in meeting international requirements related to exportation or participation in equestrian sporting events and death in some cases (Kerber et al. Citation1999; Rothschild Citation2013). The disease is endemic in Saudi Arabia. So, this study was delineated to study the clinical, haematological and therapeutic impact concerning babesiosis in Arabian horses.
2. Materials and methods
2.1. Animals
At the Qassim region, central of Saudi Arabia, 20 (13 females and 7 males) B. caballi naturally infected Arabian horses ( and ) and 10 (5 females and 5 males) parasitologically free controls were used in this study. All horses under study were over 5 years old.
2.2. Clinical examination
All horses were subjected to careful clinical examination before treatment.
2.3. Parasitological examination
Thin blood films from the ear vein, air-dried, fixed in methanol for 5 min, stained in 10% Giemsa stain and rinsed with distilled water. The smears were examined at 1000 magnification (oil immersion) where at least 50 fields were searched per slide.
2.4. Haematological examination
Anticoagulated blood samples were collected via jugular venepuncture from the infected horses (n = 20) as well as from the controls (n = 10). The samples were taken before administration of any medications. Erythrocyte and total and differential leucocyte counts and thrombocytes as well as haemoglobin concentration were measured (Coles Citation1986).
2.5. Treatment trials
To study the efficacy of imidocarb dipropionate in the treatment of equine babesiosis in naturally infected horses at different stages of the disease was carried out. Twenty B. caballi infected horses were treated using imidocarb dipropionate (Imizol, Schering-Plough Animal Health) for two treatments 24 h apart delivered intramuscularly to the neck at a dosage rate of 2.2 mg/kg body weight. Additional supportive treatments as phosphorous; 10 mg/kg body weight and vitamin B12; 500 mg/ kg bodyweight (Cobozal 10%, Arab Company For Medical Product) and two liters of 10% dextrose saline were administered for five days. Tick control by spraying the surrounding environment with diazinon (Nucedol Ultra 60 EC, Bioagripharm, Germany) was performed twice, one week apart. The clinical and parasitological status of each treated horses was evaluated for 4 weeks post-treatment.
2.6. Statistical analysis
Student t test was carried out on the obtained data. P-values less than 0.05 were considered significant.
3. Results
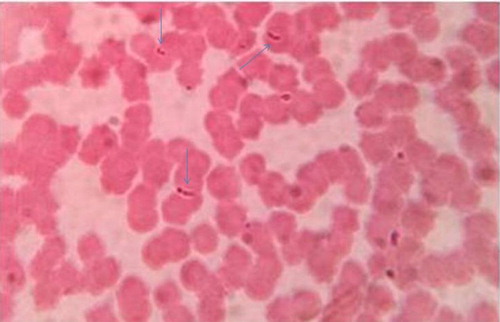
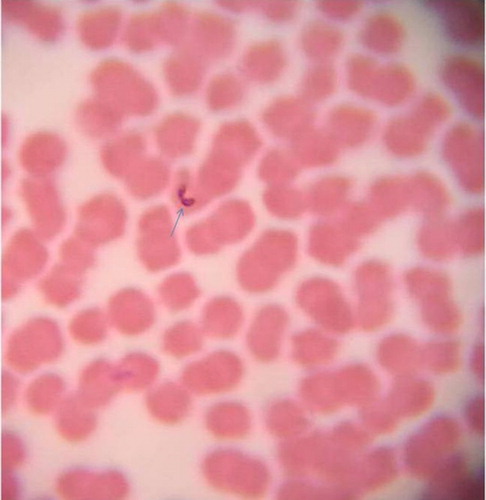
Microscopic examination of blood smears of infected horses revealed pyriform-shaped merozoites of B. caballi within erythrocytes that often form pairs joined at their posterior ( and ).
The clinical signs observed in infected horses are recorded in . In the early stage of infection, pyrexia (40.0–41.0°C), anorexia, pale of conjunctiva, weakness and depression were the most common signs. Death occurred in one late-treated horse.
Table 1. Clinical signs in B. caballi infected horses.
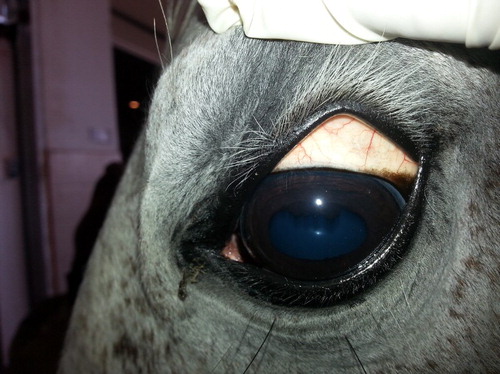
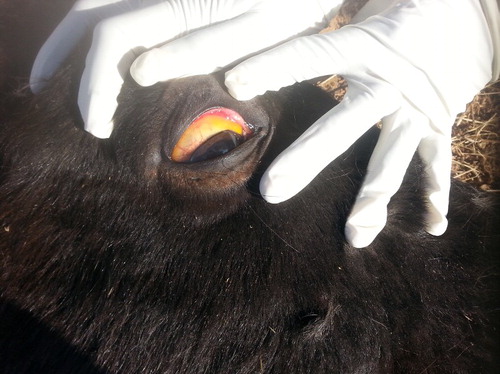
After recovery from the acute phase of babesiosis, the infection may assume a subclinical, chronic course and these animals could become carriers of the protozoans. Chronically infected horses showed signs of inappetence, pale or icteric mucous membranes ( and ) and severe loss of body weight. Oedema was observed in the limbs and at the thoracic inlet in two cases (). Temperature of animals was not affected. Pregnant mares sometimes abort at 8 months pregnancy (2 cases out of 4 pregnant mares). Haemoglobinurea was only observed in two acute infected cases and not observed in chronically infected ones.
Hematological examination revealed decrease in the erythrocyte counts, haemoglobin concentration and thrombocytes in addition to a decrease in packed cell volume in infected horses compared with the controls ().
Table 2. Haemogram in healthy and B. caballi infected horses (mean ± SD).
Treatment results revealed that early intervention using imidocarb dipropionatein in conjunction with supportive treatments as phosphorous and vitamin B12 and two liters of 10% dextrose saline were highly efficient in the elimination of the protozoan parasites from blood with a clinical improvement and an eventual 100% cure rate especially in acutely affected horses. Clinical improvement takes more time in chronic cases (four weeks) compared with acute ones (within week). Forty percent of treated horses (8 horses out of 20) showed salivation, colic and sometimes recumbence that disappeared after short time.
4. Discussion
Equine piroplasmosis (EP) is a tick-borne protozoal disease of horses, mules, donkeys and zebras that is characterized by acute haemolytic anemia (Rothschild Citation2013).
The paired merozoites joined at their posterior ends are considered to be a diagnostic feature of B. caballi infection. Similar findings were recorded previously by de Waal (Citation1992) and OIE Terrestrial Manual (Citation2014).
Considerable variation was noticed in the clinical signs of the infected horses according to the stage of infection. Death may be due to the haemolytic anemia which results from intra-erythrocytic piroplasms (Preston et al. Citation1992). Similar signs were recorded previously by Sakha (Citation2007), Uilenberg (Citation2006) and Zobba et al. (Citation2008).
Chronically infected horses showed signs of inappetence, pale or icteric mucous membranes and severe loss of body weight. Similar findings were reported previously by Potgieter et al. (Citation1992) who recorded that intrauterine infections may result in abortion and neonatal death and Teglas et al. (Citation2005) who found that diseases related to the tick-borne infections (chronic) can frequently result in abortion and decrease in weight gain. Often clinical signs of piroplasmosis are too general to allow specific diagnosis and may be confused with other diseases especially in endemic areas. In carrier horses that may act as reservoirs of infection, parasites were present in very low numbers in the blood (Böse et al. Citation1995). In addition, de Waal and van Heerden (Citation1994) noticed that the clinical signs of EP were not pathognomonic for the disease, especially in endemic areas and horses infected with B. caballi may remain carriers for up to 4 years and carrier horses maintained the life cycle of the parasites by serving as a source of transmission for ticks.
Pale or icteric mucous membranes that represent a constant feature of the disease are largely due to hyperbilirubinemia from severe intravascular haemolysis as a result of multiplication of the piroplasms inside red blood cells (Radostits et al. Citation2007). Jaundice occurred as a result of the increase in the indirect (un-conjugated) bilirubin, which is deposited in mucosal surfaces and elsewhere, imparting a yellow color to those tissues (Rothschild Citation2013). Haemoglobinurea was not a prominent feature of the disease. Similar observations were previously recorded by de Waal (Citation1992) and Friedhoff and Soule (Citation1996).
Camacho et al. (Citation2005) explained the weight losses in infected horses by the energy uptake by piroplasms cells in forms of adenosine tri-phosphate (ATP) and adenosine mono-phosphate (AMP) deprive the host (horse) of essential energy for normal anabolic processes thus contributing to the loss of weight.
Comparisons between infected and healthy horses (mean ± SD) showed a significant decrease in erythrocyte counts, haemoglobin concentration and thrombocytes in infected horses compared with the controls. Moreover, a significant decrease in packed cell volume was also seen (). Similar result was recorded previously by Alsaad (Citation2014).
Ambawat et al. (Citation1999) mentioned that the alterations in erythrocyte membrane protein and lipid content and increases in plasma malondialdehyde during severe parasitemia suggested that accumulation of oxidative ions resulting from lipid peroxidation alters the erythrocytes’ biochemical composition, leading to haemolysis. Moreover, they added that thrombocytopenia may be a result of low-grade DIC, as proposed in dogs with Babesia canis infections.
There is no safe and efficacious vaccine against equine babesiosis in Saudi Arabia and control of the disease is mainly based on the chemotherapy and tick control. Treatment results revealed that early intervention using imidocarb dipropionatein in conjunction with supportive treatments as phosphorous and vitamin B12 and two liters of 10% dextrose saline was highly efficient. Similar results were obtained previously by Bruning (Citation1996), de Wall (Citation1990), Friedhoff and Soule (Citation1996) and Schwint et al. (Citation2009). Forty percent of the treated horses showed side effects in the form of salivation, colic and sometimes recumbence. These signs which were observed in those treated horses could be attributed to the anticholinesterase activity of imidocarbe dipropionate that can result in adverse cholinergic effects. Similar observation was recorded previously by Frerichs and Holbrook (Citation1974). Tick control by spraying the surrounding environment with diazinon (Nucedol Ultra 60 EC, Bioagripharm, Germany) was performed twice, one week apart.
5. Conclusion
It can be concluded that anemia or jaundice and severe body weight loss are characteristic for equine babesiosis. Also, bloody urine which is a characteristic feature of babesiosis in other animals is not common in Arabian horses. In addition, the early intervention using imidocarb dipropionate is the key of treatment success.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Alsaad KM. 2014. Evaluation of hemogram, acute phase response, acid base balance and blood gas analysis in newborn foals infected with babesiosis. J Anim Plant Sci. 24:738–742.
- Ambawat HK, Malhotra DV, Kumar S, Dhar S. 1999. Erythrocyte associated hemato-biochemical changes in Babesia equi infection experimentally produced in donkeys. Vet Parasitol. 85:319–324. doi: 10.1016/S0304-4017(99)00110-7
- Bashiruddin JB, Camma C, Rebelo E. 1999. Molecular detection of Babesia equi and Babesia caballi in horse blood by PCR amplification of part of the 16S rRNA gene. Vet Parasitol. 84:75–83. doi: 10.1016/S0304-4017(99)00049-7
- Böse R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ. 1995. Current state and future trends in the diagnosis of babesiosis. Vet Parasitol. 57:61–74. doi: 10.1016/0304-4017(94)03111-9
- Bruning A. 1996. Equine piroplasmosis an update on diagnosis, treatment and prevention. Br Vet J. 152:139–151. doi: 10.1016/S0007-1935(96)80070-4
- Camacho AT, Guitian FJ, Pallas E, Gestal JJ, Olmeda AS, Habela MA, Telford SR, Spielman A. 2005. Theileria (Babesia) equi and Babesia caballi infections in horses in Galicia, Spain. Trop Anim Health Prod. 37:293–302. doi: 10.1007/s11250-005-5691-z
- Coles EH. 1986. Veterinary clinical pathology. 4th ed. Philadelphia and London: W.B. Saunders.
- Frerichs WM, Holbrook AA. 1974. Treatment of equine piroplasmosis (B. caballi) with imidocarb dipropionate. Vet Rec. 95:188–189. doi: 10.1136/vr.95.9.188
- Friedhoff KT, Soule C. 1996. An account on equine babesioses. Rev Sci Tech. 15:1191–1201.
- Hodgson J. 2002. Equine Exotic Diseases, A Manual for Horse Owners. RIRDC Publication No. 02/054, Printed by Union Offset, Canberra, pp. 47–49.
- Kerber CE, Ferreira F, Pereira MC. 1999. Control of equine piroplasmosis in Brazil. Onderstepoort J Vet Res. 66:123–127.
- Knowles Jr DP. 2010. Understanding piroplasmosis. Equine Disease Quarterly. 19:4–5.
- Kumar S, Kumar R. 2007. Diagnosis of Babesia equi infection: an update on the methods available. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. 2:1–14.
- Mehlhorn H, Schein E. 1998. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein. Parasitol Res. 84:467–475. doi: 10.1007/s004360050431
- Motloang MY, Thekisoe OMM, Alhassan A, Bakheit M, Motheo MP, Masangane FES, Thibedi ML, Inoue N, Igarashi I, Sugimoto C, Mbati PA. 2008. Prevalence of Theileria equi and Babesia caballi infections in horses belonging to resource poor farmers in the north-eastern Free State Province, South Africa. Onderstepoort J Vet Res. 75:141–146. doi: 10.4102/ojvr.v75i2.12
- OIE Terrestrial Manual. 2014. Equine piroplasmosis. Chapter 2.5.8.
- Potgieter FT, de Waal DT, Posnett ES. 1992. Transmission and diagnosis of equine babesiosis in South Africa. Mem Inst Oswaldo Cruz. 87:139–142. doi: 10.1590/S0074-02761992000700021
- Preston PM, Brown CG, Bell-Sakyi L, Richardson W, Sanderson A. 1992. Tropical theileriosis in Bos taurus and Bos taurus 3 Bos indicus calves. Response to infection with graded doses of sporozoites. Res Vet Sci. 53:230–243. doi: 10.1016/0034-5288(92)90115-I
- Radostits OM, Gay CC, Blood DC, Hinchliff KW. 2007. Veterinary medicine. A text book of the diseases of cattle, sheep, goats and horses. 10th ed. London: WB Saunders. 1483–1498 p.
- Rothschild CM. 2013. Equine piroplasmosis. J Equine Vet Sci. 33:497–508. doi: 10.1016/j.jevs.2013.03.189
- Russell ZE, Holly M, Bruce EL, Latimer A. 2005. Equine babesiosis – a review. Athens, GA: Department of Pathology, college of Veterinary medicine, University of Georgia. p. 3062–7388.
- Sakha M. 2007. Successful treatment of babesiosis in a horse. J Vet Res. 62:155–157.
- Schwint ON, Ueti MW, Palmer GH. 2009. Kappmeyer LS, Hines MT, Cordes RT, et al. Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob Agents Chemother. 53:4327–4332. doi: 10.1128/AAC.00404-09
- Teglas M, Matern E, Lein S, Foley P, Mahan SM, Foley J. 2005. Ticks and tick-borne disease in Guatemalan cattle and horses. Vet Parasitol. 131:119–127. doi: 10.1016/j.vetpar.2005.04.033
- Uilenberg G. 2006. Babesia: a historical overview. Vet Parasitol. 138:3–10. doi: 10.1016/j.vetpar.2006.01.035
- de Waal DT. 1992. Equine piroplasmosis: a review. Br Vet J. 148:6–14. doi: 10.1016/0007-1935(92)90061-5
- de Waal DT, van Heerden J. 1994. Equine babesiosis. In: Coetzer JAW, Thomson GR, Tustin RC editors. Infectious diseases of livestock with special reference to South Africa. vol. 1. Cape Town, South Africa: Oxford University Press, p. 293–304.
- de Wall DT. 1990. The transovarial transmission of Babesia caballi by Hyalomma truncatum. Onderstepoort J Vet Res. 57:99–100.
- Zobba R, Ardu M, Niccolini S, Chessa B, Manna L, Cocco R, Parpagliae MLP. 2008. Clinical and laboratory findings in Equine Piroplasmosis. J Equine Vet Sci. 28:301–308. doi: 10.1016/j.jevs.2008.03.005