ABSTRACT
Ricinoleic acid is obtained from castor bean and studies related to its effects on glucose and insulin dynamic are scarce in the literature. Eight male Crioulo breed horses (362 ± 16 of live weight, mean ± SD) were used in a replicated 4 × 4 Latin square design experiment that consisted of 10 days of adaptation and 7 days for data collection. The horses were allocated in each square to receive one of the following treatments: Control (0) and 1, 2, 3 and 4 g/d of ricinoleic acid (diet DM basis). Diets were composed of a commercial concentrate and Coast cross hay. Treatments did not affect DM and nutrient intake. Ricinoleic acid did not alter the nutrient digestibility, regardless of the dosage used. The quadratic effect was observed for glucose and insulin area under the curve. Ricinoleic acid quadratically affected glucose and insulin concentrations. Increasing dietary doses of ricinoleic acid altered glycemic and insulinemic responses of horses, and the present study recommends the dietary inclusion of 1.8 g of ricinoleic acid to improve horse's energetic metabolism.
1. Introduction
The functional oils are complex volatile compounds produced by aromatic plants as secondary metabolites. These compounds are characterized by strong odour, rarely coloured, fat-soluble and often obtained by steaming or hydro-distillation (Bakkali et al. Citation2008). Currently, due to several studies related to the effects of essential oils, their action mechanisms especially as an antimicrobial agent have been reported in the literature (Palazzolo et al. Citation2013).
The possible benefits of the essential oils (EO) utilization in animal nutrition are related to their action on intestinal microorganisms, since EO can control the growth of pathogen microorganisms, reduce ammonia production, increase intestinal mucus production and improve digestive capacity (Windisch et al. Citation2007; Hashemi & Davoodi Citation2011). Essential oils are reported to improve nutrient digestibility by altering the enzymatic activity (Scheurmann & Cunha Jr Citation2005), stimulating the saliva production and gastric and pancreatic juice secretions (Mellor Citation2000; Brenes & Roura Citation2010).
The castor oil derived from the beans of the castor plant (Ricinus communis) differs from other vegetal oils by owning higher hydroxide levels and high levels of ricinoleic acid (85–90% of triglycerides, Totaro et al. Citation2014). Literature reported antibacterial property and the combined analgesic and anti-inflammatory effects of R. Communis seeds extract (Vieira et al. Citation2000). The objective of this study was to evaluate the effects of increasing dietary doses of ricinoleic acid on nutrient intake and total tract digestion, glycemic and insulinemic responses of horses.
2. Materials and methods
2.1. Animals and sampling
The experiment was conducted at the Research Center of Little Farm Horses, Olimpia, Brazil. Eight male horses of Crioulo breed (362 ± 16 of live weight, mean ± SD) were used in a replicated 4 × 4 Latin square design experiment consisted of 22-day periods (10 days of adaptation, 7 days of sampling and 5 days for wash out). Throughout the experimental period, animals were housed in individual pens (12 m2). Animals were vermifuged (Moxi Duo®, Ourofino, Cravinhos, Brazil) before the beginning of the experiment.
The horses were allocated within each square to receive one of treatments: Control (0) and 1, 2, 3 and 4 g/d of ricinoleic acid (diet DM basis). Diets were composed of a commercial concentrate and Coast cross hay (). Feed was supplied twice daily at 0700 h and 1900 h (50:50 DM basis). The DM intake was set at 2.0% of live weight and forage to concentrate ratio was 50:50 (diet DM basis). Water and mineral supplement were fed ad libitum and diets were formulated according to NRC (Citation2007) to supply maintenance requirements.
Table 1. Chemical composition of forage, concentrate and basal diet (% DM, otherwise stated).
Samples of feed offered were collected daily during the last 7 days of each experimental period. Total faeces collection was performed during 120 h and a 10% aliquot (on wet basis) of the faeces collected were stored at −20°C for posterior chemical analyses. Samples of feed and faeces were dried in a forced-air oven at 55°C during 72 h to partially DM determination. Dried samples were ground in a knives Willey mill (Wiley Mill, A. H. Thomas, Philadelphia, PA, USA) to pass through 1-mm screen. Samples were analysed for neutral detergent fibre (NDF) with heat-stable α-amylase (Van Soest et al. Citation1991), DM (AOAC Citation2000, 950.15), ash (AOAC, 942.05), ether extract (EE; AOAC 920.39) and crude protein (CP; AOAC, 984.13). Starch analysis was performed according to Pereira and Rossi (Citation1995).
Blood samples were collected from the jugular vein 30 minutes before, 30, 90, 150 and 210 minutes after the morning concentrate supply (Stull & Rodiek Citation1988) on day 11 of the experimental period. Blood glucose concentration was performed according to Bergmeyer et al. (Citation1974) and blood insulin concentration was performed according to Yalow and Berson (Citation1974).
2.2. Statistical analysis
Data of nutrient total tract digestion and blood concentrations of glucose and insulin were subjected to SAS (Citation2004), after performing Shapiro–Wilk and F-test to analyse the normality of residues and the homogeneity of variances.
Total tract digestion data were evaluated by polynomial regression analysis using the PROC REG (SAS, Inst. Inc., Cary, NC, USA) and overall means were adjusted and compared through Tukey's test (significance level was set at P < .05) using PROC MIXED (SAS, Inst. Inc., Cary, NC, USA).
The blood glucose and insulin concentrations were analysed as area under the curve by using the EXP procedure (SAS, Inst. Inc., Cary, NC, USA) and polynomial regression analysis according to the dietary levels of ricinoleic acid. The glycemic and insulinemic responses were subject to repeated measures analysis of PROC MIXED (SAS, Inst. Inc., Cary, NC, USA) and were analysed by polynomial regression according to the increasing dietary doses of ricinoleic acid using PROC REG (SAS, Inst. Inc., Cary, NC, USA).
3. Results
3.1. Dry matter and nutrients intake
Dry matter and nutrients intake were not affected (P > .05) by dietary inclusion of ricinoleic acid (). The expected DM intake was 2.0% of live weight, however, the horses supplemented with 3 g/d of ricinoleic acid had DM intake of 2.45% of live weight. Horses fed 3 g/d of ricinoleic acid showed higher DM intake (640 or 8.30%) compared to those fed control.
Table 2. Effects of increasing doses (g/d) of ricinoleic acid on nutrient intake of horses.
3.2. Total tract digestion
Dietary ricinoleic acid did not affect total tract digestion of nutrients (P > .05) in horses (). The average DM total tract digestion was 58.99%. The animals supplemented with 2 or 3 g/d of ricinoleic acid had higher digestibility of CP compared to those not supplemented.
Table 3. Effects of increasing doses (g/d) of ricinoleic acid on nutrient total tract digestion of horses (% DM).
3.3. Blood glucose and insulin
Time effect was observed for blood glucose and insulin concentrations ( and , respectively). Glucose blood concentrations were increased by ricinoleic acid treatments after feeding; however, after 120 minutes of feeding time ricinoleic acid at 1 and 3 g/d showed lower blood glucose levels compared to ricinoleic acid at 0 and 2 g/d (). Blood insulin concentrations progressively increased after the morning feeding, although ricinoleic acid did not alter the concentration of this metabolite in bloodstream of horses (). Blood glucose () and insulin () concentrations had a quadratic response to increasing dietary doses of ricinoleic acid. Insulin was quadratically affected (P = 0.019) by supplying ricinoleic acid ().
Figure 1. Glucose blood concentrations of horses fed with increasing doses of ricinoleic acid according to the morning feeding time (mean ± SD).
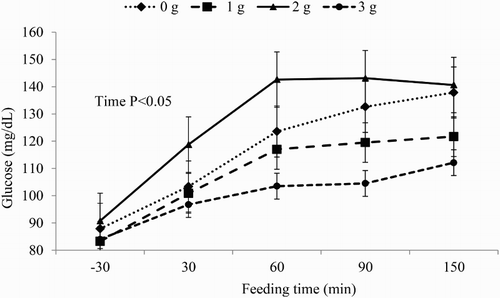
Figure 2. Insulin blood concentration of horses fed with increasing doses of ricinoleic acid according to the morning feeding time (mean ± SD).
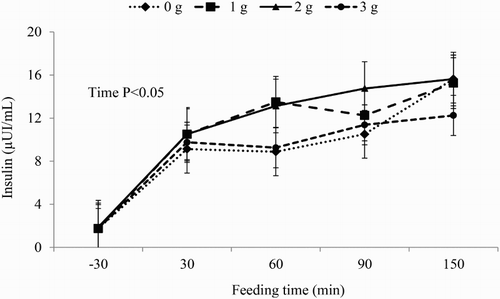
Figure 3. Observed and estimated values of the regression equation of area under the curve (AUC) of blood glucose concentrations of horses according to ricinoleic acid dose.
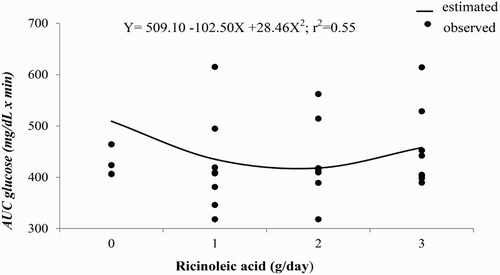
Figure 4. Observed and estimated values of the regression equation of area under the curve (AUC) of blood insulin concentrations of horses according to ricinoleic acid dose.
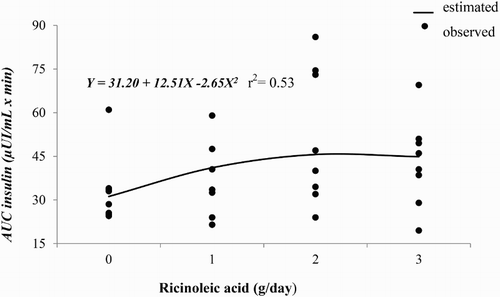
Table 4. Effects of increasing doses (g/d) of ricinoleic acid on blood glucose and insulin dynamic of horses.
4. Discussion
Dry matter and nutrient intake results of this trial may be related to the type and shape of roughage used. These characteristics may influence feed intake, and consequently the duration, speed and dynamic which digesta flows throughout the digestive tract, affecting the efficiency of microbial action and the availability of nutrients, especially the energy from forage digestion (LaCasha et al. Citation1997).
The time of meals of stabled animals is influenced by environmental factors and factors related to individual behaviour. The physical form and the frequency which the feed is delivered alter the chewing time and the time which digesta transits in intestine, affecting the time of feed intake. These changes may favour or not the occurrence of defects and digestive disorders in stabled animals (NRC Citation2007).
Several factors may affect nutrient total tract digestion including the animal individuality, physical activity, type and size of feed particles supplied, diet chemical composition (mainly the fractions of structural and no-structural carbohydrates), fibrolytic activity of intestinal microbiota and feed passage rate in caecum and hind gut (Meyer Citation1995). Despite no statistical differences in nutrient total tract digestion, CP digestion numerically increased (P = 0.22) according to the dietary doses of ricinoleic acid. Gandra et al. (Citation2012) reported improvements in protein metabolism of beef cattle fed 0, 1, 2 and 4 g/d of ricinoleic acid.
The blood glucose concentration values were considered normal as described by Meyer (Citation1995) which glycaemia during fasting ranges from 80 to 100 mg/dL, and peaks 150 mg/dL after 2–3 hours of feeding diets rich in starch or sugars. The blood glucose concentrations after feed intake is influenced by food particle size, thermal processing, protein composition, fat and fibre diet content, biochemical structure and absorption process of carbohydrates and interval between meals (Guezennec Citation1995).
The ricinoleic acid supplementation could alter a bacterial profile of caecum increasing the propionate production and consequently increase blood glucose concentrations. According to Arai et al. (Citation1994), the glucose is the major energy source in animals and the best substrate for enzymatic reaction in cells to produce ATP.
Forhead et al. (Citation1994) reported that insulin is primordial for regulation of metabolic process involving glucose. Insulin improves glucose transport into muscular tissue through redistribution of GLUT-4 protein from intracellular vesicles to cell surface (Slot et al. Citation1991), which results in increased (10–30-folds) glucose transport into cells (James et al. Citation1988). The present experiment demonstrated that animals fed 2 g/d of ricinoleic acid had higher values of blood insulin concentrations after 30 minutes of the morning feeding compared with other treatments ().
The area under the curve of blood glucose and insulin showed that 1.8 g/d of ricinoleic acid would provide the minimum of blood glucose concentration (416.81 mg/dL × min) and the maximum of blood insulin concentration (45 µIU/mL × min) which are related to the efficiency of glucose utilization by peripheral tissues. Stull and Rodiek (Citation1988) evaluated four diets with different proportions of Coast cross hay and concentrate mixture, and observed an area under the curve for glucose of 308.24 mg/h/dL, which result is lower than those found in the current study, and this fact may be an evidence of better glucose metabolism when ricinoleic acid is added in horse's diet.
5. Conclusion
Increasing dietary doses of ricinoleic acid did not alter nutrient total tract digestion of horses. However, ricinoleic acid altered the blood glucose and insulin dynamics. Based on the results of glucose and insulin dynamics, the current study recommends the inclusion of 1.8 g/d of ricinoleic acid in diets to improve energetic metabolism of horses.
Disclosure statement
No potential conflict of interest was reported by the authors
References
- AOAC (Association of Official Analytical Chemistry). 2000. Official methods of analysis. 17th ed. Arlington, VA: AOAC.
- Arai T, Washizu T, Hamada S, Sako T, Takagi S, Yasshiki K, Motoyoshi S. 1994. Glucose transport and glycolytic enzyme activities in erythrocytes of two years-old thoroughbreds undergoing training exercise. Vet Res Commun. 18:417–422. doi: 10.1007/BF01839417
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. 2008. Biological effects of essential oils – a review. Food Chem Toxicol. 46:446–475. doi: 10.1016/j.fct.2007.09.106
- Bergmeyer HU, Bernt E, Schmidt F, Storkk H, Glucose D. 1974. D-Glucose. In methods of enzymatic analysis, 3. Bergmeyer HU, editor. New York, NY: Academic Press, p. 1196.
- Brenes A, Roura E. 2010. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci. Technol. 158:1–14. doi: 10.1016/j.anifeedsci.2010.03.007
- Forhead AJ, French J, Ikin P, Fowler JN, Dobson H. 1994. Relationship between plasma insulin and triglyceride concentrations in hypertriglyceridemic donkeys. Res Vet Sci. 56:389–392. doi: 10.1016/0034-5288(94)90158-9
- Gandra JR, Nunes Gil PC, Consolo NRB, Gandra ERS, Gobesso AAO. 2012. Addition of increasing doses of ricinoleic acid from castor oil (Ricinus communis L.) in diets of Nellore steers in feedlots. J Anim Feed Sci. 21:566–576.
- Guezennec C. 1995. Oxidation rates, complex carbohydrates and exercise. Sports Med. 19:365–372. doi: 10.2165/00007256-199519060-00001
- Hashemi SR, Davoodi H. 2011. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res Commun. 35:169–180. doi: 10.1007/s11259-010-9458-2
- James DE, Brown R, Navarro J, Pileh PF. 1988. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 333:183–185. doi: 10.1038/333183a0
- LaCasha PA, Brady HA, Allen VG, Richardson RA, Pond KR. 1997. Voluntary intake and selection of Matua prairie grass, Coastal Bermuda grass, and alfalfa hay by yearling horses. Center Feed Ind. Res. Educ. Annu. Rep. No. T-5-362. p. 13. Texas Tech Univ., Lubbock.
- Mellor S. 2000. Herbs and spices promote health and growth. Pig Progress. 16:18–21.
- Meyer H. 1995. Alimentação de cavalos. São Paulo: Livraria Varela, 1995. 303 p.
- NRC (National Research Council). 2007. Nutrients requirements of horses. 6th ed. Washington, DC: NRC, 341 p.
- Palazzolo E, Laudicina VA, Germanà MA. 2013. Current and potential use of citrus essential oils. Current Org Chem. 17:3042–3049. doi: 10.2174/13852728113179990122
- Pereira JRA, Rossi Júnior P. 1995. Practical handbook of nutritional food assessment. Piracicaba: Fealq, 1995. 76 p.
- SAS Institute Inc. 2004. SAS/STAT User's Guide: Statistics. Release 9.0. Cary, NC: SAS Inst. Inc.
- Scheuermann GN, Cunha Jr A. 2005. Prospects for the use of plant products as alternative additives in poultry feed. Concórdia, SC: EMBRAPA Suínos e Aves, 56 p.
- Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. 1991. Translocation of the glucose transporter GLUT-4 in cardiac myocytes of the rat. Proc Natl Acad Sci. 88:7815–7819. doi: 10.1073/pnas.88.17.7815
- Stull CL, Rodiek AV. 1988. Responses of blood glucose, insulin and cortisol concentrations to common equine diets. Am Inst Nutr. 118:206–213.
- Totaro G, Cruciani L, Vannini M, Mazzola G, di Gioia D, Celli A, Sisti L. 2014. Synthesis of castor oil-derived polyesters with antimicrobial activity. Eur Polymer J. 56:174–184. doi: 10.1016/j.eurpolymj.2014.04.018
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber and nonstarch/polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
- Vieira C, Evangelista C, Crillo R, Lippi A, Maggi CA, Manzini S. 2000. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 9:223–228. doi: 10.1080/09629350020025737
- Windisch W, Schedle K, Plitzner C, Kroismayr A. 2007. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 86:E140–E148. doi: 10.2527/jas.2007-0459
- Yalow RS, Berson SA. 1974. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 39:1157–1175. doi: 10.1172/JCI104130
