ABSTRACT
This research was conducted to compare kinetics of gas production, methane emission, and in vitro digestibility between organic mineral (OM) and inorganic mineral (IM) in king grass (Pennisetum hybrid), in combination with natural crude tannin from neem (Azadirachta indica, AI) leaves. Treatments were as follows: T0 (king grass as a control), T1 (T0 + 3% IM), T2 (T0 + 3% OM), T3 (T0 + 2% AI), T4 (T0 + 3% IM + 2% AI), T5 (T0 + 3% OM + 2% AI), and T6 (T0 + 40 ppm monensin), and these were arranged on a completely randomized design. Data were analysed using ANOVA and orthogonal contrast test was used for comparing among treatment means. Results showed that either OM or IM supplementation significantly increased (P < .05) gas production. Total gas productions from T0, T3, T5, and T6 were lower than those of T1, T2, and T4. Total VFA, acetate, and propionate were similar in all treatments; however butyrate concentration was higher in T2 and T4 than the others. In vitro organic matter digestibility, protozoa cells number, and ammonia and methane concentrations were not influenced (P > .05) by treatments. In summary, either OM or IM improved fermentability of king grass while their combination with tannin-containing leaves reduced the fermentability without affecting methane production.
1. Introduction
Mineral is among the essential elements that plays an important role in cattle's physiological processes for growth and health maintenance. Mineral supplementation through feed is not only to prevent deficiency but also to optimize livestock production and health. Mineral is usually supplied to the livestock through mineral mixture in its inorganic form. However, a major disadvantage of using such a supplement is that the mineral is not fully absorbed due to antagonism and anti-nutritional factors (such as tannin, phytic acid, oxalate, etc.) present in the diet (Bhanderi et al. Citation2010). Organic mineral (OM) (mineral bound to organic compound) that is easily absorbed by the body is one of the important solutions to overcome insufficient availability of minerals found in forage crops or natural grasses to meet the physiological needs of livestock.
For instance, trace metal–amino acid complexes can allegedly mimic the process by which trace elements are absorbed (as metal-peptides) and thus be more available to livestock than inorganic mineral (IM) (Suttle Citation2010). Neem (Azadirachta indica) is a plant species that is rich in bioactive compounds. It was observed that neem leaf contained flavanoid and tannin (Pandey et al. Citation2014). Recently, tannin has been used to modify feed utilization by reduction in methane emission and therefore tannin is considered as a natural compound possessing methane-mitigating effect (Jayanegara et al. Citation2013). Further, Biswas et al. (Citation2002) reviewed that many bioactive compounds in neem leaf were cyclic-trisulphide and cyclic tetrasulphide which it can be used antivirus, antiparasite and support to humoral immunity. Neem leaf had also been reported as an ethnoveterinery medicine for inhibiting larvae of the sheep nose bot fly (Cepeda-Palacios et al. Citation2014) and preventing bovine strongylosis (Jamra et al. Citation2015).
It would be of interest to evaluate the effect of OM combined with neem leaf on rumen fermentation parameters including ruminal methane emission. Therefore, the objective of this experiment was aimed to evaluate the effectiveness of OM supplementation (as compared to its inorganic form) combined with neem leaf on fermentability, methane emission, and in vitro digestibility of feed forages.
2. Material and methods
2.1. Forage, tannin, and mineral preparation
A forage sample was prepared by harvesting king grass (Pennisetum hybrid) at 70 days after planting. Forage was chopped and dried at 60°C for 2–3 days (until moisture content reached 12%), then ground and sieved into 2 mm particle size. A drying method using a vacuum oven was employed according to the AOAC Official Method 934.06 (AOAC Citation1995). Natural crude tannin was prepared by drying neem (Azadirachta indica) leaves according to forage preparation as well. OM was prepared by inoculating Saccharomyces cerevisiae ATCC 9763 (50 ml) into cassava flour as substrate (1 kg) which was fortified by micro-minerals. Prior to fermentation processes, inoculum was cultivated in malt extract broth (Oxoid) and incubated for 24 h at 30°C, similar to the experiment conducted by van Rijswijck et al. (Citation2015). Micro-minerals formulated per kg substrate contained FeCl2.4H2O (0.177 g), MnCl2.4H2O (7.129 g), CuSO4.5H2O (9.810 g), ZnSO4.7H2O (12.646 g), CoCl2.6H2O (0.192 g), and KI (0.217 g). Fermentation was conducted for seven days on the substrate in the facultative condition then dried by oven at 55°C (for up to 24–48 h, moisture content around 10–12%), followed by grinding and sieving to 1 mm particle size. However, IM was prepared by the addition of micro-minerals' formula on the substrate without fermentation.
2.2. Experimental design and data analysis
Treatments that were arranged on a completely randomized design consisted of seven treatments, that is, T0 (P. hybrid as a negative control), T1 (T0 + 3% IM), T2 (T0 + 3% OM), T3 (T0 + 2% AI), T4 (T0 + 3% IM + 2% AI), T5 (T0 + 3% OM + 2% AI), and T6 (T0 + 40 ppm monensin as a positive control). Monensin was used as a positive control since the substance has as antiprotozoal agent (Kišidayová et al. Citation2009) associated with reduction of methane emission (Patra & Saxena Citation2010). Each treatment was conducted in 3 replications. Variables measured were in vitro organic matter digestibility (IVOMD), gas production kinetics, volatile fatty acids (VFAs), ammonia (NH3), methane (CH4), non-glucogenic ratio (NGR), and protozoal number. Data were analysed by the one-way ANOVA and continued with orthogonal contrast test (Gomez & Gomez Citation1984) when the treatments showed significantly different P < .05.
2.3. In vitro digestibility and fermentability evaluation
In vitro digestibility and fermentability were conducted by gas production technique (Menke et al. Citation1979) using a 100 ml glass syringe (Fortune Models, Poultren and Graft Gmbh). Sample of forage (380 mg), in- or OM (11.4 mg), natural crude tannin (7.6 mg), or monensin (0.0152 mg) (according to treatments) was placed into a syringe for pre-incubation for 24 h at 39°C. Rumen liquor from a fistulated cattle (10 ml) and buffer solution (20 ml) were inserted into each syringe and infused with CO2 gas. Seven treatments were randomly allocated to an incubator. Incubation was carried out at 39°C and gas production was observed at 0, 1, 2, 4, 6, 8, 12, 18, 24, 36, and 48 hours after incubation. Gas production kinetics was calculated following the equation Gp = B × [1 −e− k ( t − L )] (López et al. Citation1999), where Gp is the cumulative gas production at time t, B is the maximum gas production (ml/g DM), k is the gas production rate (ml/h), t is the incubation time (h), and L is the lag time (h). Determination of IVOMD was assayed according to Blümmel et al. (Citation1997). Productions of VFA and NH3 were measured at the end of the incubation. Analysis of VFA was performed by the gas chromatography method according to Friggens et al. (Citation1998) and NH3 analysis using the spectrophotometry method as described by Broderick and Kang (Citation1980). NGR value was calculated according to Zhang and Yang (Citation2012).
2.4. Methane measurement and counting protozoa
The concentration of methane from total gas production was sampled at 18 h. Each sample taken included 10 ml of gas using vacuum syringe then injected into a vacuum tube. Gas samples were analysed using gas chromatography (Shimadzu GC-14) equipped with Proparak Q column (50°C) and a flame ionization detector (150°C) as described by Liu et al. (Citation2011). Protozoa cell was counted using a hemocytometer and colouring with methylegreen formalin saline/MFS (Ogimoto & Imai Citation1981). The MFS solution was composed of 100 ml of 35% formaldehyde solution, 900 g of distilled H2O, 0.6 g of methyl green, and 8 g of NaCl.
3. Results and discussion
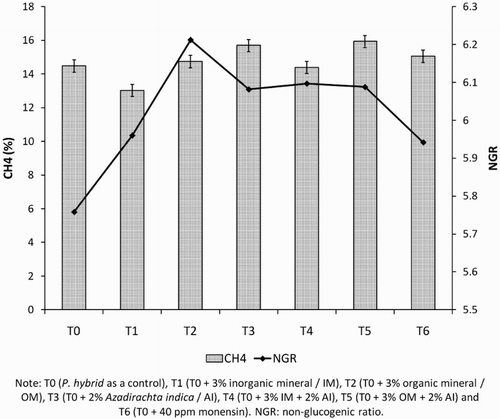
Supplementation of micromineral (organic or organic form) combined with tannin from neem leaves (Azadirachta indica) significantly (P < .05) affected cumulative (Gp), maximum (B), gas production rate (k), and butyrate. However, the treatment did not affect the IVOMD, total VFA, lag time (L), ammonia, and protozoa (). Furthermore, methane production from feed combined with tannin seems to be lower than the others and NGR value tends to be higher than control ().
Table 1. In vitro digestibility, fermentability of king grass treated by mineral, tannin leaves, and monensin.
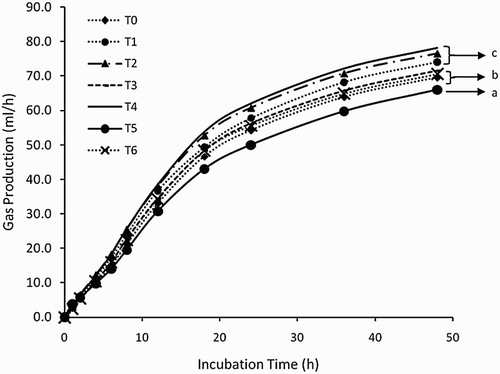
Supplementation of OM and tannin increased butyrate acid and tended to improved (P ≈ .109) total VFA production. However, ammonia and protozoa number were not influenced by treatment. Gas production kinetics from forage-supplemented IM (T1), OM (T2), and IM + tannin (T4) showed higher gas production than control and tannin (T3), OM + tannin (T5), and monensin (T6).
Increasing gas production, VFA, and butyrate of forage supplemented by T2 or T4 indicated that OM presence contributed to support ruminal microbes activity in producing VFA. In contrast, addition of tannin and OM (T5) showed reducing VFA which had an antagonism effect on fermentability. Antagonistic mechanism related to chemical characteristic of tannin to bind organic compound consequences reduction of OM by rumen microbe rumen. Tannin (condensed) has a complex strongly binding with metal ions, carbohydrates, and proteins (Porter Citation1992). Moreover, supplementation of condensed tannin reduced organic matter digestion in rumen. Lorenz et al. (Citation2014) revealed that protein as organic matter could be precipitated by tannin in rumen. It was indicated by gas production and VFA from substrate supplemented with tannin (T3 and T5) less than without tannin addition.
Furthermore, the binding effect of tannin to inhibit protozoa growth in rumen implied completion utilization of mineral by ruminal bacteria. Bacteria were supplied enough mineral diet while protozoa were inhibited by supplementation of tannin from neem leaves. Biswas et al. (Citation2002) reported the presence of many kinds of bioactive compounds in A. indica such as azadirachtin, polysaccharide, cyclic-trisulphide/tetrasulphide, and glycoside which have antibacterial, antiviral, and anti-inflammatory properties. In ruminal metabolism, tannin (contained in neem leaves) was able to inhibit methanogenic bacteria and protozoa (Patra & Saxena Citation2010); as a consequence it improved feed utilization by increasing fermentability. In contrast, Vasta et al. (Citation2010) reported that the number of protozoa increased by tannin supplementation in sheep that consumed a concentrated diet. It might be related to adaptation factors and nutrient composition in the diet.
Cieslak et al. (Citation2013) reported that tannins may inhibit growth, and affect development and activity of the population of methanogens indirectly (by reducing the number of protozoa associated with methanogens) and directly (by affecting methanogens). Tannin might also increase propionate production by affecting methanogens through reduced competition for hydrogen utilization. Moreover, the insignificant protozoa population or methane production on addition of natural crude tannin from neem leaves might be due to low dosage of the tannin. Bhatta et al. (Citation2014) revealed that A. indica leaves decreased methane or protozoa number with the minimum dosage of 25% in dry matter basis.
Based on , the effectivity of supplementing IM (T1), IM + tannin leaves (T4), and OM (T2) with higher gas production than monensin and control indicated that either tannin with organic or IM potential in replacing monensin. However, in this result monensin treatment was similar to control as well as the previous study reported by Smith et al. (Citation2010) that VFA and methane in vitro production could not be influenced by addition of monensin up to 0.6 mg/L. Aderinboye et al. (Citation2012), monensin had also potential to increase feed digestibility by increasing propionate proportion that could be associated with reduction of methanogenesis activity. Feed digestion improvement was related to the reduction in methane followed by increase in NGR value (). Although the treatment was not affected by in vitro methane production, supplementation of A. indica leaves should be evaluated by an in vivo experiment which is related to the complexity processes in ruminal digestion.
Figure 2. Kinetics curve of gas production from king grass treated by an/organic mineral, tannin, and monensin. T0 (P. hybrid as a control), T1 (T0 + 3% inorganic mineral/IM), T2 (T0 + 3% organic mineral/OM), T3 (T0 + 2% Azadirachta indica/AI), T4 (T0 + 3% IM + 2% AI), T5 (T0 + 3% OM + 2% AI), and T6 (T0 + 40 ppm Monensin). Different letters, a, b, c, mean significant differences (P < 0.05).
4. Conclusion
Supplementation of organic or IM improved in vitro fermentability of forage (based on total gas production, total VFA, and butyrate concentration) while their combination with tannin-containing leaves reduced the fermentability without affecting methane production. OM could be considered for use as an additive to support rumen fermentation.
ORCID
Ahmad Sofyan http://orcid.org/0000-0002-0578-4671
Additional information
Funding
References
- Aderinboye RY, Onwuka CFI, Arigbede OM, Oduguwa OO, Aina ABJ. 2012. Effect of dietary monensin inclusion on performance, nutrient utilization, rumen volatile fatty acid concentration, and blood status of West African dwarf bucks fed with basal diets of forages. Trop Anim Health Prod. 44:1079–1087. doi: 10.1007/s11250-011-0043-7
- AOAC. 1995. Official methods of analysis. 16th ed. Arlington, VA: Association of Official Analytical Chemists, International.
- Bhanderi BM, Pande AM, Parnerkar S. 2010. Influence of organic and inorganic forms of trace minerals supplementation at different doses on daily weight gain and serum mineral levels in male calves. Livestock Res Rural Dev. 22: 143.
- Bhatta RM, Saravanan M, Baruah L, Prasad CS. 2014. Effect of graded level of tannin-containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. J Appl Microbiol. 118:557–564. doi: 10.1111/jam.12723
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. 2002. Biological active-ties and medicinal properties of neem (Azadirachta indica). Curr Sci. 82:1336–1345.
- Blümmel M, Steingass H, Becker K. 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br J Nutr. 77:911–921. doi: 10.1079/BJN19970089
- Broderick GA, Kang JH. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
- Cepeda-Palacios R, Servín R, Ramírez-Orduña JM, Ascencio F, Dorchies P, Angulo-Valadez CE. 2014. In vitro and in vivo effects of neem tree (Azadirachta indica A. Juss) products on larvae of the sheep nose bot fly Oestrus ovis L. Díptera: Oestridae). Vet Parasitol. 200:225–228. doi: 10.1016/j.vetpar.2013.11.018
- Cieslak A, Szumacher-Strabel M, Stochmal A, Oleszek W. 2013. Plant components with specific activities against rumen methanogens. Animal. 7:253–265. doi: 10.1017/S1751731113000852
- Friggens NC, Oldham JD, Dewhurst RJ, Horgan G. 1998. Proportions of volatile fatty acids in relation to the chemical composition of feeds based on grass silage. J Dairy Sci. 81:1331–1344. doi: 10.3168/jds.S0022-0302(98)75696-6
- Gomez KA, Gomez AA. 1984. Statistical procedures for agricultural research. 2nd ed. New York: Wiley.
- Jamra N, Das G, Singh P, Haque M. 2015. Anthelmintic efficacy of crude neem (Azadirachta indica) leaf powder against bovine strongylosis. J Parasitic Dis. 39: 786–788. doi: 10.1007/s12639-014-0423-9
- Jayanegara A, Marquardt S, Wina E, Kreuzer M, Leiber F. 2013. In vitro indications for favourable non-additive effects on ruminal methane mitigation between high-phenolic and high-quality forages. Br J Nutr. 109:615–622. doi: 10.1017/S0007114512001742
- Kišidayová S, Laukova A, Jalč D. 2009. Comparison of nisin and monensin effects on ciliate and selected bacterial populations in artificial rumen. Folia Microbiol. 54:527–532. doi: 10.1007/s12223-009-0076-8
- Liu H, Vaddella V, Zhou D. 2011. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J Dairy Sci. 94:6069–6077. doi: 10.3168/jds.2011-4508
- López S, France J, Dhanoa MS, Mould F, Dijkstra J. 1999. Comparison of mathematical models to describe disappearance curves obtained using the polyester bag technique for incubating feeds in the rumen. J Anim Sci. 77:1875–1888.
- Lorenz MM, Alkhafadji L, Stringano E, Nilsson S, Mueller-Harvey I, Udén P. 2014. Relationship between condensed tannin structures and their ability to precipitate feed proteins in the rumen. J Sci Food Agric. 94:963–968. doi: 10.1002/jsfa.6344
- Menke KH, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. 1979. The estimation of the digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci. 92:217–222. doi: 10.1017/S0021859600086305
- Ogimoto K, Imai S. 1981. Atlas of Rumen microbiology. Tokyo: Japan Scientific Societies Press; p. 158.
- Pandey S, Verma KK, Singh M. 2014. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int J Pharm Pharm Sci. 6:444–447.
- Patra AK, Saxena J. 2010. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry. 71:1198–1222. doi: 10.1016/j.phytochem.2010.05.010
- Porter LJ. 1992. Structure and chemical properties of the condensed tannins. Plant Polyphenols Basic Life Sci. 59:245–258. doi: 10.1007/978-1-4615-3476-1_14
- van Rijswijck IMH, Dijksterhuis J, Wolkers-Rooijackers JCM, Abee T, Smid EJ. 2015. Nutrient limitation leads to penetrative growth into agar and affects aroma formation in Pichia fabianii, P. kudriavzevii and Saccharomyces cerevisiae. Yeast. 31:89–101.
- Smith DR, DiLorenzo N, Leibovich J, May ML, Quinn MJ, Homm JW, Galyean ML. 2010. Effects of sulfur and monensin concentrations on in vitro dry matter disappearance, hydrogen sulfide production, and volatile fatty acid concentrations in batch culture ruminal fermentations. J Anim Sci. 88:1503–1512. doi: 10.2527/jas.2009-2498
- Suttle NF. 2010. Mineral nutrition of livestock. 4th ed. Oxforshire, UK: CAB International; p. 284–545.
- Vasta V, Yanez-Ruiz DR, Mele M, Serra A, Luciano G, Lanza M, Biondi L, Priolo A. 2010. Bacterial and protozoal communities and fatty acid profile in the rumen of sheep fed a diet containing added tannins. Appl Environ Microbiol. 76:2549–2555. doi: 10.1128/AEM.02583-09
- Zhang DF, Yang HJ. 2012. Combination effects of nitrocompounds, pyromellitic diimide, and 2-bromoethanesulfonate on in vitro ruminal methane production and fermentation of a grain-rich feed. J Agric Food Chem. 60:364–371. doi: 10.1021/jf203716v