ABSTRACT
The aim of the present study was to evaluate the effects of a commercial antioxidant blend (CAB; Loxidan® TD100) and rosemary powder (RP) on performance, caecal microflora and humoral immunity of broilers fed with two different dietary poultry fat (PF) levels. Using a 3 × 2 factorial experiment, 180 male Ross 308 broiler chicks were assigned to 6 groups with 3 replicates per group, during a 42-day production cycle according to each following treatment: 3.5% (Treatment 1, T1) or 6% (Treatment 2, T2) PF without antioxidants; 0.1% CAB to 3.5% (Treatment 3, T3) or 6% (Treatment 4, T4) PF; and 0.1% RP added to 3.5% (Treatment 5, T5) or 6% (Treatment 6, T6) PF. All birds were vaccinated against Newcastle and infectious bursal disease virus. During starter, grower or finisher periods the weight gain was higher in T2 than T1. Feed efficiency was also improved during the first two periods at 6% PF level. On day 42, a lower number of Escherichia coli colonies on caecal contents were observed when 6% PF and CAB or RP were added. Plasma antibody titre against infectious bursal disease virus was higher in T2 than T1 and in groups T6 and T5 than T2 or T1. Both additives showed advantages, mainly in broilers fed with 6% PF.
1. Introduction
Nowadays oils and fats are one of common feed ingredients of broilers. The inclusion of fat, mainly triacylglycerols, in broiler diet until 40 g/kg (Leeson & Summer Citation2005) shows several advantages. Fat is a high energy source, improves the diet palatability and fat–vitamin absorption, increases the intestinal absorption of all nutrients reducing the passage rate of digesta, modulates immunity, modifies meat composition and quality, and can be a relatively economic feed (sub)product from crop, livestock and fishery industries (Baiao & Lara Citation2005; Nayebpor et al. Citation2007; Febel et al. Citation2008; Kellems & Church Citation2010). However, oxidation (oxidative rancidity) is the main deterioration process in fats and oils and lipid-based feedstuffs. The oxidation process is related to a decrease in nutritional value, feed palatability and broiler performance, and can also cause health problems (Baiao & Lara Citation2005; Tavarez et al. Citation2011)
Adding antioxidants to poultry feed is one of the most efficient strategies to protect the sensitive nutrients in feedstuffs. Some natural antioxidants and synthetic or commercial antioxidant blend (CAB) compounds for feed inclusion were recently studied and available in order to use in the poultry industry (Tavarez et al. Citation2011; Delles et al. Citation2014; Lu et al. Citation2014).
Whereas synthetic additives can be used in intensive animal production, natural antioxidants can be advantageous for organic poultry production, and their effects on animal physiology, including performance and beneficial or detrimental bacteria prevailing on gut, should be exhaustively evaluated. Rosemary (Rosmarinus officinalis L.) is an aromatic herb. Natural major active compounds are carnosol, rosmanol and their acid forms or flavonoids (Ibañez et al. Citation2003). This plant shows antioxidant and antimicrobial qualities and can modify bird physiology (Moreno et al. Citation2006). This additive can be supplied at broiler diet as rosemary powder (RP) form (Ghazalah & Ali Citation2008) or using their essential oils (Yesilbag et al. Citation2011). Recently, we have tested in our laboratories the effect of RP, as feed additive, on broilers performance and gut gross morphometry (Rostami et al. Citation2015). However, to our knowledge, the effects of RP on bird physiology were not sufficiently evaluated in broilers fed at different levels of poultry fat (PF).
On the other hand, the infectious bursal disease (IBD) and Newcastle disease are viral infections observed worldwide, which cause significant economic losses in the broiler production industry (Miller et al. Citation2009, Müller et al. Citation2012). Other than vaccination and biosecurity measures, new approaches are necessary in order to improve broiler immunity and resistance to virus infection.
The main aims of the present study were to evaluate and compare the effects of RP and CAB, (Loxidan® TD 100) incorporated on diets with different PF levels, on performance, caecum Escherichia coli microflora, haematological traits and humoral immune response against IB and Newcastle diseases virus of broiler chickens.
2. Material and methods
2.1. Animals and housing
A total of 180-day-old male Ross 308 broiler chicks were allocated to 18 land cages (150 cm × 100 cm × 60 cm; 6 groups × 3 replicates; 10 birds in each group) and fed 6 diets for 42 days during 2013. The mean birth body weights were similar between each cages. They were given free access to water and a feed throughout experiment period.
At placement time, ambient temperature was controlled at 32°C and decreased periodically to 24°C at 3 weeks of age and was maintained at 24°C until the termination of the investigation.
Humidity was added to the barn atmosphere via a water spray to maintain relative humidity between 55% and 65%.
Air circulation within the poultry barn was facilitated by three wall-mounted 60 cm diameter fans on one end of the barn and 160 cm diameter wall-mounted fans on the other end of the barn to establish tunnel ventilation.
Lighting was provided by 23 w fluorescent tubes in ceiling fixtures. Constant light was provided on day 1, but on day 2, lighting was established at 21 h per day until the end of the study.
A vaccination programme against Avian Influenza disease (1st day of age; Avian Influenza – H9N2; Razi Co, Karaj, Iran), Infectious Bronchitis disease (1st, 20th and 33rd day of age; Infectious Bronchitis Virus – H120; Razi Co, Karaj, Iran), IBD (16th and 29th day of age; Gumboro IBD071IR; Razi Co, Karaj, Iran) and Newcastle disease (8th and 29th day of age; Newcastle lentogenic vaccine; strains Hitchner B1 and La Sota; Razi Co, Karaj, Iran) virus was practiced, in order to simulate a classical vaccination programme.
All procedures have been approved by the Authors’ Institution’s Ethic Committee, and care was taken to minimize the number of animals used.
2.2. Diets and experimental treatments
A three-phase feeding programme was used in this investigation and consisted of provision of starter feed from 1st to 10th day, grower feed from 11th to 24th day and finisher feed from 25th to 42nd day. The composition of the diets is given in . The diets met or exceeded Ross 308 catalogue recommendations. All experimental diets were provided in powder form.
Table 1. Feed ingredients and nutrient analysis of used diets during experiment.
The CAB compounds (Loxidan® TD 100) were a mix of 6% Propyl gallate (E 310), 2.4% ethoxyquin (E 324), 17% butylated hydroxytoluene (E 321) and 25% citric acid (E 330), according to the manufacturer-provided information (Lohmann Animal Health, Germany).
The RP and CAB and/or bentonite were added to each diet according each treatment.
The experimental design, a 3 × 2 factorial arrangement, included six treatments with three replicates for each treatment. Environmental conditions were similar for all following treatments: Treatment 1 (T1) – diets included 3.5% PF (extracted viscera oil) without antioxidants (0%); Treatment 2 (T2) – diets included 6.0% PF without antioxidants (0%); Treatment 3 (T3) – diets included 3.5% PF and Loxidan® TD 100 powder as antioxidant (0.1% in diet); Treatment 4 (T4) – diets included 6.0% PF and Loxidan® TD 100 powder as antioxidant (0.1% in diet); Treatment 5 (T5) – diets included 3.5% PF and RP as antioxidant (0.1% in diet) and Treatment 6 (T6) – diet included 6.0% PF and RP as antioxidant (0.1% in diet).
2.3. Studied traits
2.3.1. Performance
On the 7th, 10th, 24th and 42nd day of age, body weight and feed intake were measured. Feed efficiency (total feed intake/total body weight gain) during each period was calculated.
2.3.2. Immunity
Humoral immune response of chickens to the Newcastle vaccine was sampled on 29th day of age and measured based on haemagglutination-inhibition test (Allan & Gough Citation1974). Humoral immune response of chickens to the IB vaccine was sampled also on 29th day of age and measured based on ELISA method (IDEXX Laboratories, B.V., The Netherlands), according to manufacturer’s instructions.
2.3.3. Microbiological cultures
Agar plates were streaked with caecum contents on 42nd day from one bird of each replicate, and sent to the laboratory. To determinate E. coli growth and colony counts, the agar plates streaked on the site were used. Collecting tubes were weighed, wrapped in aluminium sheet and autoclaved for 10 min. The culture medium was prepared and 24 h before collecting samples were poured into the Petri dish. Metilan Blou (EMB, 1.01347.0500) to culture E. coli was used. Samples were transferred to the laboratory in the listed tubes and again weighed. The amount of sample in each tube was calculated from the difference between these two values. Tubes were shaken for approximately 30 min. The action was performed for bacteria isolated from caecum contents and preparation of suspension. One millilitre was removed from the prepared suspension and was added into 9 ml buffer phosphate saline in the other tube. So the concerned suspension was prepared from dilutions 10–1 and serial dilutions were done (10–2, 10–3, 10–4, 10–5 and 10–6). One hundred microlitre was removed from (10–4, 10–5 and 10–6) dilutions and poured into the Petri dish previously prepared containing the medium and completely distributed to all parts of the medium. Counting bacteria in Petri dishes was done by colony counter. Bacterial counts were reported as logarithm number of bacteria per 1 g sample.
2.4. Statistical analyses
Data were analysed by analysis of variance using a 3 × 2 factorial arrangement with three antioxidant type treatments (no antioxidant, RP powder and Loxidan® TD 100) and two dietary fat-level treatments (3.5% and 6.0% in diet), using a two-way ANOVA procedure (SPSS Citation1997) and based on Yijk = μ + Ai + Bj + ABij + eijk formula. The Duncan post hoc test was used if the initial test result was significant at p < .05.
Statements of significance were based on p < .05.
3. Results
During the starter period, broilers fed with 6.0% PF showed a higher feed intake on 7th day than fed with 3.5% PF (183.4 versus 175.1 ± 2.5 g, respectively; ± SEM; p < .05). On 10th day, the body weight was also higher when birds were fed with 6.0% PF (271.5 g) than fed with 3.5% PF (266.0 ± 1.7 g; p < .05). However, no significant differences (p > .05) were observed between the six treatments for both antioxidants variables or for feed efficiency, throughout the starter period.
During the grower and finisher periods, the body weight gain was higher (p < .05) in groups T5 or T6 than in group T1 (). However, in overall (from 1st to 42nd day), the body weight (2542.9 ± 121.4 g), feed intake (5369.1 ± 337.7 g) and feed efficiency (2.67 ± 0.18) of broilers were similar (p > .05) between all groups.
Table 2. Performance means between groups of 180 Ross 308 broilers fed with diets containing the different levels of oil and antioxidants during the grower and finisher periods.
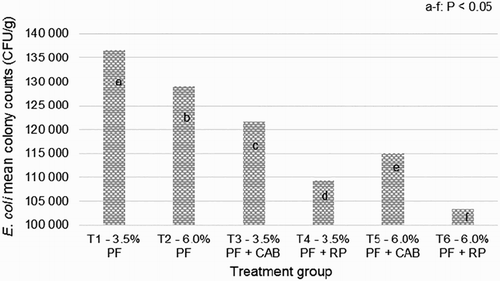
On 42nd day, CAB or RP feed additives reduced (p < .05) the E. coli colonies number in caecum ().
Figure 1. Caecal Escherichia coli mean colony counts (SEM ± 1 420) on 42nd day of age in 18 Ross 308 broilers fed with diets containing the different levels of oil and antioxidants. PF – poultry fat; CAB – commercial antioxidant blend (Loxidan® TD 100 at 0.1% in diet); RP – rosemary powder at 0.1% in diet.
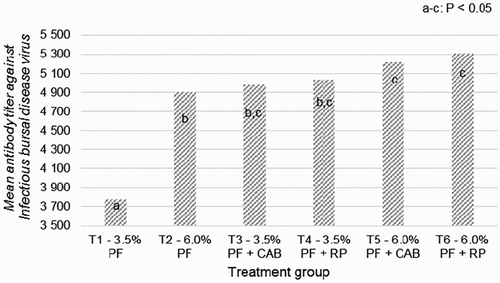
Both RP and CAB feed additives also improved antibody titre against IBD virus (). Inversely, antibody titre against Newcastle disease virus remained similar (p > .05) in all groups (T1 = 4.67 ± 0.61 lg2).
Figure 2. Immune response mean (SEM ± 235.9) after vaccination against infectious bursal disease virus of 18 Ross 308 broilers fed with diets containing the different levels of oil and antioxidants. PF – poultry fat; CAB – commercial antioxidant blend (Loxidan® TD 100 at 0.1% in diet); RP – rosemary powder at 0.1% in diet.
4. Discussion
In summary, the present study demonstrated that both CAB and RP and diets supplemented with 6.0% PF can be advantageous mainly in order to modulate the caecal coliform microflora and improve humoral immunity against IB in broilers. Although final performances were similar between groups, diets supplemented with 6.0% PF also can promote the bird weight in specific growth periods, improving feed efficiency. Additionally, both antioxidants can increase the body weight during the finisher period in broilers fed with 6% PF.
The higher feed intake and body weight on 10th day of age observed in birds fed with 6.0% PF than fed with 3.5% PF during the starter period suggest that the fat digestibility was not a growth-restrictive factor, and is in consonance with the results obtained by Noy and Sklan (Citation1995) and Zelenka et al. (Citation1997). However, a limitation of fat digestion and absorption in young chickens due low duodenal lipase levels was observed by Al-Marzooqi and Leeson (Citation1999). Lipase secretion increased 20–100-fold until 21st day (Noy & Sklan Citation1995).
Although in our study all performances were similar (p > .05) between groups at the end of production cycle, the body weight gain was higher in broilers fed with 6.0% PF than 3.5% PF when only starter, grower or finisher period was considered. Ghazalah et al. (Citation2008) also observed that dietary 5% PF improve performances (body weight, body weight gain and feed conversion ratio) in broilers under heat stress conditions. In their study, this performance also increased when metabolizable energy was improved. However, in the present study, metabolizable energy presumably remained constant in both dietary PF levels and the feed efficiency improved during each period. Nobakht (Citation2012) has not observed adverse effects on performance when broilers were supplemented with 6% PF, but vitamin E, a strong antioxidant, interacted negatively with PF.
In our study, a positive effect of both additives and 6.0% PF was observed on E. coli colony counts of caecal contents, a habitual commensal bacteria. This number reduction is very important due to the potential control of several potential (non-) pathogenic E. coli strains in caecum, a major gut segment for bacterial fermentation and nutrients absorption (Seidavi et al. Citation2010). Our results also suggest that both additives can help the modulation of caecal microflora in order to substitute antimicrobial substances as growth promoters.
In the present study, the humoral response against IBD, but not against Newcastle disease, was improved by CAB, RP or 6.0% PF. An improvement of antibody titre against IB virus was also observed by Nayebport et al. (Citation2007) when 2–4–6% soybean oil was increased in diet. However, antibody titre against IBD virus or Newcastle disease virus was not enhanced using dietary rosemary oils at 100 mg/kg level (Abd El-Latif et al. Citation2013). Maybe the humoral immunity enhancement observed in our study was related with the presence of polyunsaturated fatty acids (PUFA) from PF. Al-Khalifa et al. (Citation2012) suggested that broiler chickens diets rich in n-3 PUFA can have detrimental effects on immunity with and consequently risk of infection should be determined. However, dietary n-3 PUFA enrichment can improve resistance and cellular immune response against IBD, also increasing performance in a dose-dependent manner (Maroufyan et al. Citation2012).
5. Conclusion
Our results suggest that CAB and RP additives can be used in diets with 6.0% PF in order to improve broilers health, modulating the caecal microflora and the humoral immune against IBD virus. Both CAB and RP additives also can interact with dietary PF improving the bird body weight and can be used in organic and conventional, respectively.
Acknowledgements
This paper is obtained from M.Sc. thesis of the first author at Islamic Azad University, Sanandaj Branch, Sanandaj, Iran. We are grateful to the Islamic Azad University, Sanandaj Branch, Sanandaj, Iran for support.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
João Simões http://orcid.org/0000-0002-4997-3933
References
- Abd El-Latif AS, Saleh NS, Allam TS, Ghazy EW. 2013. The effects of rosemary (Rosemarinus officinalis) and garlic (Allium sativum) essential oils on performance, hematological, biochemical and immunological parameters of broiler chickens. Brit J Poult Sci. 2:16–24.
- Al-Khalifa H, Givens DI, Rymer C, Yaqoob P. 2012. Effect of n-3 fatty acids on immune function in broiler chickens. Poult Sci. 91:74–88. doi: 10.3382/ps.2011-01693
- Allan WH, Gough RE. 1974. A standard haemagglutination inhibition test for Newcastle disease. (1). Comparison of macro and micro methods. Vet Rec. 95:120–123. doi: 10.1136/vr.95.6.120
- Al-Marzooqi W, Leeson S. 1999. Evaluation of dietary supplements of lipase, detergent, and crude porcine pancreas on fat utilization by young broiler chicks. Poult Sci. 78:1561–1566. doi: 10.1093/ps/78.11.1561
- Baião NC, Lara LJC. 2005. Oil and fat in broiler nutrition. Rev Bras Ciênc Avíc. 7:129–141. doi: 10.1590/S1516-635X2005000300001
- Delles RM, Xiong YL, True AD, Ao T, Dawson KA. 2014. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult Sci. 93:1561–1570. doi: 10.3382/ps.2013-03682
- Fébel H, Mézes M, Pálfy T, Hermán A, Gundel J, Lugasi A, Balogh K, Kocsis I, Blázovics A. 2008. Effect of dietary fatty acid pattern on growth, body fat composition and antioxidant parameters in broilers. J Anim Physiol Anim Nutr. 92:369–376. doi: 10.1111/j.1439-0396.2008.00803.x
- Ghazalah AA, Abd-Elsamee MO, Ali AM. 2008. Influence of dietary energy and poultry fat on the response of broiler chicks to heat therm. Inter J Poult Sci. 7:355–359. doi: 10.3923/ijps.2008.355.359
- Ghazalah AA, Ali AM. 2008. Rosemary leaves as a dietary supplement for growth in broiler chickens. Inter J Poult Sci. 7:234–239. doi: 10.3923/ijps.2008.234.239
- Ibañez E, Kubátová A, Señoráns FJ, Cavero S, Reglero G, Hawthorne SB. 2003. Subcritical water extraction of antioxidant compounds from rosemary plants. J Agric Food Chem. 51:375–382. doi: 10.1021/jf025878j
- Kellems RO, Church DC. 2010. Livestock feeds and feeding. 6th ed. Boston, MA: Prentice Hall.
- Leeson S, Summer JD. 2005. Commercial poultry nutrition. 3rd ed. Nottingham: Nottingham University Press.
- Lu T, Harper AF, Zhao J, Dalloul RA. 2014. Effects of a dietary antioxidant blend and vitamin E on growth performance, oxidative status, and meat quality in broiler chickens fed a diet high in oxidants. Poult Sci. 93:1649–1657. doi: 10.3382/ps.2013-03826
- Maroufyan E, Kasim A, Ebrahimi M, Loh TC, Bejo MH, Zerihun H, Hosseni F, Goh YM, Farjam AS. 2012. Omega-3 polyunsaturated fatty acids enrichment alters performance and immune response in infectious bursal disease challenged broilers. Lipids Health Dis. 11, 15. http://doi.org/10.1186/1476-511X-11-15
- Miller PJ, Decanini EL, Afonso CL. 2009. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect Genet Evol. 10:26–35. doi: 10.1016/j.meegid.2009.09.012
- Moreno S, Scheyer T, Romano CS, Vojnov AA. 2006. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Res. 40:223–231. doi: 10.1080/10715760500473834
- Müller H, Mundt E, Eterradossi N, Islam MR. 2012. Current status of vaccines against infectious bursal disease. Avian Pathol. 41:133–139. doi: 10.1080/03079457.2012.661403
- Nayebpor M, Hashemi A, Farhomand P. 2007. Influence of soybean oil on growth performance, carcass properties, abdominal fat deposition and humoral immune response in male broiler chickens. J Anim Vet Adv. 6:1317–1322.
- Nobakht A. 2012. The effects of different levels of poultry fat with vitamin E on performance and carcass traits of broilers. Afr J Agric Res. 7:1420–1424.
- Noy Y, Sklan D. 1995. Digestion and absorption in the young chick. Poult Sci. 74:366–373. doi: 10.3382/ps.0740366
- Rostami H, Seidavi A, Dadashbeiki M, Asadpour Y, Simões J. 2015. Effects of dietary Rosmarinus officinalis powder and vitamin E at different levels on the performance and gut gross morphometry of broiler chickens. Braz J Poult Sci. 17: 23–30
- Seidavi AR, Mirhosseini SZ, Shivazad M, Chamani M, Sadeghi AA, Pourseify R. 2010. Detection and investigation of Escherichia coli in contents of duodenum, jejunum, ileum and cecum of broilers at different ages by PCR. Asia-Pac J Mol Biol Biotech. 18:321–326.
- SPSS. 1997. SPSS Base 7.5 for Windows. SPSS, Chicago, IL.
- Tavárez MA, Boler DD, Bess KN, Zhao J, Yan F, Dilger AC, McKeith FK, Killefer J. 2011. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult Sci. 90:922–930. doi: 10.3382/ps.2010-01180
- Yesilbag D, Eren M, Agel H, Kovanlikaya A, Balci F. 2011. Effects of dietary rosemary, rosemary volatile oil and vitamin E on broiler performance, meat quality and serum SOD activity. Br Poult Sci. 52:472–482. doi: 10.1080/00071668.2011.599026
- Zelenka W, Knaus W, Aichinger F, Lettner F. 1997. Effects of different dietary fat sources on performance and carcass characteristics of broilers. Anim Feed Sci Techn. 66:63–73. doi: 10.1016/S0377-8401(96)01126-1
