ABSTRACT
There is a definite pattern of the growth of seminiferous tubules at different post natal ages in mammals. The present work involves recording the changes of the seminiferous tubules, the cellular components of the seminiferous epithelium and the Leydig cells in male Assam goats. Different micrometrical parameters of the seminiferous tubules were found to increase with the increase in age of the male goats and they showed a significant (P < .05) increase between the age of 6 and 8 months. Also, the micrometrical parameters of different spermatogenic cells showed a significant (P < .05) increase between 6- and 8-month-old goats. The Leydig cells could be marked in the intertubular tissue right from birth. Various micrometrical parameters of the Leydig cells were found to increase with the advancement of age of the male goats. The nuclear diameter of the Leydig cells did not show a definite pattern during their postnatal growth. The other micrometrical measurements of the Leydig cells denoted their highest values at the age of 8 months.
1. Introduction
Postnatal development of the seminiferous epithelium has been investigated in various animal species, viz. pig (Van Straaten et al. Citation1978) and domestic cat (Elcock & Schoning Citation1984). The seminiferous tubules seem to exert some poorly understood effects on Leydig cell structure and steroidogenic activity (Parvinen Citation1982). Moreover, macrophages that are present in the testis intertubular tissue have been suggested to enhance Leydig cell steroidogenic activity in vitro (Neaves et al. Citation2002). It has also been stated that a testicular luteinizing hormone releasing hormone-like factor probably secreted by the Sertoli cells has the potential to exert a wide range of stimulatory and inhibitory effects on Leydig cell function (Sharpe Citation1984). Mammalian Leydig cells emerge in two successive waves – the first leading to a foetal generation, second beginning at puberty and leading to adult Leydig cell population (Wrobel Citation1984). Again, the Leydig cells were very less in the interstitial tissue of neonatal buffalo calves up to the age of six months (Ramaya et al. Citation2007). It is thought that still unidentified correlations may persist among testicular compartments, Leydig cell organelles and testosterone secretory capacity in the domestic animals. All these results strongly suggest that some percentage of the Leydig cell steroidogenic activity is regulated by the intratesticular factors emanating in part from structures other than the Leydig cells.
The Assam goat is the most versatile caprine species reared mainly for meat production. The chevon of this animal is less fibrous and highly delicious. Assam goat is found in the state of Assam and in the hilly tracts of the other North-Eastern states. This study is the first report enlightening the development of the testis of this animal in terms of the morphometrical parameters of different cellular components of the seminiferous epithelium during the postnatal period.
2. Materials and methods
A total of 72 male Assam goats from birth to the age of 10 months were used in this study. The animals were divided into six age groups – viz. group-I (0-day), group-II (2 months), group-III (4 months), group-IV (6 months), group-V (8 months) and group-VI (10 months) – consisting of twelve animals in each group. The age of the goats were estimated from birth records. Each animal was weighed using Spring Balance to record the body weight. The animals were sedated by giving intramuscular injection of Siquil (Triflupromazine Hydrochloride) @ 1 mg/Kg body weight and subsequently anaesthetized by administering intravenous injection of Intravel Sodium (Pentobarbital Sodium) @ 15 mg/Kg body weight (Hall et al. Citation2000). After induction of proper level of anaesthesia, the testicles were incised out by performing open method of castration as per standard protocol (duly approved by Institutional Animal Ethics Committee, Assam Agricultural University, Khanapara, Guwahati). Subsequently, the animals were given proper post-operative treatment and care. Tissue pieces were collected from three different regions of the testis (viz., upper, middle and lower) and subsequently fixed in the prepared Bouin’s solution (Luna Citation1968). All the tissues were processed for paraffin sections by alcohol-xylene method (Luna Citation1968) using ceder wood oil. Sections were cut at 5 μ thickness using a Rotary Microtome (Thermo Germany) and stained with various stains – viz. Haematoxylin & Eosin, Masson’s Trichrome stain for collagen, Weigert’s method for elastic fibres, Gomori’s method for reticular fibres (Luna Citation1968), PAS-Haematoxylin method for nuclear staining and Lilley’s Allochrome method for basement membrane (Humason Citation1967). All the micrometrical parameters of the Leydig cells were recorded in H&E stained slides as follows.
Thickness of tunica albugenia: Recorded in 20 randomly selected cross sections of apparently round sex cords/ seminiferous tubules per animal (Franca & Godinho Citation2002).
External diameter of the sex cords/ tubules: These measurements were taken in 20 randomly selected cross sections of apparently round seminiferous cords/tubules per animal (Franca & Godinho Citation2002; Neaves et al. Citation2002).
Height of the seminiferous tubular epithelium: Epithelial height was measured in the same tubules utilized for determining the tubular diameter.
Leydig cell nuclear diameter: Under light microscope, the Leydig cell nuclear diameter could be regarded as spherical (Wrobel Citation1990). It was recorded in 20 cross sections of seminiferous tubules per animal (Franca et al. Citation2000).
No. of Sertoli cells per cross section of the seminiferous cord/tubule: 20 round seminiferous cords/tubules were considered at random per animal (Franca et al. Citation2000).
No. of spermatogonia per tubule: 10 nos. of tubules were considered at random (Prakash et al. Citation2008).
Sertoli cell nuclear diameter: 20 Sertoli cell nuclei were measured for recording their diameter per animal (Elizabeth et al. Citation2002).
Leydig cell nuclear volume: This was calculated from mean nuclear diameter (D) using the formula of Franca et al. (Citation2000) as follows:
Nuclear volume (NV) = 1/6 × Π × D3 (expressed in μ3).
Numerical density or Number of cell per mm3 of testis (NV):
Numerical density (NV) of various tissue components were recorded by point count method using square lattice of 100 intersections (ocular grid) silver lined for better visuality (Leica, Germany). Number count or numerical density (Nv), per unit area of testis was calculated as per Prakash et al. (Citation2008)where NA is the average no. of cells in the reference area; A is the area of the reticule in mm2 (area of the ocular grid in mm2); D is the mean diameter of cell in mm; t is the thickness of the section in mm.
(j) Volume density (Vv) of a tissue component: It was calculated as per Handagama et al. (Citation1988) as shown below:
Pt denotes total no. of points in the test grid.
(k) Absolute volume of the Leydig cell: It was calculated by multiplying the volume density of Leydig cell with testis volume (Handagama et al. Citation1988).
(l) Absolute volume of seminiferous tubule/cord: It was calculated by multiplying the volume density (VV) of seminiferous tubule or cord with testis volume (Handagama et al. Citation1988).
(m) Number of various spermatogenic cells per seminiferous tubules:
Number of spermatogonia per seminiferous tubule.
Number of primary spermatocytes per seminiferous tubule.
Number of secondary spermatocytes per seminiferous tubule.
Number of round spermatid per seminiferous tubule.
Number of Sertoli cell nuclei per seminiferous tubule.
These were counted in 10 round or nearly round cross sections of seminiferous tubules chosen at random per animal (Segatell et al. Citation2004).
(n) No. of Leydig cells per testis: It was calculated as per Handagama et al. (Citation1988).
(o) The ratios of various cells of the seminiferous tubules were estimated for determination of different functional aspects as per Segatell et al. (Citation2004). These measurements were recorded in 10 numbers of randomly selected seminiferous tubules per animal as follows.
2.1. Statistical analysis
Statistical analysis was performed using the programme SPSS, version 10.0 (SPSS Inc., Chicago, IL, USA) (Snedecor & Cochran Citation1994). Differences between species were examined for significance using Mann–Whitney U-tests. The level of significance was set at P ≤ .05, whereas P ≤ .01 was defined as highly significant.
3. Results and discussion
In the present work, the diameter of the sex cords/seminiferous tubules () showed an increasing pattern with advancing age of the animals () as also recorded earlier in rams (Sergeev & Zaboloskii Citation1976), cattle (Wrobel et al. Citation1988) and male Tokara goat (Nishimura et al. Citation2000). The diameter of the seminiferous tubules in 10-month-old Assam goats was recorded to be 210.03 ± 3.98 µ, which was similar to that reported in other domestic goats (Oke et al. Citation1984; Yadav & Sharma Citation1994; Franca & Russel Citation1998; Nishimura et al. Citation2000). The present study recorded a highly significant (P < .01) increase of the diameter of the sex cords/seminiferous tubules between various age groups. More precisely, a significant increase in diameter between 4- and 6-month-old goats indicated a rapid growth of the tubules before the onset of puberty in male Assam goats. On the contrary, Baishya et al. (Citation1986) reported that in male goats aged 0–90 days of age, there was an increase in diameter of the seminiferous tubules with the advancement of age, but the rate of increase was not significant.
Figure 1. Values of different parameters of the sex cords/seminiferous tubules of the male Assam goats at different post natal ages.
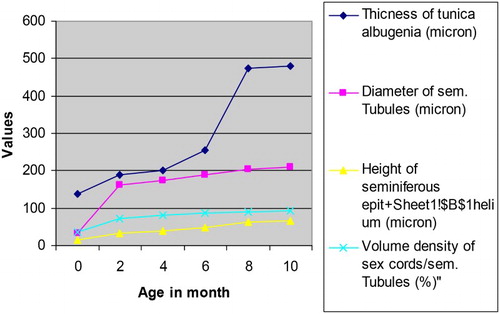
Table 1. Micrometrical values (Mean ± SE) of the sex cords/seminiferous tubules of testis in male Assam goats at different ages.
The height of the seminiferous epithelium increased significantly (P < .01) as age advanced from 0 to 10 months in the male goats. The increase in height of seminiferous epithelium was found to be more marked between 4 and 6 months of age, which corresponded with the period around puberty. The present finding was in conformity with that reported by Nishimura et al. (Citation2000) and Zahid et al. (Citation2002) in male Tokara goats and male Teddy goats, respectively.
The seminiferous tubules comprised the main compartment of the testis and their volume densities ranged from 70 to 90% of the testis parenchyma in most mammals investigated (Russel et al. Citation1990; Franca & Russel Citation1998). In this regard, the values obtained for the volume densities of the seminiferous tubules of Assam goats at 10 month of age (92.09 ± 2.39%) was at slightly higher level when compared to most mammals. In domestic cat (Franca & Godinho Citation2002) and Gerbil (Segatell et al. Citation2004), the volume densities of the seminiferous tubules were reported to be 90 and 92%, respectively. However, the data in regard to this micrometrical parameter found in the available literature for other breed of goat were around 80–85% (Oke et al. Citation1984; Yadav & Sharma Citation1994).
The Leydig cells secreted steroids and pheromones that were important for other reproductive functions such as sexual behaviour and maintenance of sexual accessory glandular function (Franca & Godinho Citation2002). In the present investigation, the nuclear diameter and volume of the Leydig cells were found to be increased from birth to 2 months of age and then it reduced at 4 month of age () (). This reduction in diameter and volume of the Leydig cell nuclei might be accounted for the presence of the increased amount of growing smooth endoplasmic reticulum destined for production of steroid hormones before the onset of puberty (Zirkin et al. Citation1980). Similar finding was also reported by Lee et al. (Citation2004) in goats.
Table 2. Different micrometrical values (Mean ± SE) of the Leydig cells of testis of male Assam goats at different ages.
When compared to most mammals, the volume densities of the Leydig cells in goats were reported as very low (Russel Citation1996; Franca & Russel Citation1998). Again, there was very little knowledge regarding the reason why dramatic variation was observed for Leydig cell volume density ranging from 1% in rams to 35% in Cappy boars (Johnson & Neaves Citation1981). The present study recorded the overall volume density of the Leydig cells in a day-old to 10-month-old Assam goats as 6.07 ± 0.49%. The volume density of Leydig cell was found to be the maximum in 8-month-old goats, which could be correlated with the more amount of smooth endoplasmic reticulum within the cytoplasm of these cells and its capacity to secrete testosterone hormone in the male goats at this age (Zirkin et al. Citation1980). However, remarkably more Leydig cell volume density was reported in boars, particularly in pre-pubertal testis, which was characteristic of the porcine species (Van Straaten & Wensing Citation1978; Franca et al. Citation2000).
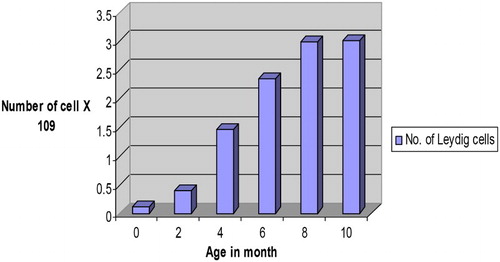
The total number of Leydig cells per testis was recorded to be ranging from 0.31 ± 0.29 × 109 cells at birth (group-I) to 3.75 ± 0.59 × 109 cells in 10 months old goats (group-VI) (). Maximum number of Leydig cells per testis was recorded at 10 month of age (group-VI). The histological part of this study on testis confirmed the age of onset of puberty in Assam goats to be at 6 month of age. Karmore et al. (Citation2001) also reported that the number of Leydig cells was less in pre-pubertal than in pubertal and post-pubertal goats. Similar trend of Leydig cell number per testis was also reported in pigs (Franca et al. Citation2000). Because the body and testicular weights, and sperm production per testis in goats increased substantially after the pubertal period (Lee et al. Citation2004), the demands for steroids and other substances secreted by Leydig cells were probably higher, requiring more Leydig cells per testis. In the present study, the Leydig cells showed a significant (P < .05) increase () in their number per testis between 4 and 6 month of age (groups-III & IV) probably due to combat with the increased demand of testosterone hormone approaching the onset of puberty in Assam goats. However, the onset of puberty in goats was reported to be variable being 3.5 ± 0.40 months in Black Bengal bucks (Samsuddin Citation1996) and 29.00 ± 0.57 weeks in Beetal × Assam local male kids (Kakoti Citation1999). Again, Hafez (Citation1987) reported that, under normal breeding conditions, goats attained puberty at the age of 6–7 months. The mean number of Leydig cells in 10-month-old Assam goats was recorded to be 3.01 ± 0.59 × 109 cells, which was more than the same (1.72 ± 0.5 × 109) reported in adult Alpine bucks (Leal et al. Citation2004).
The Sertoli cell was the major controller of testis development and efficiency of spermatogenesis (Hess et al. Citation1993). Again, the FSH is considered to be the main mitogenic factor responsible for Sertoli cell divisions. The Sertoli cell number established during the prepubertal period determined the final testicular size and the number of sperm produced in sexually mature animals (Orth et al. Citation1988). This occurred because each Sertoli cell could support only a limited number of germ cells, with the support capacity being variable and species specific (Franca & Russel Citation1998).The nuclear diameter of the Sertoli cells in the present study increased with the advancing age of the male kids, being significant in the pre-pubertal period (up to 4 month of age) in Assam goats. Similar increase was also reported during post natal development of the testis in pigs (Franca et al. Citation2000). However, paucity of relevant literature in ruminants pertaining to this parameter restricted the comparison of present findings.
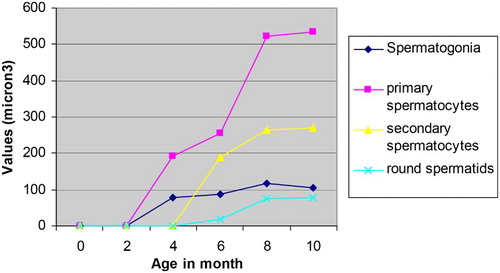
The nuclear volumes of spermatogonia, primary spermatocytes, secondary spermatocytes and round permatids of Assam goats increased with increasing age of the male kids (). The nuclear volume of the spermatogonia varied significantly (P < .05) between 4 and 6 months of age while the same for primary spermatocytes varied significantly between 6 and 8 months of age (). The variation in nuclear volumes of secondary spermatocytes and round spermatids were recorded to be significant (P < .05) between 6 and 8 months of age in the male goats. The overall nuclear volume of spermatogonia, primary spermatocytes, secondary spermatocytes and round spermatocytes in Assam goats from 0-day through 10 month of age were recorded as 96.45 ± 13.71, 375.34 ± 75.34, 241.19 ± 13.88 and 57.63 ± 16.09 µ3, respectively. Information pertaining to this parameter was found to be scant in the available literature. However, the mean nuclear volume of primary spermatocytes recorded in the present study in 10-month-old (group-VI) goats (533.27 ± 15.29 µ3) was comparable to the same observed in adult donkeys (410 ± 4.00 µ3) and in mules (384.33 ± 14.10 µ3), respectively (Neaves et al. Citation2002).
Table 3. Nuclear volume (Mean ± SE in µ3) of different spermatogenic cells of the seminiferous epithelium of testis of male Assam goats at different ages.
The mean numbers of Types A & B spermatogonia per seminiferous tubule increased in the male kids in advancing age groups (). Again, the number of primary spermatocytes increased significantly (P < .05) between 4, 6 and 8 months of age (groups-III, IV & V), which might be to aid formation of more number of progenitor cells from them during and just after puberty. The secondary spermatocytes showed their appearance in the testes at 6 months of age (group-IV) and their number in the seminiferous tubules did not vary significantly between the older age groups as the secondary spermatocytes appeared for a very transient period in the testicular tissue and immediately they became transformed into the round spermatids (Dellmann Citation1981; Franca & Russel Citation1998). The mean number of the round and elongated spermatids increased significantly (P < .5) between 6 and 8 months of age (groups-IV & V). However, the mean number of elongated spermatids showed a slight reduction in their number at 10 months of age (group-VI), which was not significant between the preceding age groups. This minimum reduction in the number of elongated spermatids per seminiferous tubule in 10-month-old goats (group-VI) might be the part of the process approaching towards establishing an adult value of these cells in the testes of Assam goats at this age. In pigs, the number of germ cell per seminiferous cord/tubular cross section was very low from birth to 4 months of age. A very dramatic increase in various populations of germ cells per cross section of seminiferous tubule occurred from 4 to 5 months of age, but the number of various germ cells showed a tendency to stabilize after 7 months of age (Franca et al. Citation2000).
Table 4. Number (Mean ± SE) of different cells per cross section of the seminiferous cord/tubule of testis of male Assam goats at different ages.
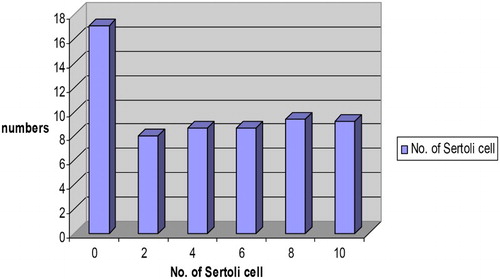
The mean number of Sertoli (Sustentacular) cells per cross section of sex cord recorded in the male Assam goat kids at birth was the highest among all the age groups under study (). The number of these cells was reduced at 2 months of age (group-II), remained almost same at 4 and 6 months of age (groups-III & IV) and then showed a slight rise in their number at 8 months of age (group-V). Subsequently, the mean number of Sertoli cells per cross section of the seminiferous tubule reduced at 10 months of age. These findings were in close corroboration with earlier reports of Sinowatz and Amselgruber (Citation1986) in cattle and Franca et al. (Citation2000) in pigs. Fernanda et al. (Citation2006) reported the presence of 6.6 number of Sertoli cells per seminiferous tubule in wild boar. The increased number of Sertoli cells per cross section of the sex cord recorded in the present work in day-old kids might be the species-specific characteristics of goats, which need further works in this aspect as no available literature could be traced to compare with the present findings.
Figure 4. Number of Sertoli cells (×109) per cross section of the seminiferous tubule of the testes in Assam goats at different post natal ages.
Germ cell loss (apoptosis) played an important role in the seminiferous epithelial homeostasis by limiting the number of sperm cells produced, which could be estimated by comparing the ratios between germ cells counted during specific steps of spermatogenesis (Johnson Citation1991). Estimation of ratio between pachytene primary spermatocytes and Type A spermatogonia in the present work () revealed that the efficiency of spermatogonial mitosis increased noticeably from 4 months of age onwards, with the maximum being in 10-month-old goats (group-VI). The overall rate of spermatogenesis as measured by estimating the ratio between round spermatids and Type A spermatogonia was recorded from 6 months of age (group-IV) onwards. The rate increased significantly (P < .05) between 6- and 8 month-old goats (groups-IV & V). Again, the Meiotic Index (ratio between round spermatids and pachytene primary spermatocytes) recorded in the present study in Assam goats ranged from 1.33 ± 0.02 at 6 months to 3.06 ± 0.16 in 10 month-old goats, denoting a decrease in germ cell loss during meiosis in older goats. The overall Meiotic Index in Assam goats was recorded as 2.45 ± 0.56, indicating that 38.75% of cell loss occurs during meiosis as one pachytene spermatocyte produces four round spermatids (Segatell et al. Citation2004). This value of cell loss was similar to that observed for most mammalian species investigated (Franca & Russel Citation1998).
Table 5. Ratio (Mean ± SE) of different spermatogenic cells of the seminiferous epithelium of testis of male Assam goats at different ages.
The efficiency of Sertoli cells increased from 6 (group-IV) to 10 months (group-VI) of age in Assam goats and the ratio of round spermatid and Sertoli cell nuclei showed a significant (P < .05) increase between 6 and 8 months of age (groups-IV & V). The Sertoli cell efficiency was found to be the maximum in 10-month-old Assam goats as almost 16.12 spermatids were found for each Sertoli cell. The overall number of 13 spermatids was found for each Sertoli cell in all the 3 age groups studied (viz. groups-IV, V & VI) in the present work. Similar value was also recorded in Gerbil (Segatell et al. Citation2004). However, the present value for efficiency of Sertoli cell was relatively high when compared with other mammalian species (Wing & Chrstensen Citation1982; Franca & Russel Citation1998).
The volume densities of different spermatogenic cells of the testis increased with advancing age (), thereby occupying more volumes in the seminiferous epithelium needed for enhanced physiological functions of the testes in pubertal and post-pubertal male Assam goats ( and ). Relevant information pertaining to this parameter was found to be very scant in the available literature. However, Franca et al. (Citation2000) reported similar findings during post natal development in pigs.
Figure 5. Volume densities of various spermatogenic cells of the seminiferous tubules of the testes in Assam goats at different post natal ages.
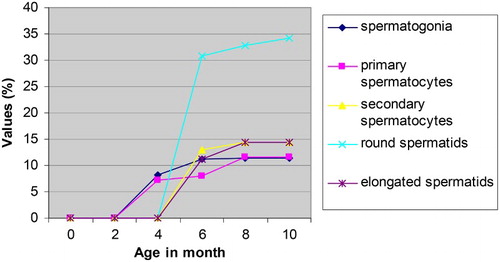
Figure 6. Photomicrograph of the testis in a four-month-old male kid showing Leydig cells (arrows) in the intertubular tissue. H & E, 1000X.
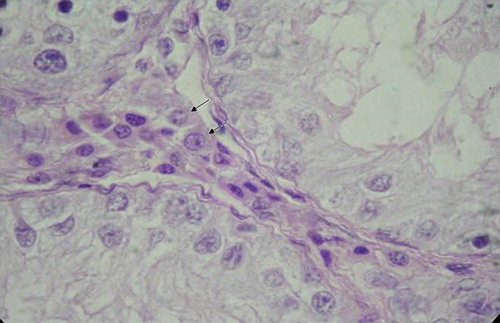
Table 6. Volume densities (Mean ± SE in %) of different cells of the seminiferous epithelium of testis of male Assam goats at different ages.
References
- Baishya G, Ahmed S, Bhattacharya M. 1986. A correlative study on biometry and histomorphometry of Male gonad and thyroid gland (0-90 days) in Assam goat (Capra hircus). Indian Vet J. 63:928–932.
- Dellmann H. 1981. Endocrine system. In: Dellmann H, Brown EM, editors. Textbook of veterinary hitology. Philadelphia: Lea and Febiger; p. 371–399.
- Elcock LH, Schoning P. 1984. Age related changes in the cat testis and epididymis. Am J Vet Res. 45:2380–2384.
- Elizabeth SN, Garcia HC, Franca LR. 2002. Comparative testis morphometry and seminiferous cycle length in Donkeys and Mules. Biol Reprod. 67:247–255.
- Fernanda FL, Almeida M, Leal C, Franca LR. 2006. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa). Biol Reprod. 75:792–799.
- Franca LR, Godinho CL. 2002. Testis morphology, seminiferous epithelium cycle length and daily sperm production in domestic cat (Felis catus). Biol Reprod. 68:1554–1561.
- Franca LR, Russel LD. 1998. The testis of domestic animals. In: Martinez F, Regadera J, editors. Male reproduction, a multidisciplinary overview. 1st ed. Madrid: Churchill Livingstone; p. 197–219.
- Franca LR, Silva Jr. VA, Garcia HC, Garcia SK, Debeljick L. 2000. Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod. 63:1629–1636.
- Hafez ESE. 1987. Reproductive cycle. In: Reproduction in farm animals. 5th ed. Bombay: K.M. Verlghese Company; p. 113–114.
- Hall LW, Clarke KW, Trim CM. 2000. Wright’s veterinary anesthesia and analgesia. 10th ed. London: ELBS and Baillierre Tindall.
- Handagama SM, Ariyanta LC, Manem TV, Haupt RL. 1988. Differentiation of adult Leydig cells in neonatal rat testis is arrested by hypothyroidism. Biol Reprod. 59:351–357.
- Hess RA, Cooke PS, Bunick D, Kirby JD. 1993. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli cell and germ cell number. Endocrinology. 132:2607–2613.
- Humason GL. 1967. Animal tissue techniques. San Francisco, CA: W.H. Freeman and Company; p. 304–312.
- Johnson L. 1991. Seasonal differences in equine spermatogenesis. Biol Reprod. 44:284–291.
- Johnson L, Neaves WB. 1981. Age related changes in the Leydig cell population, seminiferous tubules and sperm production in stallions. Biol Reprod. 24:703–712.
- Kakoti D. 1999. Studies on body measurements, testicular biometry, age at puberty, sexual behaviour and semen characteristics in Beetal×Assam local male kids [M.V.Sc. Thesis]. Assam Agril. University, Khanapara, Guwahati, India.
- Karmore SK, Bhamburkar VR, Banubakode B, Dalvi RS, Waghaye Y. 2001. Age wise changes in the histological and histochemical status of the interstitial cells in goat (Capra hircus). Indian J Vet Anat. 13:49–52.
- Leal MC, Becker-Silva SC, Garcia CG, Franca LR. 2004. Sertoli cell efficiency and daily sperm production in goats (Capra hircus). Anim Reprod. 1:122–128.
- Lee J, Lee B, Lee S. 2004. Studies on spermatogenesis in Korean native goat. Korean J Vet Res. 25:91–101.
- Luna LG. 1968. Manual of histological staining methods of armed forces institute of pathology. 3rd ed. New York: McGraw Hill Book Co.; p. 153–173.
- Neaves ES, Garcia H, Franca LR. 2002. Comparative testis morphometry and seminiferous epithelium cycle length in donkeys and mules. Biol Reprod. 67:247–255.
- Nishimura S, Okano K, Yasukouchi K, Gotoh T, Tabata S, Iwamoto H. 2000. Testis developments and puberty in the male Tokara (Japanese native) goat. Anim Reprod Sci. 64:127–131.
- Oke BO, Ugwuegbu SO, Akusu MO. 1984. Morphometric study of the testis of the West African Dwarf goat. Bull Anim Health Prod South Africa. 32:57–60.
- Orth JM, Gunsalus GL, Lamperti AA. 1988. Evidence from Sertoli cell depleted rats indicate that spermatid number in adults depends on number of Sertoli cells produced during perinatal development. Endocrinology. 122:787–794.
- Parvinen M. 1982. Regulation of the seminiferous epithelium. Endocrinology. 3:404–417.
- Prakash S, Prithiviraj E, Suresh S. 2008. Developmental changes of seminiferous tubule in prenatal, postnatal and adult testis of Bonnet monkey (Macaca radiata). Anat Histol Embryol. 37:19–23.
- Ramaya PJ, Kumar V, Pathak D, Singh O, Sethi RS. 2007. Histomorphological studies on the testes of neonatal buffalo calves (Bubalus bubalis). Indian J Vet Anat. 19:7–12.
- Russel LD. 1996. Mammalian Leydig cell structure. In: Payne AH, Hardy MP, Russel LD, editors. The Leydig cell. Viena: Cache River Press; p. 43–96.
- Russel LD, Ren HP, Sinha-Hikim I, Schulze W, Sinha-Hikim AP. 1990. A comparative study in twelve mammalian species of volume densities, volumes and numerical densities of selected testis compartments, emphasizing those related to the Sertoli cells. Am J Anat. 188:21–30.
- Samsuddin M. 1996. Puberty in Black Bengal bucks (Capra hircus). Congress Programme of the Int. Congress of Anim. Reprod., Sydney, Australia, p. 14–19.
- Segatell TM, Franca LR, Pinheiro PFF, Alemida CCD, Martinez M, Martinez FE. 2004. Spermatogenic cycle length and spermatogenic efficiency in the Gerbill (Meriones unguiculatus). J Androl. 25:210–222.
- Sergeev NI, Zaboloskii VA. 1976. Age changes in the development of the rete testis in rams. Doklady Vsesoyuznoi Akademii Sel’skokhozyaistvennykh Nauk. 12:26–28.
- Sharpe RM. 1984. Intratesticular factors controlling testicular function. Biol Reprod. 30:29–49.
- Sinowatz F, Amselgruber W. 1986. Postnatal development of bovine Sertoli cells. Anat Embryol. 174:413–423.
- Snedecor GW, Cochran WG. 1994. Statistical methods. 9th ed. Ames: Iowa State University Press; p. 124–130.
- Van Straaten HW, Wensing CJG, Van-Straaten HWM. 1978. Leydig cell development in the testis of the pig. Biol Reprod. 18:86–93.
- Van Straaten WM, Wensing CJG. 1978. Leydig cell development in the testis of the pig. Biol Reprod. 18:86–93.
- Wing TY, Chrstensen K. 1982. Morphometric studies on rat seminiferous tubules. Am J Anat. 165:13–25.
- Wrobel KH. 1984. The postnatal development of the bovine Leydig cell population. Reprod Domestic Anim. 25:51–60.
- Wrobel KH. 1990. The postnatal development of the bovine Leydig cell population. Reprod Domestic Anim. 25:51–60.
- Wrobel KH, Dostal S, Schimmel M. 1988. Postnatal development of the tubular lamina propria and the intertubular tissue in the bovine testis. Cell Tissue Res. 252:639–653.
- Yadav SK, Sharma DN. 1994. Seminiferous tubule length in normal buffalo bulls and bucks. Indian J Anim Sci. 9:293–296.
- Zahid IA, Lodhi LA, Rehman NU, Akhtar MS. 2002. Effects of gossypol on micrometry of testis of Teddy male goat. Pak Vet J. 27:55–62.
- Zirkin BR, Ewing L, Kromann N, Cochran RC. 1980. Testosterone secretion by rat, rabbit, guinea pig, dog and hamster testes perfused in vitro: correlation with Leydig cell ultrastructure. Endocrinology. 107:1867–1874.