ABSTRACT
The mRNA expression profiles of Hsp-90 alpha, Hsp-90 beta and Hsp-60 were evaluated in coloured broiler chickens, that is, Punjab broiler-2 (PB-2) and naked neck (NN), exposed to 2°C increased incubation temperature for 3 h each on 16th, 17th and 18th days of incubation. Another set of eggs were incubated at normal conditions that were utilized as control. A total of 432 chicks, 216 in each breed (PB-2; NN) and treatment (heat exposed; normal), randomly distributed were reared at high ambient temperatures (32–45°C) during summer in battery brooders. Birds were sacrificed on 0 and 28th days post-hatch and different tissues (heart, liver, muscle, spleen and bursa) were collected to assess the mRNA expression. Heat exposure during incubation significantly (P ≤ .05) influenced the expression of Hsp-90 alpha in spleen of day old; in heart, liver and spleen at 28 days in PB-2 chicken. Hsp-90 alpha mRNA expression was not significant among the tissues at different ages in NN chicken. Hsp-90 beta did not show any significant variations in gene expression across the tissues at both the age groups either in NN or in PB-2 chicken. Heat exposure had significant effect in the expression patterns of Hsp-60 at 28 days in heart, liver and spleen in PB-2 chicken. The mRNA expression of all the three genes significantly (P ≤ .05) varied in different tissues across the age groups in both PB-2 and NN chickens. The study concluded that thermal adaptation during the embryonic stage to higher incubation temperature reduced Hsp gene expression in different tissues in postnatal life during high ambient temperature which might result in increased heat tolerance capacity of the broiler chickens.
1. Introduction
Thermal adaptation during embryogenesis to high temperature has been one of the options to mitigate the heat stress during the postnatal life of chicken to improve the heat tolerance, especially in broiler chicken. Temperature is one of the most important factors that exerts a negative influence on the performance of poultry and causes huge losses in terms of loss of productivity, reduced reproductive efficiency, increased stress, reduced immune competence and increased investment costs to mitigate the effects of climate change (Rajkumar et al. Citation2011): reduced growth rate, feed efficiency, intestinal injury, egg shell quality and survivability (Mashaly et al. Citation2004; Quinteiro-Filho et al. Citation2010). Poultry species are more vulnerable to heat stress due to increased environmental temperature, as birds can tolerate a narrow zone of temperature range; 18–24°C is the thermo-neutral zone for the birds. Increase in temperature beyond this range due to environmental or other metabolic factors will lead to cascading effects on thermoregulation and could be lethal to the birds. When chickens are exposed to high ambient temperature with high humidity, the thermoregulatory processes get impaired, heat dissipation affected, feed consumption and growth rate reduced resulting in increased mortality.
Thermal manipulation during embryogenesis (prenatal) induces physiological memory due to epigenetic adaptation to high temperature eliciting improved thermotolerance during the postnatal life (Yahav Citation2009). Thermotolerance in birds was achieved by exposing birds to neonatal heat stress (Yahav & Hurwitz Citation1996). Cyclical higher incubation temperature appears to improve the heat tolerance in chickens (Yahav Citation2009), depending on the length and period of exposure. The central and peripheral nervous thermoregulatory mechanism and other body functions are well developed during the later stage of incubation (Tzschentke Citation2007) which enables the embryos to adjust to the short-term increase in temperature without any negative effects on hatching performance. Sudden changes in the temperature are the earliest and most common phenomenon that cell has to cope with to preserve their structural and enzymatic integrity (Nadeau & Landry Citation2006). The variation in environmental temperature will generally impose stress on the embryo, which may result in the evolution of adaptive genetic mechanisms to combat with extreme temperatures (Hoffmann & Parsons Citation1991). Heat shock proteins (Hsps) are a set of proteins synthesized in response to physical, chemical or biological stresses, including heat exposure (Staib et al. Citation2007). The stimulated thermal tolerance degree is related with the expression of Hsps (Krebs & Bettencourt Citation1999).
The role of these proteins in the thermotolerance phenomenon in poultry is highly complex and has not been fully elucidated. The purpose of the present study was to investigate the possible effects of increased incubation temperature in the later stages of incubation on Hsp-90 alpha, 90 beta and 60 mRNA expressions in various tissues in coloured broiler chickens.
2. Materials and methods
2.1. Location
The experiment was conducted at ICAR – Directorate of Poultry Research, Hyderabad, Telangana, India, which is located on Deccan plateau, positioned between 17°23′N and 78°28′E at a height of 500 m from sea level. The environmental temperature ranges from 12°C in winter to 45°C in summer, The experiment was approved by the Institutional Animal Ethics Committee (IAEC).
2.2. Experimental population
Chicks from Punjab broiler-2 (PB-2) and naked neck (NN) were utilized for studying the effect of higher incubation temperature during the later stages of incubation (16th–18th day) on the expression of Hsp genes during post-hatch life of chicks. PB-2, a synthetic coloured broiler line was under long-term selection for high 5-week body weight and 40-week part period egg production which is being utilized for the development of improved broiler chicken varieties. NN is an important ecotype of chicken distributed along the hot humid coastal regions of India and is known for its heat tolerance. The Na gene was introduced into broiler population and the base population was developed after four successive generations of backcrossing and is maintained under mild selection pressure for six-week body weight for the last nine generations.
A total of 1002 eggs collected from PB-2 (636) and NN (366) lines were divided into 2 groups randomly. One group (heat exposed: HE) of 318 (PB-2) and 183 (NN) eggs was exposed to higher temperature (39.5°C), 2°C above the normal incubation temperature (37.5°C) for 3 h on 16th, 17th and 18th days of incubation. The relative humidity was maintained at 65% during the exposure. Other group (Normal: N) with same number of eggs was incubated under standard incubation conditions. A total of 432 chicks, 216 in each breed (PB-2; NN) and treatment (HE, N) out of 623 chicks produced, randomly distributed were reared at high ambient temperatures (32–45°C) during summer in battery brooders.
2.3. Rearing and management
The chicks were randomly distributed at the rate of 6 birds per battery brooder cage (60 × 75 cm) placed in an open-sided house with 2 breeds, 2 treatments and 18 replicates (2 × 2 × 18). The chicks were reared from day old to four weeks of age under standard management practices in battery brooders. The chicks were offered broiler ration (2900 K cal: ME, 22%: CP) ad libitum throughout the experiment period. The chicks were vaccinated against Marek’s disease (1st day), Newcastle disease (7th day) and infectious bursal disease (14th and 24th days). The ambient temperature ranged from 32°C to 45°C during the experiment period.
2.4. Collection of samples
On 0 and 28th days, six chicks from each group were sacrificed by decapitation and blood was collected. After exsanguinations, the birds were manually eviscerated and tissue samples (heart, liver, muscle, spleen and bursa) were collected aseptically and stored at −70°C until further use.
2.5. RNA extraction and cDNA synthesis
Total RNA was extracted from tissue samples using TRI reagent (Sigma Aldrich) according to the standard protocol. The purity of the RNA was determined by measuring absorbance in an ultraviolet spectrophotometer at 260 and 280 nm. cDNA was synthesized using high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). First-strand cDNA was synthesized from 1.5 μg of each total RNA samples using a random primer and reverse transcriptase enzyme according to the manufacturer’s protocol.
2.6. Real-time quantitative polymerase chain reaction
The expression of mRNA was quantified by the SYBR green method using an Mx-3000P spectro flourometric thermocycler (Stratagene, USA). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. The first-strand cDNAs were used as template to amplify a gene-specific primers for Hsp-90 alpha, Hsp-90 beta and Hsp-60 and reference gene GAPDH. The reactions were performed in a 25 μl volume of SYBR green master mix (Sigma, USA) with 10 pM of each primer (). The amplification protocol used was as follows: initial denaturation at 95°C for 2 min, followed by 40 cycles of cyclic denaturation at 94°C for 30 s, annealing at 59°C for 1 min and a final extension at 72°C for 15 s. Primer amplification efficiency was assessed from the standard curve generated by using serial 10-fold dilution of transcribed RNA. Regression of Ct values of standard curve was carried out to determine the amplification efficiency.
Table 1. Primer sequences used for amplification of gene fragments.
2.7. Statistical analysis
Relative quantification of gene expression was estimated using Ct values. The Ct value is the fractional cycle number at which the amount of amplified target reaches a fixed threshold. The ΔCt values are the difference between the Ct values of target gene and endogenous control subtracted from 40 (total cycles). The higher ΔCt values were interpreted as the numerical values indicating high gene expression (Mac Kinnon et al. Citation2009). The gene expression data were analysed on a four-factorial analysis of variance, the main effects (breed, tissue, treatment and age) and their interactions were evaluated using the general linear model procedure of SAS 9.2 software. Significant treatment means were further subjected to Tukey’s post hoc test.
3. Results
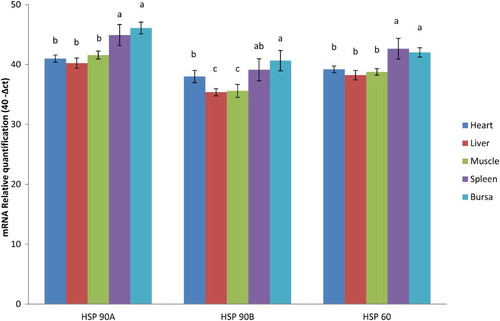
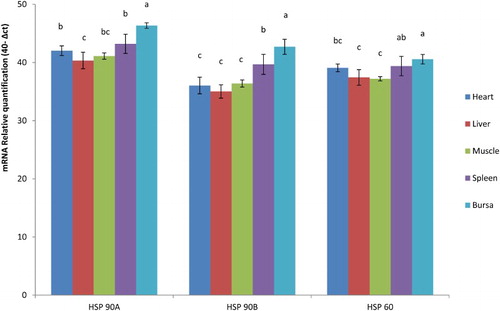
The concentrations of Hsp-90 alpha, 90 beta and 60 mRNA expression with respect to internal control (GAPDH), in various tissues of N and HE birds at different ages in NN and PB-2 are presented in . Heat exposure during incubation significantly (P ≤ .05) influenced the expression of Hsp-90 alpha in spleen of day old; in heart, liver and spleen at 28 days in PB-2 chicken (). Hsp-90 alpha mRNA expression was not significant among the tissues at different ages in NN chicken. Hsp-90 beta did not show any significant variations of gene expression across the tissues at both the age groups either in NN or in PB-2 chicken. Heat exposure had significant effect in the expression patterns of Hsp-60 in PB-2 chicks at 28 days in heart, liver and spleen, while muscle tissue and bursa had non-significant variations. In NN, there were no significant differences in expression patterns of Hsp-60 mRNA ().
Table 2. Hsp-90 alpha m-RNA expression in heat-exposed and normal broiler chickens.
Table 3. Hsp-60 mRNA expression in heat-exposed and normal broiler chickens.
The mRNA expression of all the three genes significantly (P ≤ .05) varied in different tissues across the age groups in both PB-2 and NN chickens. The Hsp-90 alpha and Hsp-60 mRNA expression was significantly (P ≤ .05) higher in spleen and bursa in NN chicken compared to other tissues (heart, liver and muscle) which had significantly (P ≤ .05) lower expression levels (). The liver and muscle had significantly (P ≤ .05) lower mRNA expression for Hsp-90 beta, while bursa had significantly (P ≤ .05) higher expression (), however, the expression pattern was similar in spleen and heart in NN chicken. In PB-2, the mRNA expression of all the three genes was significantly (P ≤ .05) higher in bursa (). Hsp-90 alpha mRNA expression was significantly (P ≤ .05) lower in liver. Hsp-90 beta and Hsp 60 gene expressions were significantly lower in heart, liver and muscle ().
4. Discussion
All living organisms (prokaryotes and eukaryotes) respond to environmental stressors at the cellular and molecular levels by expression of Hsps (Lindquist Citation1986; Morimoto et al. Citation1990). These Hsps help to maintain the metabolic and structural integrity of the cells and exert protective function for some tissues against some stressors such as heat stress, ischaemia and cytotoxicity (Eichler et al. Citation2005; Hagiwara et al. Citation2007). The heat shock proteins (Hsp-90 alpha, 90 beta and 60) mRNA expression revealed significant variation in heart, liver and spleens of N and HE groups in PB-2 chicken which were lower in HE birds indicating the possible impact of thermal adaptation except in liver (). The heat-exposed birds could adapt better to the environmental temperature stress resulting in reduced expression of Hsp genes, while the Hsp gene expression was significantly higher in normal birds confirming the hypothesis that thermal adaptation during embryogenesis improves the heat tolerance in postnatal life of birds. Hyperthermia induced by exposure to heat appears to increase the activity and amount of heat shock transcription factor enhancing the Hsps mRNA synthesis leading to high concentrations in normal birds, while the birds already exposed could tolerate the hyperthermia and effectively reduced the heat shock transcription factor resulting in low levels of gene expression and better tolerance to heat stress.
All cells respond to increased temperature of 3–5°C above the normal by rapid gene transcription and subsequent mRNA translation to yield highly conserved Hsps to protect them from heat stress (Locke & Noble Citation1995). The response of cellular mechanisms to the long-term effect of thermal conditioning is complex and contradicting observations were reported in earlier studies. One school of thought suggesting the high Hsp expression is associated with better heat tolerance (Wang & Edens Citation1993, Citation1998) while the other is low level of expression (Yahav et al. Citation1997). The present findings were in accordance with Yahav et al. (Citation1997) wherein thermal conditioning during early age induced thermotolerance by adaptation and subsequent low level of Hsp expression. The mechanism to combat heat stress in the present study involves reducing hyperthermia by protecting the body tissues from being exposed to stress, which might be partly due to the stability of tissues to hyperthermia due to earlier conditioning. These variations may be due to the differences in protocols of thermal conditioning. If both tissue stability to hyperthermia and measures to reduce hyperthermia are standardized in thermal conditioning, it would be beneficial to the poultry farmers in hot tropical climates. The results revealed that ambient temperature during the rearing period in thermally conditioned birds was not perceived as a stress factor at the cellular level due to pre-adaptation to increased temperature during the embryonic stage, therefore, reduced Hsp mRNA expression in the birds.
The level of Hsps mRNA expression in different tissues varied which might be due to varied metabolic functions of different organs under the influence of thermal adaptation during embryogenesis, as pre-exposure to high temperature induces physiological memory due to epigenetic adaptation to high temperature resulting in improved thermotolerance during the postnatal life (Yahav Citation2009). The level of Hsps mRNA in various tissues, namely, heart, liver, bursa, spleen and muscle revealed higher expression in spleen and bursa () The expression of Hsps is tissue dependent in chicken subjected to heat treatment (Leandro et al. Citation2004; Zhen et al. Citation2006; Gan et al. Citation2013) which was true in the present study also. Blake et al. (Citation1990) reported that different tissues respond differently to heat and/or cold stress and with age, which substantiates the present findings. Nakai and Morimoto (Citation1993) reported that differences in the response of the tissues could be related to the differences in the levels of the heat shock factors (HSFs). Under the influence of stressful conditions HSFs are responsible for the activation of the heat shock element, which is an upstream promoter sequence in the heat shock gene (Mizuno et al. Citation1997).
The expression pattern of all the three Hsps is similar in all the tissues except heart, in which heat-exposed chicks recorded higher expression at day old and 28 days of age in PB-2 chickens. This might be due to the increased metabolic activity of the heart in exposed chicks. Increased expression of Hsp 60, 90 and 70 in heart was observed in heat-exposed chicks after 2 h heat exposure and led to the localization of Hsps in myocardial tissue (Yan et al. Citation2009). The localization of Hsps in myocardial cells during the pre-exposure might have enhanced the expression levels in heart in the present study also.
5. Conclusion
In conclusion, thermal adaptation during the embryonic stage to higher incubation temperature reduces Hsp gene expression in different tissues in postnatal life and increases the heat tolerance capacity of the birds in broiler chickens.
Acknowledgements
We thank the Director, Directorate of Poultry Research for his constant encouragement and support. The technical help received from Dr Daryab Singh, Hatchery Manager, Dr S.K. Bhanja, Farm Manager and all other technical staffs is duly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Blake MJ, Gershon D, Fargonli J, Holbrook NJ. 1990. Discordant expression of heat shock proteins mRNAs in tissue of het-stressed rats. J Biol Chem. 265:15275–15279.
- Eichler T, Ransom RF, Smoyer WE. 2005. Differential induction of podocyte heat shock proteins by prolonged single and combination toxic metal exposure. Toxicol Sci. 84:120–128. doi: 10.1093/toxsci/kfi048
- Gan JK, Zhang DX, He DL, Zhang XQ, Chen ZY, Luo QB. 2013. Promoter methylation negatively correlated with mRNA expression but not tissue differential expression after heat stress. Genet Mol Res. 12(1):809–812. doi: 10.4238/2013.March.15.1
- Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T, Yoshioka H. 2007. Association between heat stress protein 70 induction and decreased pulmonary fibrosis in an animal model of acute lung injury. Lung. 185:287–293. doi: 10.1007/s00408-007-9018-x
- Hoffmann AA, Parsons PA. 1991. Evolutionary genetics of environmental stress. Oxford: Oxford University Press.
- Krebs RA, Bettencourt BR. 1999. Heat shock protein variation and the evolution of thermotolerance in Drosophila. Am Zool. 39(6):910–919. doi: 10.1093/icb/39.6.910
- Leandro NS, Gonzales E, Ferro JA, Ferro ML, Givisiez PE, Macari M. 2004. Expression of heat shock proteins in broiler embryo tissues after acute cold or heat stress. Mol Reprod Dev. 67:172–177. doi: 10.1002/mrd.10397
- Lindquist S. 1986. The heat shock responses. Annu Rev Biochem. 55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443
- Locke M, Noble EG. 1995. Stress protein. 1. The exercise response. Can J Appl Physiol. 20:155–167. doi: 10.1139/h95-011
- MacKinnon KM, He H, Nerren JR, Swaggerty CL, Genovese KJ, Kogut MH. 2009. Expression profile of toll-like receptors within the gastrointestinal tract of 2-day-old Salmonella enteriditis-infected broiler chickens. Vet Microbiol. 137(3–4):313–319. doi: 10.1016/j.vetmic.2009.01.024
- Mashaly M, Hendricks GL, Kalama MA, Gehad AE, Abbas AO, Patterson PH. 2004. Effect of heat stress on production parameters and immune response of commercial laying hens. Poult Sci. 83:889–894. doi: 10.1093/ps/83.6.889
- Mizuno S, Ishii A, Murakami Y, Akagawa H. 1997. Stress dose dependent suppression of heat shock protein gene expression by inhibiting protein synthesis during heat shock treatment. Cell Struct Funct. 22:7–13. doi: 10.1247/csf.22.7
- Morimoto RI, Tisicres A, Georgopoulus C. 1990. The stress response function of the proteins and perspective. In: Morimoto RI, Tisicres A, Georgopoulus C. editors. Stress proteins in biology and medicine. New York, NY: Cold Spring Harbor Laboratory Press; p. 1–36.
- Nadeau SI, Landry J. 2006. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. In: Csermely P, Vigh L. editors. Molecular aspect stress response: chaperones, membranes networks. New York, NY, Springers, Vol. 594, p. 100–113.
- Nakai A, Morimoto RI. 1993. Characterization of a novel chicken heat shock transcription factor heat shock factor 3 suggests new regulatory pathway. Mol Cell Biol. 13:1983–1997. doi: 10.1128/MCB.13.4.1983
- Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sá LRM, Ferreira AJP, Palermo-Neto J. 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci. 89:1905–1914. doi: 10.3382/ps.2010-00812
- Rajkumar U, Reddy MR, Rama Rao SV, Radhika K, Shanmugam M. 2011. Evaluation of growth, carcass, immune response and stress parameters in naked neck chicken and their normal siblings under tropical winter and summer temperatures. Asian Australas J Anim Sci. 24:509–516. doi: 10.5713/ajas.2011.10312
- Staib JC, Quindry JC, French JP, Criswell DS, Powers SK. 2007. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am J Physiol Regul Integr Comp Physiol. 292:432–439. doi: 10.1152/ajpregu.00895.2005
- Tzschentke B. 2007. Attainment of thermoregulation as affected by environmental factors. Poult Sci. 86:1025–1036. doi: 10.1093/ps/86.5.1025
- Wang S, Edens FW. 1993. Stress-induced heat-shock protein synthesis in peripheral leukocytes of turkeys (Meleagris Galopavo). Comp Biochem Phys B. 106:621–628. doi: 10.1016/0300-9629(93)90370-J
- Wang S, Edens FW. 1998. Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poult Sci. 77:1636–1645. doi: 10.1093/ps/77.11.1636
- Yahav S. 2009. Alleviating heat stress in domestic fowl: different strategies. World’s Poult Sci J. 65:719–732. doi: 10.1017/S004393390900049X
- Yahav S, Hurwitz S. 1996. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult Sci. 75:402–406. doi: 10.3382/ps.0750402
- Yahav S, Straschnow A, Plavnik I, Hurwitz S. 1997. Blood system response of chickens to changes in environmental temperature. Poult Sci. 76:627–633. doi: 10.1093/ps/76.4.627
- Yan J, Bao E, Yu J. 2009. Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature. Res Vet Sci. 86:533–538. doi: 10.1016/j.rvsc.2008.09.002
- Zhen FS, Du HL, Xu HP, Luo QB. 2006. Tissue and allele specific expression of hsp 70 gene in chicken basal and heat stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br Poult Sci. 47:449–455. doi: 10.1080/00071660600827690