ABSTRACT
The objective of this study was to investigate single nucleotide polymorphisms in the calpastatin (CAST) gene and to test their association with meat quality traits in Hyla, Champagne, and Tianfu Black rabbit breeds. We detected one single nucleotide polymorphism (g.16441502 C > T) located at 67 bp in intron 3 of the CAST gene in Chromosome 11. The three rabbit populations had intermediate levels of genetic diversity in the CAST gene. The statistical analysis indicated that rabbits with the TT genotype had a significantly greater yellowness at 0 and 24 h postmortem than those with the CC genotype (P < .05) in the longissimus dorsi muscle. Rabbits with CC genotype had higher intramuscular fat content than those with CT and CC genotypes in both longissimus dorsi and biceps femoris muscles in the three breeds (P < .05). Our results indicated that the CAST gene may be used as a possible candidate for marker-assisted selection in rabbit meat breeding programmes.
1. Introduction
Calpastatin (CAST) was first identified in 1978 and exists widely in muscle cells (Nishiura et al. Citation1978; Waxman & Krebs Citation1978). CAST is an endogenous inhibitor which controls the activities of calpains with addition of Ca2+ (Kwak et al. Citation1993). Calpastatins are rich in proline and glutamate but poor in aromatic amino acids (Murachi Citation1983). Koohmaraie et al. (Citation2002) showed that the rate of protein degradation postmortem affected the meat quality. Variation in CAST abundance influences postmortem aging rates in different muscles (Ouali & Talmant Citation1990; Geesink et al. Citation1992). Recently, some CAST SNPs have been used as commercial genetic markers by livestock industries (Johnston & Graser Citation2010). Calvo et al. (Citation2014) showed that a new single nucleotide polymorphism in the CAST gene is associated with beef tenderness. Cafe et al. (Citation2010) provided further evidence that selection based on CAST gene markers may improve meat tenderness in Brahman cattle. Further, Castro et al. (Citation2016) reported that several SNP in the CAST gene showed significant effects on the b* and hue* parameters of the longissimus thoracis et lumborum and semitendinosus muscles in Brahman and Brahman crossbred cattle. The CAST gene has also been found to have a large effect on pork quality (Rohrer et al. Citation2012) and muscle fibre traits in chicken (Liu et al. Citation2008; Zhang et al. Citation2012). However, there has been little research on association between SNPs and rabbit meat quality traits.
The hybrid Hyla breed has shown high performance for daily weight gain, feed efficiency, and dressing per cent (Chiericato et al. Citation1993). The Champagne breed has displayed a high growth rate in young rabbits and was the most productive among medium-sized breeds (Bolet et al. Citation2004). The Tianfu Black is a Chinese indigenous breed that is popular among Chinese breeders. The aim of this study was to discuss a single nucleotide polymorphism in the CAST gene and to test its association with meat quality traits in the Hyla, Champagne, and Tianfu Black rabbit breeds.
2. Materials and methods
2.1. Animals
A total of 372 rabbits of both sexes (185 males and 180 females) from 3 breeds (including 138 Hyla, 139 Champagne, and 88 Tianfu Black) were used in this study. Rabbits were reared in individual cages after weaning at 6 weeks of age. Rabbits were managed following standard practices and had free access to a commercial diet. Slaughter occurred at 70 days of age. Ear tissue samples were collected for DNA extraction. Carcasses were kept at 4°C for 24 h. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University.
2.2. Meat quality traits
Meat quality measurements were: (1) pH at 0 and 24 h postmorterm, (2) colour at 0 and 24 h postmortem, and (3) intramuscular fat (IMF) at 24 h postmortem. The samples were taken from the longissimus dorsi and biceps femoris muscles. The pH measurements were taken with a probe using a pH meter (Model PH-STAR CPU, Meister®, Germany). The probe was inserted directly into the muscle to a depth of 3 mm. Colour data were expressed in terms of Lightness (L*), redness (a*), and yellowness (b*) (Van Laack et al. Citation2000). Lightness (L*) ranged from black (0) to white (100), redness (a*) ranged from green (−60) to red (+60), and yellowness (b*) ranged from blue (−60) to yellow (+60). The IMF was analysed using the modified Soxhlet method (AOAC Citation1980).
2.3. Detection of SNP and genotyping
Genomic DNA was extracted using AxyPrep Genomic DNA Miniprep Kit (Axygen, USA) and stored at −20°C. The polymerase chain reaction (PCR) primers for the CAST gene were designed by the Primer Premier 5 software based on the rabbit gene sequence (Ensembl accession NO. ENSOCUG00000007802). The PCR primers for the CAST gene were CAST-F: CATTAGGCCGTTCCAATCAGC and CAST-R: CCTATGTAGCAGCCCGGTTATTC. These PCR primers were used to amplify a 655 bp fragment, including exon 3, intron 3, and exon 4, in the CAST gene. The 25 μL reaction mixture contained 50 ng genomic DNA, 1.5 mM MgCl2, 1 µM of each primer, 200 µM dNTPs (dATP, dTTP, dCTP, and dGTP), and 0.4 units of Taq DNA polymerase (MBI). The PCR protocol was as follows: initial denaturation at 95°C for 4 min, followed by 35 cycles of denaturing at 95°C for 45 s, annealing at 56.0°C for 45 s, extension at 72°C for 45 s, and a final extension at 72°C for 10 min. Then, the PCR products were purified and directly sequenced in both directions with a BigDye Terminator sequencing kit (Applied Biosystems, Foster City, CA, USA) on a 3700 DNA sequencer. Sequences were subsequently analysed using program DNAMAN (version 5.2.2).
2.4. Statistical analysis
Genotype frequencies and allele frequencies for Hyla, Champagne, and Tianfu Black were obtained using the usual procedures (Falconer et al. Citation1996; Yeh et al. Citation1997). Observed genotype frequencies within each breed were compared with their respective expected frequencies under Hardy–Weinberg equilibrium (HWE) and tested for significant departures from HWE with software POPGENE (Ver. 3.2) (Yeh et al. Citation1997) using a likelihood ratio test. In addition, gene heterozygosity (He), effective allele numbers (Ne), and polymorphism information content (PIC) were estimated for each of the three breeds using expressions found in Nei and Roychoudhury (Citation1974) and Botstein et al. (Citation1980).
Preliminary statistical analyses included the fixed effects of breed, genotype, sex, and interactions between breed and genotype and between sex and genotype in the linear model for meat quality traits. However, the interactions between breed and genotype and between sex and genotype were non-significant; thus they were excluded from the model. Consequently, the final linear model to analyse the meat quality traits was as follows:
where Yijkl was a meat quality trait, μ was the overall mean for each trait, Bi was the breed effect, Gj was the genotype effect, Sk was the fixed sex effect, and eijkl was the random error. Least squares means and their standard errors were computed for all genotype effects, and pairwise comparisons among them were made using Bonferroni t-tests. Computations were carried out using the general linear model procedure of SPSS 21 (IBM, Armonk, NY, USA).
3. Results and discussion
3.1. Genotypic frequencies, allelic frequencies, and population genetic indexes
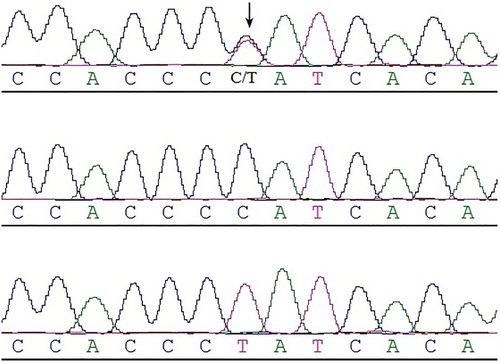
The genotyping of the SNP was successfully implemented using PCR and DNA sequencing. shows a sequencing map of the novel SNP (g.16441502 C > T) in the Hyla, Champagne, and Tianfu Black rabbit breeds. We detected one single nucleotide polymorphism located at 67 bp in intron 3 of the CAST gene in Chromosome 11. The genotype and allele frequencies, chi-square test, heterozygosity (He), effective number of allele (Ne), and PIC were calculated and summarized in . The T allele showed a high prevalence in these breeds. The minor allele frequencies (MAF ranged from 0.2391 to 0.3777) showed that this SNP was polymorphic (MAF > 0.05) in these three breeds. Chi-square tests showed that genotypic frequencies were in HWE (P > .05) in three rabbit populations. The three rabbit populations had intermediate levels of genetic diversity according to their PIC values (0.2977 for Hyla, 0.3596 for Champagne, and 0.3522 for Tianfu Black).
Table 1. Genotypic and allelic frequencies, χ2 value test and diversity parameter for the CAST gene in rabbits.
3.2. Associations analysis between the SNP and meat quality
The results of the association analysis between the g.16441502 C > T SNP found here and rabbit meat quality traits are shown in and . Least squares means in showed that rabbits with the TT genotype had a significantly greater and
than rabbits with the CC genotype (P < .05), but similar values for pH (0 h, 24 h), L* (0 h, 24 h), and a* (0 h, 24 h) in the longissimus dorsi muscle. No significant differences among the three genotypes were found for pH (0 h, 24 h), L* (0 h, 24 h), a* (0 h, 24 h), and b* (0 h, 24 h) in biceps femoris muscle (; P > .05). Thus, rabbits with CC genotype had higher IMF than rabbits with CT and TT genotypes in both longissimus dorsi and biceps femoris muscles among these three rabbit breeds ( and ; P < .05).
Table 2. Least square means for CAST genotype effects on meat pH, colour, and IMF traits in rabbit longissimus dorsi muscle.
Table 3. Least square means for CAST genotype effects on meat pH, colour, and IMF traits in rabbit biceps femoris muscle.
Colour, IMF content, and pH value are all typical meat quality parameters (Dalle Zotte Citation2002; Li et al. Citation2013). The pH values here were similar to those of Hulot and Ouhayoun (Citation1999), who found that pH was almost neutral in live rabbits but it decreased rapidly after slaughter. The higher pH in fresh rabbit meat may imply a higher level of muscle glycogen reserves (Sabuncuoglu et al. Citation2011). In our study, colour values were similar to values reported in previous research (Trocino et al. Citation2002; María et al. Citation2006). The small differences in colour trait values between longissimus dorsi and biceps femoris muscles here partially agreed with the results reported by Chiericato et al. (Citation1996), who found that yellowness was not consistent across different muscles. The result for IMF indicated that the C > T SNP here was closely associated with QTL, affecting rabbit meat quality. Koohmaraie (Citation1992) indicated that the biological activity of the CAST gene played an important role in the tenderization in beef, pork, and lamb because the calpain–CAST system is involved in the degradation of important proteins, and also, the system, a Ca2+-activated protease family, may stimulate more rapid glycolysis and pH decline. Chung et al. (Citation2001) found that CAST genotypes in intron 6 of the CAST gene influenced (P < .05) CAST activity in Angus cattle. Thus, it is possible that the g.16441502 C > T SNP found in intron 3 here may be involved in regulation of transcriptional and post-transcriptional levels of gene expression of the CAST gene in rabbits. Further work is necessary with larger rabbit populations to elucidate the mechanisms involved in this gene’s effect on rabbit meat quality.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC. 1980. Official methods of analysis. 13th ed. Washington (DC): Association of Official Analytical Chemists.
- Bolet G, Brun JM, Lechevestrier S, Lopez M, Boucher S. 2004. Evaluation of the reproductive performance of eight rabbit breeds on experimental farms. Ani Res. 53:59–65.
- Botstein D, White RL, Skolnick M, Davis RW. 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 32:314–331.
- Cafe LM, McIntyre BL, Robinson DL, Geesink GH, Barendse W, Pethick DW, Thompson JM, Greenwood PL. 2010. Production and processing studies on calpain-system gene markers for tenderness in Brahman cattle: 2. Objective meat quality. J Anim Sci. 88:3059–3069.
- Calvo JH, Iguácel LP, Kirinus JK, Serrano M, Ripoll G, Casasús I, Joy M, Pérez-Velasco L, Sarto P, Albertí P, Blanco M. 2014. A new single nucleotide polymorphism in the calpastatin (CAST) geneassociated with beef tenderness. Meat Sci. 96:775–782.
- Castro S, Ríos M, Ortiz Y, Manrique C, Jiménez A, Ariza F. 2016. Association of single nucleotide polymorphisms in CAPN1, CAST and MB genes with meat color of Brahman and crossbreed cattle. Meat Sci. 117:44–49.
- Chiericato GM, Rizzi C, Rostellato V. 1993. Effect of genotype and environmental temperature on the performance of the young meat rabbit. World Rabbit Sci. 1:119–125.
- Chiericato GM, Rizzi C, Rostellato V. 1996. Meat quality of rabbits of different genotypes reared in different environmental conditions. Proceedings of the 6th World Rabbit Congress; Toulouse (France), p. 141–145.
- Chung HY, Davis ME, Hines HC. 2001. Genetic variants detected by PCR-RFLP in intron 6 of the bovine calpastatin gene. Anim Genet. 32:53.
- Dalle Zotte A. 2002. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest Prod Sci. 75:11–32.
- Falconer DS, Mackay TFC, Frankham R. 1996. Introduction to quantitative genetics. 4th ed. Trends Genet. 12:280.
- Geesink GH, Ouali A, Tassy C, Smulders FJM. 1992. Tenderization, calpain/calpastatin activities and osmolality of 6 different beef muscles. Int Congr Meat Sci Technol Proc. 38:363–366.
- Hulot F, Ouhayoun J. 1999. Muscular pH and related traits in rabbits: a review. World Rabbit Sci. 7:15–36.
- Johnston DJ, Graser HU. 2010. Estimated gene frequencies of GeneSTAR markers and their size of effects on meat tenderness, marbling, and feed efficiency in temperate and tropical beef cattle breeds across a range of production systems. J Anim Sci. 88:1917–1935.
- Koohmaraie M. 1992. The role of Ca2+ dependant proteases (calpains) in post mortem proteolysis and meat tenderness. Biochimie 74:239–245.
- Koohmaraie M, Kent MP, Shackelford SD, Veiseth E, Wheeler TL. 2002. Meat tenderness and muscle growth: is there any relationship? Meat Sci. 62 (Special Issue S1):345–352.
- Kwak KB, Chung SS, Kim OM, Kang MS, Ha DB, Chung CH. 1993. Increase in the level of m-calpain correlates with the elevated cleavage of filamin during myogenic differentiation of embryonic muscle cells. BBA – Mol Cell Res. 1175:243–249.
- Li X, Ekerljung M, Lundström K, Lundén A. 2013. Association of polymorphisms at DGAT1, leptin, SCD1, CAPN1 and CAST genes with color, marbling and water holding capacity in meat from beef cattle populations in Sweden. Meat Sci. 94:153–158.
- Liu AF, Liu YP, Jiang XS, Li L, Du HR, Zhu Q. 2008. Studies of single nucleotide polymorphism of CAST gene and its association with muscle fiber traits in chicken. Chinese J Vet Anim Sci. 29:437–442.
- María GA, Buil T, Liste G, Villarroel M, Sañudo C, Olleta JL. 2006. Effects of transport time and season on aspects of rabbit meat quality. Meat Sci. 72:773–777.
- Murachi, T. 1983. Calpain and calpastatin. Trends Biochem Sci. 8:167–169.
- Nei M, Roychoudhury A. 1974. Sampling variances of heterozygosity and genetic distance. Genetics 76:379–390.
- Nishiura L, Tanaka K, Yamato S, Murachi T. 1978. The occurrence of an inhibitor of Ca2+-dependent neutral protease in rat liver. Biochem J. 84:1657–1659.
- Ouali A, Talmant A. 1990. Calpains and calpastatin distribution in bovine, porcine, and ovine skeletal muscles. Meat Sci. 28:331–348.
- Rohrer GA, Nonneman DJ, Miller RK, Zerby H, Moeller SJ. 2012. Association of single nucleotide polymorphism (SNP) markers in candidate genes and QTL regions with pork quality traits in commercial pigs. Meat Sci. 92:511–518.
- Sabuncuoglu N, Coban O, Lacin E, Ceylan ZG, Ozdemir D, Ozkan A. 2011. Effect of pre-slaughter environment on some physiological parameters and meat quality in New Zealand rabbits (Oryctolagus cuniculus). Trop Anim Health Pro. 43:515–519.
- Trocino A, Xiccato G, Queaque PI, Sartori A. 2002. Effect of transport duration and sex on carcass and meat quality of growing rabbits. Proceedings of the Second Rabbit Congress of the America; La Habana (Cuba). p. 232–235.
- Van Laack RL, Liu CH, Smith MO, Loveday HD. 2000. Characteristics of pale, soft, exudative broiler breast meat. Poultry Sci. 79:1057–1061.
- Waxman L, Krebs EG. 1978. Identification of two protease inhibitors from bovine cardiac muscle. J Biol Chem. 253:5888–5891.
- Yeh FC, Yang R, Boyle TBJ, Ye Z, Mao JX. 1997. The user-friendly shareware for population genetic analysis. Edmonton (Canada): Biology and Biotechnology Center, University of Alberta.
- Zhang ZR, Jiang XS, Du H, Zhu Q, Li XC, Yang CW, Liu YP. 2012. Characterization of the expression profiles of calpastatin (CAST) gene in chicken. Mol Biol Reports 39:1839–1843.