ABSTRACT
Concerns about antibiotic residues in milk and emergence of antimicrobial resistant pathogens necessitate exploration of alternative therapeutic strategies for the treatment of mastitis. This study aims to investigate anti-infective properties of a Thai traditional polyherbal formula, namely Ya-Sa-Marn-Phlae (YSMP), its herbal components (Curcuma longa, Areca catechu, Oryza sativa, and Garcinia mangostana), and representative chemical constituents (catechin, α-mangostin, and curcumins). Ethanol extracts of YSMP and G. mangostana, and α-mangostin exhibited potent antibacterial effects against Staphylococcus spp. isolated from mastitis cows with MIC values of 1–32 µg/mL. These tested agents inhibited biofilm formation of the isolates on both polypropylene (hydrophobic) and glass (hydrophilic) surfaces. The current study indicated that YSMP had strong antibacterial activity and anti-biofilm abilities against the tested isolates similar to that of α-mangostin and G. mangostana. The anti-staphylococcal effects were confirmed with both scanning and transmission electron microscopes. The main abnormalities in the microstructure of the treated cells were the severe alterations of the cell wall with the formation of holes and morphological disorganization. Therefore, it might be proposed that G. mangostana is the major active component of YSMP and α-mangostin may be used as an active marker compound for YSMP to indicate its activity against bovine mastitis-isolated staphylococci.
1. Introduction
Mastitis is an inflammation of the mammary gland most often caused by bacterial intramammary infections. Based on bacteriological etiologic agents, mastitis can be categorized as contagious mastitis, mainly caused by Staphylococcus aureus and Streptococcus agalactiae and environmental mastitis, generally infected by Escherichia coli, Streptococcus dysgalactiae, and Streptococcus uberis (Gruet et al. Citation2001). This disease is considered one of the most significant causes of economic loss in the dairy industry due to reduced milk production, discarded milk, and additional inputs to reduce the level of mastitis (Hogeveenet al. Citation2011). Global economic losses due to mastitis are estimated more than US$ 2 billion per year (Huijps et al. Citation2008). The national economic loss in India due to mastitis was approximately US$ 2.6 billion annually (Joshi & Gokhale Citation2006), whereas in the United Kingdom and Northern Ireland the annual losses were £300 million and £14 million, respectively (Hillerton & Berry Citation2005). Although the uses of both disinfectant preparations and antibiotics to treat and prevent udder infections play a key role in bovine mastitis control, this method was cited as a major reason for milk contamination (Erskine et al. Citation2003). The presence of drug residues in milk could lead to the selection of antibiotic-resistant strains of bacteria (Sandegren Citation2014) as well as provoke allergic reactions in some hypersensitive individuals (Tan et al. Citation2009). According to the aforementioned concerns, effective alternative strategies are needed for controlling mastitis in dairy cows.
An increasing interest has emerged for therapeutic use of non-toxic traditional medicinal plants as alternative and inexpensive drugs for treating bovine mastitis. A few studies indicate that plant-derived compounds such as trans-cinnamaldehyde, eugenol, carvacrol, thymol, citral, and geraniol exhibited good results for inhibiting mastitis-causing pathogens (Ananda Baskaran et al. Citation2009; Aiemsaardet al. Citation2011). Ya-Sa-Marn-Phlae (YSMP) consists of Curcuma longa (rhizome), Areca catechu (seed), Oryza sativa (seed), and Garcinia mangostana (pericarp). This polyherbal preparation has been traditionally used for treatment of wounds and inflammatory skin conditions by topical application. Because of its in vitro-notable antibacterial activity against clinical isolates of S. aureus with minimum inhibitory concentration (MIC) value of 4 µg/mL, YSMP might be a potential natural antibacterial agent when used as a disinfectant (Chusri, Jittanon et al. Citation2013). An ethanol extract of YSMP had no cytotoxic effect and exhibited good anti-inflammatory and anti-oxidant activities. Its ethanol extract additionally possessed anti-biofilm activity against Staphylococcus epidermidis (Chusri, Settharaksa et al. Citation2013) and Pseudomonas aeruginosa (Chusri, Sompetch et al. Citation2013).
In this study, we aimed to compare antibacterial effects of an ethanol extract of YSMP, ethanol extracts of its individual plant components, and their major representative components which were catechin (Wang et al. Citation1997), α-mangostin (Mahabusarakam et al. Citation1987), and curcumins (Arajo & Leon Citation2001) against coagulase-negative and -positive staphylococci isolated from subclinical and clinical mastitis cows in dairy farms in Phatthalung province, Thailand. Biofilm-forming ability of Staphylococcus spp. is considered to be a major virulence factor affecting their pathogenesis in mastitis. Therefore in vitro testing of the activity of effective agents on the staphylococcal isolates was additionally carried out.
2. Materials and methods
2.1. Plant materials and preparation of extracts
A selected herbal recipe, YSMP was first prescribed by Mr Somporn Chanwanisakul, a traditional Thai medical doctor at a traditional Thai medicine hospital, Prince of Songkla University, Hat Yai, Thailand. This recipe is traditionally used for wound healing and consists of C. longa (rhizome), A. catechu (seed), O. sativa (seed), and G. mangostana (pericarp). The medicinal plants were washed with distilled water, dried at 60°C overnight, and separately ground into fine powder. The formulation of YSMP consisting of 75 g of each herbal component was prepared as previously described (Chusri, Settharaksa et al. Citation2013). Powders (300 g) of each medicinal plants and the recipe were then separately soaked in 95% ethanol (1500 mL) for 7 days at 25°C. After filtrations through a Whatman No. 1 paper, the filtrates were concentrated using a rotary evaporator, and kept at 55°C until they were completely dry. Extraction yields (%; w/w) of YSMP, C. longa, A. catechu, O. sativa, and G. mangostana were 6.45, 9.88, 7.89, 0.46, and 3.54, respectively.
Catechin (Sigma-Aldrich, USA), alpha-mangostin (Sigma-Aldrich, USA), and curcumins (Sigma-Aldrich, USA), were additionally tested as active chemical constituents of A. catechu, G. mangostana, and C. longa, respectively. Stock solutions at concentration of 200 mg/mL for dried ethanol extracts and 1 mg/mL for the active compounds were prepared by dissolving in 1 mL of dimethyl sulfoxide (DMSO) (Merck, Germany) and stored at −20°C.
2.2. Tested pathogens
In this study 14 isolates of Staphylococcus spp., S. aureus ATCC 25923, and a biofilm-positive strain, S. epidermidis ATCC 35984 were provided by the Department of Microbiology, Faculty of Science, Prince of Songkla University. The tested pathogens were isolated from milk samples of subclinical mastitis cows in dairy farms in Phatthalung province, Thailand in June-July 2010. The subclinical mastitis cases were considered when cows had bacteriologically positive milk samples with at least one the California Mastitis Test (CMT)-positive quarter (Ajariyakhajorn et al. Citation2003). Colonies were cultured on blood agar plates and tentatively identified according to morphological features, pigment production, type of haemolysis produced, Gram staining, catalase test, and characteristic growth on mannitol salt agar (Oxoid, UK) which was used as a selective and differential medium for isolation and identification (Asfour & Darwish Citation2011). The coagulase test was performed with rabbit plasma and the results were recorded after 4 and 24 h of incubation at 37°C. S. aureus ATCC 25923 and a biofilm-positive strain, S. epidermidis ATCC 35984 were used for quality control. Antibiotic susceptibility test was performed by the disk diffusion method using a panel of antimicrobial agents, including amikacin (30 µg/disc), ampicillin (10 µg/disc), erythromycin (15 µg/disc), gentamicin (10 µg/disc), oxacillin (1 µg/disc), penicillin (10 unit/disc), rifampicin (5 µg/disc), tetracycline (30 µg/disc), and vancomycin (30 µg/disc) (CLSI Citation2008). The antibiotics were purchased from Becton Dickinson Microbiology Systems (Sparks, MD, USA).
2.3. Determination of MIC
Cultures for experiments were prepared from 24 h Mueller–Hinton broth (MHB, Difco, France) and the suspensions were subsequently diluted with fresh MHB to achieve a bacterial culture concentration corresponding to 106 CFU/mL. MICs of the formula extracts were tested by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (Citation2011). A 96-well sterile microtiter plate (Nunc, Denmark) was prepared by dispensing 100 µL of the inoculated broth plus an aliquot of 100 µL of twofold serial dilutions of the tested extracts, catechin, curcumin, or alpha-mangostin. The tested final concentrations of the extracts, catechin, curcumin, and alpha-mangostin in the microtitre plate were in the range of 15.6-1000, 3.9-250, 3.9–250, and 0.2–10 μg/mL, respectively. An aliquot of 100 µL of 1% DMSO was employed as a negative control. The plate was incubated for 24 h at 37°C and the bacterial growth was measured by recording the absorbance at 620 nm, using a microplate reader (Tecan Sunrise, Tecan Austria).
2.4. Time kill assay
Assays for the rate of killing bacteria by both the ethanol extracts and the active constituents were performed using a modified plating technique. Bacterial inoculum at concentration of 106 CFU/mL (1 mL) was mixed with 1 mL of MHB containing each tested agent at final concentrations of 0.5MIC, MIC, 2MIC, and 4MIC. The MICs of YSMP, A. catechu, C. longa, G. mangostana, α-mangostin, and curcumin against representative isolates, S. epidermidis ATCC 35984/BCNS018/BCPS031 were 7/15/31, 250/250/250, 125/250/250, 3/7/7, 1/10/10, and 31/250/250 µg/mL, respectively. The tubes were incubated at 37°C and samples (100 µL) were taken at 0, 2, 4, and 8 h. The serial 10-fold dilutions of each sample were made and plated on Mueller–Hinton agar (MHA, Difco, France). After incubation at 37°C for 24 h, emergent bacterial colonies were counted, CFU per millilitre calculated, and compared with the count of the culture control without the extract.
2.5. Biofilm formation of Staphylococcus spp. isolates
Biofilm productions of both coagulase-negative and -positive staphylococci isolates (n = 14) were first screened by a modification of spectrophotometric technique as previously established (Limsuwan & Voravuthikunchai Citation2008). Briefly, well-isolated colonies grown overnight at 37°C on tryptic soy agar (TSA) were inoculated in tryptic soy broth (TSB) supplemented with 2% (w/w) d-glucose (TSBGlc) at 37°C for 24 h. The cultures were then diluted 200-fold with fresh TSBGlc, divided into 200-µL aliquots, and added into a flat-bottomed 96-well polystyrene microtiter plate (Nunc, Roskilde, Denmark). Following incubation at 37°C for 48 h, planktonic cells were removed and the remaining biofilm was gently washed three times for one minute with 250 µL of phosphate-buffered saline (PBS; pH 7.4). The biofilm mass was stained with 200 µL of 0.1% (w/v) crystal violet solution for 30 min at room temperature. The excess dye was then washed off three times with 200 µL of PBS and allowed to dry. The stained biofilm was re-suspended using 200 µL DMSO and quantified in a microplate reader at 620 nm. Wells containing 200 µL of fresh TSBGlc were used to exclude a possible contamination and serve as a blank control. All experiments were performed at least in triplicate. The biofilm-forming ability of the isolates could be classified into three groups (Taponen al. Citation2003), isolates that formed (i) fully established biofilm with >75% of the biomass of the positive control, (ii) moderately adherent biofilm with 25–75% biomass, or (iii) weak biofilm with <25% of the biomass of the positive control.
2.6. Anti-biofilm property
Effects of the recipe extract and its effective constituents on biofilm formation of coagulase-positive staphylococci NPRC BCPS031 and the biofilm-positive strain, S. epidermidis ATCC 35984 were investigated (Chusri, Settharaksa et al. Citation2013). The bacterial cultures were diluted 200-fold in TSBGlc, divided into 100-µL aliquots, and added into a flat-bottomed 96-well polystyrene microtiter plate (Nunc, Roskilde, Denmark). An aliquot of 100 µL of YSMP extract (3.9–250 μg/mL), G. mangostana extract (3.9–250 μg/mL), or alpha-mangostin (0.2–10 μg/mL) prepared in TSBGlc was subsequently added into the 96-well microtiter plate. After incubation at 37°C for 48 h, the effect of the tested agents on the biofilm mass of the pathogens was evaluated using a colourmetric assay, as described above. The wells containing the media and the tested agents were included as control. In parallel experiments, the bacterial growth after the treatment of these agents was additionally quantified using the microplate reader.
2.7. Their effects on bacterial cell morphology
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were taken to elucidate the morphology of bacterial cells. Coagulase-negative staphylococci cells (BCNS 18) were pretreated with the ethanol extracts of YSMP (15 µg/mL), G. mangostana (7 µg/mL), A. catechu (250 µg/mL), C. longa (250 µg/mL), and alpha-mangostin (10 µg/mL) for 18 h. The treated cells were harvested (5000 rpm for 5 min), washed twice with PBS pH7.4, and then prepared for the transmission electron microscope (TEM; JEM 100 CX II; JEOL, Japan) and SEM (5800LV, JEOL, Japan), as previously described (Chusri and Voravuthikunchai Citation2009; Chusri, Jittanon et al. Citation2013).
3. Results and discussion
3.1. Anti-staphylococcal activity of YSMP and its components
Our ongoing research aimed to investigate the antibacterial properties of traditional herbal formulations and to analyse the activity of the individual compound or the herbal components in the formulations, with the aim of informing eventual quality control analysis of the formulation for use as medicines. We found that the ethanol extract of ‘YSMP’ possessed the ability to inhibit and eradicate human clinical isolates of S. aureus. This study further showed that the formula extract as well as the ethanol extract of G. mangostana, its medicinal plant component, showed excellent results for inhibiting coagulase-negative and -positive staphylococci isolated from bovine mastitis cases.
The majority of the tested isolates were coagulase-negative staphylococci. It may be because of coagulase-negative staphylococci were the predominant bacteria involved in bovine mastitis (Mordmuang & Voravuthikunchai Citation2015; Suriyasathaporn Citation2011).
The isolates of Staphylococcus used in this study were susceptible to amikacin, gentamicin, and tetracycline (). A multidrug resistant pattern, which meant that an isolate was resistant to three or more classes of antimicrobials, was not seen in all Staphylococcus spp. isolates, but 78% of the isolates were resistant to at least one drug. Most of the tested isolates were resistant to penicillin, oxacillin, and ampicillin which occurred in 50–71% of the tested isolates.
Table 1. Antibiotic susceptibility patterns of coagulase-positive staphylococci and coagulase-negative staphylococci isolated from bovine mastitis.
As shown in , the ethanol extracts of YSMP and G. mangostana, and α-mangostin exhibited a notable antibacterial effect towards all tested staphylococcal isolates with MIC values ranging between 1 and 32 µg/mL. The ethanol extracts of C. longa and A. catechu, and curcumins were less effective against the bovine pathogens (MIC values = 32 to >250 µg/mL) than the ethanol extracts of YSMP, whereas O. sativa ethanol extract and catechin did not show any antimicrobial activity (MIC values >1000 µg/mL). Exposures of BCPS, BCNS, and S. epidermidis to these agents at 0.5–4 times of their MIC caused bacteriostatic effects, whereas the extracts of YSMP and G. mangostana at 4x MIC were able to kill the tested pathogens at approximately 2–4 log reduction within 2 h ().
Figure 1. Time kill assay of YSMP (a), A. catechu Linn. (b), C. longa Linn. (c), G. mangostana Linn. (d), curcumin (e), and α-Mangostin (f) against S. epidermidis ATCC 35984, coagulase-negative staphylococci NPRC BCNS018, and coagulase-positive staphylococci NPRC BCPS031. MHB containing 1% of DMSO was added as a positive control. MICs of YSMP, A. catechu, C. longa, G. mangostana, α-Mangostin, and curcumin against S. epidermidis ATCC 35984/BCNS018/BCPS031 were 7/15/31, 250/250/250, 125/250/250, 3/7/7, 1/10/10, and 31/250/250 µg/mL, respectively.
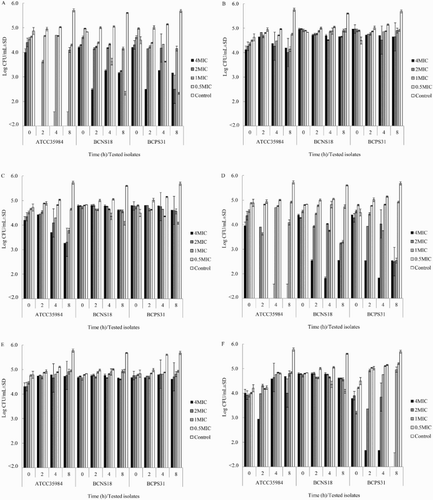
Table 2. MICs of ethanol extracts of YSMP, its herbal components, and active constituents against bovine mastitis-isolated coagulase-positive staphylococci (BCPS) and coagulase-negative staphylococci (BCNS).
As proposed by Cos et al. (Citation2006) and Kuete (Citation2010), MIC values of a plant extract should be below 100 µg/mL and below 25 µg/mL for plant-derived pure compounds. Based on those criteria, only YSMP and G. mangostana extracts can be categorized as a useful antibacterial agent, as they have developed MIC varying between 3–15 and 4–32 μg/mL, respectively. Although bovine mastitis-isolated staphylococci appeared to be more resistant to the tested agents than the reference strains, the MIC values of the disinfectants against coagulase-positive staphylococci was the same as that of coagulase-negative staphylococci. Similar results have been found by our group when testing the activity of the ethanol extract of YSMP against methicillin-resistant S. aureus (MRSA) and -susceptible S. aureus involved in nosocomial infections (Chusri, Jittanon et al. Citation2013). The results from MIC values showed that C. longa, A. catechu, curcumin, and catechin had the anti-staphylococcal activity approximately 10 times less than that of YSMP extracts. Thus, combining more than one plant may increase the effectiveness of this formula and this might be due to the synergistic interaction of the herbal components. This finding was also similar to the previous ethnobotanical surveys indicated that traditional medical practitioners use a combination of more than one plant for treating diseases (Simbo, Citation2010; Neamsuvan et al. Citation2012). Although considerable information is available on the anti-staphylococcal properties of G. mangostana (Chomnawanget al. Citation2009), C. longa (Kimet al. Citation2005), and A. catechu (Zhanget al. Citation2009), those studies were carried out to evaluate antibacterial activity of the plants against human-isolated clinical strains of Staphylococcus spp. Similar to our study, ethyl acetate extract of C. longa (Kimet al. Citation2005), curcumin (Guneset al. Citation2014), and turmeric oil (Negi al. Citation1999) were found to possess moderate antibacterial activity against MRSA (MIC = 100–1000 µg/mL). Our results are similar to that reported by Karphrom et al. (Citation2009) as the ethanol extract of A. catechu also exhibited moderate anti-staphylococcal activity (MIC = 780 µg/mL), whereas G. mangostana extracts exhibited remarkable antibacterial effect on MRSA (MIC = 39–80 µg/mL) (Asaiet al. Citation1995; Voravuthikunchai & Kitpipit Citation2005). α-Mangostin, the major constituent and most studied bioactive xanthone from this plant, had an activity against MRSA with MIC of 1.57–12.5 µg/mL (Asai et al. Citation1995). It is interesting to note that YSMP had strong antibacterial activities against the tested isolates similar to that of α-mangostin and G. mangostana. It might be proposed that pericarp of G. mangostana is the major active component in the formulation of YSMP. Alpha-mangostin may be used as an active marker compound for YSMP to indicate its activity against bovine mastitis-isolated staphylococci.
3.2. Biofilm inhibition potency of YSMP and its components
According to several reports, the biofilm-forming ability of Staphylococcus spp. is considered as an important virulence factor that facilitates the persistence of the pathogen in the udder, contributes to the evasion of host immunological defense, and impairs antibiotic therapy (Oliveira et al. Citation2006; Babra et al. Citation2013). Biofilm-forming ability of 14 isolates of Staphylococcus spp. isolated from mastitis cows is presented in . Most isolates were characterized as weak or non-biofilm producers, whereas coagulase-negative staphylococci NPRC BCNS018, 027, and 044 and coagulase-positive staphylococci NPRC BCPS031 were categorized as moderate biofilm producers.
Table 3. Biofilm-forming ability of coagulase-positive staphylococci and coagulase-negative staphylococci isolates.
Coagulase-positive staphylococci NPRC BCPS031 was the most efficient strain in terms of biofilm production, it was thus selected for assessment of anti-biofilm activity of YSMP, G. mangostana, and α-mangostin. Their effects on biofilm production are shown in . The inhibitory effects of the agents on biofilm formation of coagulase-positive staphylococci NPRC BCPS031 and the biofilm producer, S. epidermidis ATCC 35984 (data not shown) had a similar pattern. At concentrations ranging from 3.9 to 250 µg/mL, both YSMP and G. mangostana extracts inhibited >50% of the biofilm formation of the isolates in a dose-dependent manner. At the tested concentrations of 3.9–31.3 µg/mL, YSMP inhibited the biofilm formation of the pathogens on polystyrene surfaces up to 48 h with a weak growth inhibition effect. The biofilm inhibition percentages ranged from 86.4 ± 1.1% to 45.7 ± 3.9%. Sub-growth inhibition at concentration as low as 3.9 µg/mL of YSMP and G. mangostana and 5 µg/mL of α-mangostin had 45.7 ± 3.9%, 26.7 ± 1.8%, and 48.9 ± 0.8% inhibition on biofilm formation of coagulase-positive staphylococci NPRC BCPS031, respectively. The inhibitory effects of YSMP on the biofilm formation of the isolates were greater than that of the extract of G. mangostana and α-mangostin.
Figure 2. Effect of different concentrations of YSMP (a), G. mangostana (b), and α-Mangostin (c) on the bacterial growth (white bars) and biofilm formation (black bars) of coagulase-positive staphylococci NPRC BCPS031. Note: Each bar indicates the percentage of the means of inhibition ± SE for three independent experiments performed in duplicate.
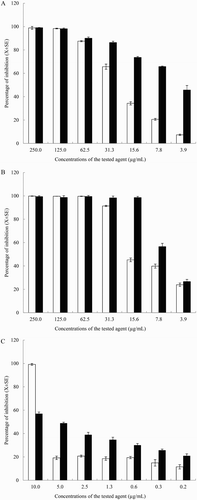
Our data reveal that YSMP is a potential biofilm prevention agent against bovine-associated coagulase-positive staphylococci. These results were similar to observations with biofilm-forming strain of P. aeruginosa (Chusri, Sompetch et al. Citation2013). Moreover, previous study has revealed the anti-biofilm potency of YSMP against the biofilm-positive strain, S. epidermidis ATCC 35984 (Chusri, Settharaksa et al. Citation2013). Even though the anti-biofilm activity of YSMP and its components on mastitis-causing bacteria has never been investigated, curcumins and essential oil of C. longa displayed anti-biofilm potencies on Streptococcus mutans (Lee et al. Citation2011), Vibrio spp. (Packiavathyet al. Citation2013), and P. aeruginosa (Rudrappa & Bais Citation2008), while, α-mangostin has been reported to have an activity against streptococci (Nguyen & Marquis Citation2011).
3.3. Morphological changes in coagulase-negative staphylococci on exposure to YSMP and its components
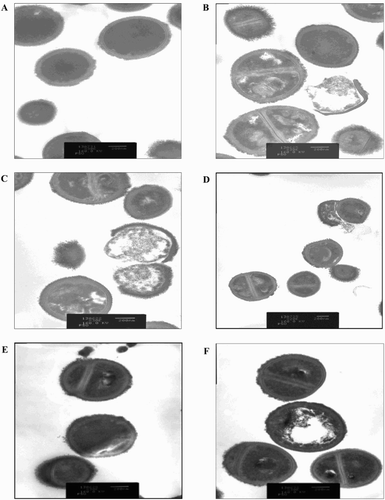
Regarding YSMP and G. mangostana extracts, SEM and TEM images demonstrated that the treated cells seemed to burst upon exposure, open holes, and deep craters in the bacterial cells and/or their envelope were found ((b) and (c) and (b) and (c)). Treatment of staphylococciwith A. catechu and C. longa extracts were slightly changed their cell morphology compared to untreated control ((a) (d) and (e) and 4(a), (d) and (e)).The mechanism of action of YSMP and the interactions of its components are still unclear, but Koh et al. (Citation2013) showed that α-mangostin which may serve as a biological marker of YSMP directly affects cytoplasmic membrane of S. aureus. We showed for the first time that YSMP which is commonly applied for wound treatment possesses a potential as an alternative compound for the action against staphylococci involved in bovine mastitis.
Figure 3. Scanning electron micrograph of BCNS 18 after treated with four MIC of YSMP (b), G. mangostana (c), A. catechu (d), C. longa (e), and alpha-mangostin (f). BCNS 18 (a) was growth in TSB used as a control. MICs of YSMP, G. mangostana, C. longa, A. catechu, and alpha-mangostin against BCNS 18 were 15, 7, 250, 250, and 10 µg/mL, respectively.
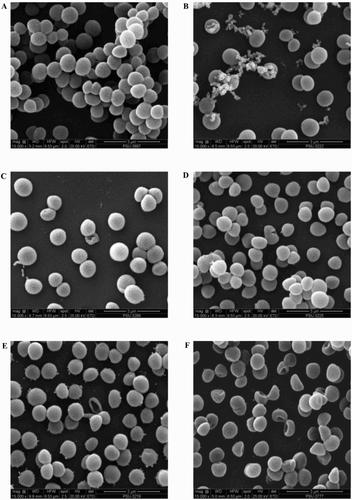
Figure 4. Transmission electron micrograph of BCNS 18 after treated with four MIC of YSMP (b), G. mangostana (c), A. catechu (d), C. longa (e), and alpha-mangostin (f). BCNS 18 (a) was growth in TSB used as a control. MICs of YSMP, G. mangostana, C. longa, A. catechu, and alpha-mangostin against BCNS 18 were 15, 7, 250, 250, and 10 µg/mL, respectively.
4. Conclusion
The results presented in this article also indicate that G. mangostana is the major active component of YSMP and further investigation will be needed to correlate the activity of either YSMP, G. mangostana, or their constituents. More studies investigating the effects of this antibacterial substance in milk and in vivo mastitis model are therefore warranted and currently being carried out in our laboratory.
Acknowledgements
We are thankful to Mr David John Leather for editing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aiemsaard J, Aiumlamai S, Aromdee C, Taweechaisupapong S, Khunkitti W. 2011. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res Vet Sci. 91:e31–e37. doi: 10.1016/j.rvsc.2011.01.012
- Ajariyakhajorn K, Samngamnim S, Boonsern T, Inchaisri C, Thirapatsakun T, Farnsworth RJ. 2003. Mastitis in small dairy holders of Nakornpathom Provinces, Thailand. Proceedings of the 11th International Symposium of the Word Association of Veterinary Laboratory Diagnosticians and OIE Seminar on Biotechnology; 2003 November 9–13, Bangkok, Thailand. p. 120–121.
- Ananda Baskaran S, Kazmer GW, Hinckley L, Andrew SM, Venkitanarayanan K. 2009. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J Dairy Sci. 92:1423–1429. doi: 10.3168/jds.2008-1384
- Arajo C, Leon L. 2001. Biological activities of Curcuma longa L. Mem I Oswaldo Cruz. 96:723–728. doi: 10.1590/S0074-02762001000500026
- Asai F, Tosa H, Tanaka T, Iinuma M. 1995. A xanthone from pericarps of Garcinia mangostana. Phytochemistry. 39:943–944. doi: 10.1016/0031-9422(95)00042-6
- Asfour HAE, Darwish SF. 2011. Phenotypic and genotypic detection of both mecA- and blaZ-genes mediated beta-lactam resistance in Staphylococcus strains isolated from bovine mastitis. Global Vet. 6:39–51.
- Babra C, Tiwari JG, Pier G, Thein TH, Sunagar R, Sundareshan S, Isloor S, Hegde NR, de Wet S, Deighton M, et al. 2013. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 58:469–474. doi: 10.1007/s12223-013-0232-z
- Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. 2009. Antibacterial activity of Thai medicinal plants against methicillin-resistant. Staphylococcus aureus. Fitoterapia. 80:102–104. doi: 10.1016/j.fitote.2008.10.007
- Chusri S, Jittanon W, Maneenoon K Voravuthikunchai SP. 2013. An effective antibiofilm agent against Pseudomonas aeruginosa biofilm from traditional Thai herbal recipes used for wound treatments. Microb Drug Resist. 19:337–343. doi: 10.1089/mdr.2012.0252
- Chusri S, Settharaksa S, Chokpaisarn J, Limsuwan S, Voravuthikunchai SP. 2013. Thai herbal formulas used for wound treatment: a study of their antibacterial potency, anti- inflammatory, antioxidant, and cytotoxicity effects. J Altern Complem Med. 19:671–676. doi: 10.1089/acm.2012.0625
- Chusri S, Sompetch K, Mukdee S, Jansrisewangwong S, Srichai T, Maneenoon K, Limsuwan S, Voravuthikunchai SP. 2013. Inhibition of Staphylococcus epidermidis biofilm formation by traditional Thai herbal recipes used for wound treatment. Evid-Based Compl Alt. 2012:159797. doi:10.1155/2012/159797.
- Chusri S, Voravuthikunchai SP. 2009. Detailed studies on Quercus infectoria Olivier (nutgalls) as an alternative treatment for methicillin-resistant Staphylococcus aureus infections. J Appl Microbiol. 106:89–96. doi: 10.1111/j.1365-2672.2008.03979.x
- Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; Approved Standard-Third Edition. CLSI document M31-A3, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087–1898 USA.
- Clinical and Laboratory Standards Institute. 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087 USA.
- Cos P, Vlietinck AJ, Berghe DV, Maes L. 2006. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 106:290–302. doi: 10.1016/j.jep.2006.04.003
- Erskine RJ, Wagner S, DeGraves FJ. 2003. Mastitis therapy and pharmacology. Vet Clin N Am-Food A. 19:109–138. doi: 10.1016/S0749-0720(02)00067-1
- Gruet P, Maincent P, Berthelot X, Kaltsatos V. 2001. Bovine mastitis and intramammary drug delivery: review and perspectives. Adv Drug Deliver Rev. 50:245–259. doi: 10.1016/S0169-409X(01)00160-0
- Gunes H, Gulen D, Mutlu R, Gumus A, Tas T, Eren Topkaya A. 2014. Antibacterial effects of curcumin: an in vitro minimum inhibitory concentration study. Toxicol Ind Health, doi:10.1177/0748233713498458.
- Hillerton JE, Berry EA. 2005. Treating mastitis in the cow-a tradition or an archaism. J Appl Microbiol. 98:1250–1255. doi: 10.1111/j.1365-2672.2005.02649.x
- Hogeveen H, Huijps K, Lam TJ. 2011. Economic aspects of mastitis: new developments. New Zeal Vet J. 59:16–23. doi: 10.1080/00480169.2011.547165
- Huijps K, Lam TJ, Hogeveen H. 2008. Costs of mastitis: facts and perception. J Dairy Res. 75:113–120. doi: 10.1017/S0022029907002932
- Joshi S, Gokhale S. 2006. Status of mastitis as an emerging disease in improved and periurban dairy farms in India. Ann NY Acad Sci. 1081:74–83. doi: 10.1196/annals.1373.007
- Karphrom A, Suknaisilp S, Pradeepasaena P, Tantratian S. 2009. Anti-microbial activities of betel nut (Areca catechu Linn) seed extracts. International Conference on the Role of Universities in Hands-On Education. Rajamangala University of Technology Lanna, Chiang-Mai, Thailand, August 23–29.
- Kim KJ, Yu HH, Cha JD, Seo SJ, Choi NY, You YO. 2005. Antibacterial activity of Curcuma longa L. against methicillin-resistant Staphylococcus aureus. Phytother Res. 19:599–604. doi: 10.1002/ptr.1660
- Koh JJ, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, Tang C, Saraswathi P, Verma C, Tan DT, et al. 2013. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Bioph Acta. 1828:834–844. doi: 10.1016/j.bbamem.2012.09.004
- Kuete V. 2010. Potential of Cameroonian plants and derived products against microbial infections. Planta Medica. 76:1479–1491. doi: 10.1055/s-0030-1250027
- Lee KH, Kim BS, Keum KS, Yu HH, Kim YH, Chang BS, Ra JY, Moon HD, Seo BR, Choi NY, You YO. 2011. Essential oil of Curcuma longa inhibits Streptococcus mutans biofilm formation. J Food Sci. 76:H226–H230. doi: 10.1111/j.1750-3841.2011.02427.x
- Limsuwan S, Voravuthikunchai SP. 2008. Boesenbergia pandurata (Roxb.) Schltr., Eleutherine americana Merr. and Rhodomyrtus tomentosa (Aiton) Hassk. as anti- biofilm producing and antiquorum sensing in Streptococcus pyogenes. FEMS Immunol Med Mic. 53:429–436. doi: 10.1111/j.1574-695X.2008.00445.x
- Mahabusarakam W, Wiriyachitra P, Taylor WC. 1987. Chemical constituents of Garcinia mangostana. J Nat Prod. 50:474–478. doi: 10.1021/np50051a021
- Mordmuang A, Voravuthikunchai SP. 2015. Rhodomyrtus tomentosa (Aiton) Hassk. leaf extract: an alternative approach for the treatment of staphylococcal bovine mastitis. Res Vet Sci. 102:242–246. doi: 10.1016/j.rvsc.2015.07.018
- Neamsuvan O, Tuwaemaengae T, Bensulong F, Asae A, Mosamae K. 2012. A survey of folk remedies for gastrointestinal tract diseases from Thailand’s three southern border provinces. J Ethnopharmacol. 144:11–21. doi: 10.1016/j.jep.2012.07.043
- Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. 1999. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agr Food Chem. 47:4297–4300. doi: 10.1021/jf990308d
- Nguyen PT, Marquis RE. 2011. Antimicrobial actions of alpha-mangostin against oral streptococci. Can J Microbiol. 57:217–225. doi: 10.1139/W10-122
- Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL. 2006. Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol. 118:133–140. doi: 10.1016/j.vetmic.2006.07.008
- Packiavathy IA, Sasikumar P, Pandian SK, Veera Ravi A. 2013. Prevention of quorum- sensing-mediated biofilm development and virulence factors production in Vibrio spp. by curcumin. Appl Microbiol Biot. 97:10177–10187. doi: 10.1007/s00253-013-4704-5
- Rudrappa T, Bais HP. 2008. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agr Food Chem. 56:1955–1962. doi: 10.1021/jf072591j
- Sandegren L. 2014. Selection of antibiotic resistance at very low antibiotic concentrations. Upsala J Med Sci. 119:103–107. doi: 10.3109/03009734.2014.904457
- Simbo DJ. 2010. An ethnobotanical survey of medicinal plants in Babungo, Northwest Region, Cameroon. J Ethnobiol Ethnomed. 6:8. doi:10.1186/1746-4269-6-8.
- Suriyasathaporn W. 2011. Epidemiology of subclinical mastitis and their antibacterial susceptibility in smallholder dairy farms, Chiang Mai Province, Thailand. J Anim Vet Adv. 10:316–321. doi: 10.3923/javaa.2011.316.321
- Tan X, Jiang YW, Huang YJ, Hu SH. 2009. Persistence of gentamicin residues in milk after the intramammary treatment of lactating cows for mastitis. J Zhejiang Univ-Sc B. 10:280–284. doi: 10.1631/jzus.B0820198
- Taponen S, Jantunen A, Pyorala E, Pyorala S. 2003. Efficacy of targeted 5-day combined parenteral and intramammary treatment of clinical mastitis caused by penicillin-susceptible or penicillin-resistant Staphylococcus aureus. Acta Vet Scand. 44:53–62. doi: 10.1186/1751-0147-44-53
- Voravuthikunchai SP, Kitpipit L. 2005. Activity of medicinal plant extracts against hospital isolates of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infec. 11:510–512. doi: 10.1111/j.1469-0691.2005.01104.x
- Wang CK, Lee WH, Peng CH. 1997. Contents of phenolics and alkaloids in Areca catechu Linn. during maturation. J Agr Food Chem. 45:1185–1188. doi: 10.1021/jf960547q
- Zhang X, Mei WL, Zeng YB, Liu J, Dai WJ, Dai HF. 2009. Phenolic constituents from the fruits of Areca catechu and their anti-bacterial activities. J Trop Subtro Bot. 17:74–76.
