ABSTRACT
The effects of different levels of Leucaena leucocephala and Manihot esculenta leaves’ supplementation on in vitro gas production, rumen fermentation and microbial populations and urinary purine derivatives (PD) were investigated. Seven treatment groups – T1:C/rice straw (RS) (40:60) (Control); T2:C/RS/leucaena leaves (40:45:15); T3:C/RS/leucaena leaves (40:30:30); T4: C/RS/leucaena leaves (40:15:45); T5:C/RS/cassava leaves (40:45:15); T6:C/RS/cassava leaves (40:30:30) and T7:C/RS/cassava leaves (40:15:45) – were used in this experiment. In the in vitro study, acetate, propionate, butyrate and total VFA were found to increase significantly in T7. No significant difference was observed in in vitro gas production except the control diet although in vitro dry matter digestibility (IVDMD) was recorded significantly decreased. Determination of urinary PD, rumen fermentation and microbial population were done using 21 local Boer goats. Rumen NH₃-N, acetic and total VFA production were found to improve (P < .05) in all supplemented group. Significant (P < .05) reduction was noted in the urinary allantoin production and total PD at T7. The populations of total protozoa and Ruminococcus flavefacien had significantly increased (P < .05) while Ruminococcus albus, and Fibrobacter succinogenes were significantly reduced (P < .05) in supplemented group. In conclusion, the result from the present study suggested that 25% of L. leucocephala leaves diet and 50% of M. esculenta leaves diet can be incorporated in the goat diet so as to improve the nutritive value of poor quality diet.
Introduction
Supplementing high proteinaceous legumes in animal fed with poor quality feed would increase the animal feed nutritional value. Proteinaceous legumes such as Leucaena leucocephala and Manihot esculenta are abundantly found in the tropics and were considered as high quality leguminous forage due to its high protein content with good amino acid profile. The usage of these forages in the animal feed shows auspicious result in growth rate, dry matter (DM) digestibility (Adejumo & Ademosun Citation1991), DM intake (Srivastava & Sharma Citation1998) for leucaena and DM intake, nutrient digestibility and weight gain (Phengvichith & Ledin Citation2007; Hue et al., Citation2008) for M. esculenta regardless of feeding forms and methods. However, the problem with both of the forages is the content of anti-nutritive factors that may exhibit health impairment in ruminants if consumed excessively. These secondary compounds similarly to mimosine in L. leucocephala and hydrogen cyanide in M. esculenta affect the nutritive value of forages and animal consumed them. As a result, this factor may decrease their nutritional value as sole feed but increased their value as supplemental feed to low quality forages as well as agricultural by-product. Mimosine, as found in L. leucocephala, and hydrogen cyanide in M. esculenta as well as tannin in both the forages have limited their utilization as sole feed in animal diets (Aganga & Tshwenyane Citation2003). The functions of anti-nutritive factors in plants are mainly for nutrient storage or as a means of protecting their structure and reproductive elements (Harborne Citation1989). For local farmers to utilize them effectively, manipulation of both forages is necessary to overcome or reduce the presence of anti-nutritive elements before it can be safely incorporated into the ruminant feed. In the tropics, wilting process has been a common practice for most forages. There is a need to look for other possible alternatives, for example, varying the level of supplementation, which could be easily practised by farmers compared to the common practices. Hence, feeding of L. leucocephala and M. esculenta leaves at different levels of supplementation in the basal-diets has been considered as one of the alternatives. In previous studies, the effect of L. leucocephala and M. esculenta leaves’ supplementation in the diet were mostly observed in terms of animal performances and health (Tan et al. Citation2011). However, rumen microbial population as affected by different level of L. leucocephala and M. esculenta leaves diet remains to be explored, especially on the rapid method of quantification by using an independent culture method –real-time PCR (qPCR), which is faster and more accurate in enumerating rumen microbial populations. Therefore, this present study was conducted to evaluate the effect of L. leucocephala and M. esculenta leaves supplemented diets on the rumen fermentation profiles, urinary purine derivatives (PD) and rumen microbial populations in goats.
Materials and methods
Treatment diets
Seven groups of treatment diets – which are T1: concentrates (C)/rice straw (RS) (40:60) (Control); T2: C/RS/leucaena leaves (40:45:15); T3:C/RS/leucaena leaves (40:30:30); T4: C/RS/leucaena leaves (40:15:45); T5:C/RS/cassava leaves (40:45:15); T6:C/RS/cassava leaves (40:30:30) and T7: C/RS/cassava leaves (40:15:45) – were used in this experiment. The chemical compositions of the experimental diets are shown in .
Table 1. Chemical composition of L. leucocephala and M. esculenta leaves supplemented diets (Mean ± SE).
Animal and rumen fluid sampling for in vitro experiment
Two rumen fistulated goats (Boer crossed-bred) with approximately 30 kg body weight (BW) were used as rumen fluid donors. The goats were fed twice daily with a diet containing a fixed amount of alfalfa hay, with a concentrate forage ratio of 60:40. The sample of rumen fluid was taken before morning feeding and immediately strained through two layers of muslin cloth before being kept in an anaerobic environment container.
In vitro gas production and rumen fermentation characteristic
In vitro gas production of the treatment diets was evaluated according to the described method by Menke and Steingass (Citation1988) with some modifications. Approximately, 200 mg of oven-dried and milled treatment diet feed samples were weighed and placed into a 100 ml well-lubricated glass syringes. A buffered mineral solution containing 1:2 ratio of strained rumen fluid for mineral buffer was prepared in a water bath at 39°C, with continuous stirring in an anaerobic condition. Then, 30 ml of the buffered rumen fluid solution was placed into 100 ml glass syringes containing samples of the treatment diets. Each of the treatment diets was evaluated in triplicate. The syringes were then maintained at a constant temperature of 39°C for 72 h. Gas production was recorded at 2, 4, 6, 8, 10, 12, 24, 48, and 72 h of incubation. Standard hay (University of Hohenheim, Stuttgart, Germany) was used to calibrate the in vitro gas production system with an average gas production of 49.6 ml/200 mg of standard. After 72 h, the rumen fluids were collected and measured for rumen pH, ammonia-nitrogen (NH₃-N), volatile fatty acid (VFA) as well as for in vitro dry matter digestibility (IVDMD).
Animal and sample preparation for in vivo experiment
In vivo experiment was conducted on 21 male Boer cross-bred goats (25 ± 5 kg BW) at the Farm 2, Universiti Putra Malaysia. During the experiment, the animals were housed individually in a metabolic crate. All the animals were fed twice daily and have continuous access to water at all times. All animals were randomly assigned into seven treatment groups according to a completely randomized experimental design. Each of the treatment groups consisted of three animals. The animals were subjected to an adaptation period for 7 days and were continued with the administration of the treatments diet for 10 days. At day-17, rumen fluids were sampled via stomach tubing before feeding and the samples obtained were analysed for determination of rumen pH, NH₃-N, VFA and for quantification of rumen microbial populations. For PD analysis, urine samples were taken daily throughout the 5-day experimental period and were pooled to obtain 1 sample urine per goat, as mentioned in Chen and Gomez (Citation1995).
Determination of rumen pH and in vitro DM digestibility
The pH value of the rumen fluids was measured by using a portable pH metre (EcoTestr™ pH 1 from Eutech Instruments (P) Ltd.). After the 72 h incubation, the rumen content from the syringes were transferred into a sintered glass and the rumen fluid residues were collected and dried at 105°C until constant weight. IVDMD was assessed by the differences in the weight of in vitro substrate before and after incubation.
Determination of rumen NH₃-N and VFA content
The rumen fluids from the in vitro and in vivo study were centrifuged at 6000 × g for 10 min. The supernatant was collected and analyses of rumen NH₃-N and VFA were carried out as described by Parsons et al. (Citation1984) and Filipek and Dvořák (Citation2009), respectively.
Determination of urinary PD
Urinary PD was determined according to the procedure in Balcells et al. (Citation1992). Urine samples were diluted with 0.1 M ammonium dihydrogen phosphate (NH₄H₂PO₄) in a 1:20 ratio. Then, the samples were filtered through 0.45 μm filter before being analysed by high performance liquid chromatography (Agilent 1100 Series High performance Liquid Chromatography System, Agilent Technologies, USA) with two 4.6 mm × 250 mm C-18 reverse-phase column (Spherisorb) and the effluent were monitored at 205 nm.
Determination of rumen microbial populations by qPCR
The populations of total bacteria, methanogens, total protozoa and three major cellulolytic bacteria (Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogenes) were determined by qPCR. PCR amplification was carried out by obtaining genomic DNA from rumen fluid and the extraction of the total DNA from the rumen fluid sample was carried out by using the QIAamp® DNA stool mini kit (Qiagen Ltd, Crawley, West Sussex, UK) following the manufacturer’s instructions.
An absolute quantification of rumen microbes was achieved based on the standard curve method in real-time PCR. Standard curves were constructed from the amplification of known amounts of target microbes DNA. The qPCR master mix was prepared for a total volume of 25 μL using the QuantiFast® SYBR® Green PCR kit (Qiagen Inc., Valencia, USA) consisted of 12.5 μL of 2 × SYBR Green Master Mix, 1 μL of 10 μM forward primers, 1 μL of 10 μM reverse primer, 2 μL of DNA samples and 8.5 μL of nuclease-free water for each reaction. Each sample was analysed in triplicates. Primers used in the PCR amplifications for targeted rumen microbial populations are detailed in . Real-time PCR quantification was done by using BioRad CFX96 Real-time PCR system (BioRad, USA) with optical grade plates. The qPCR cycling conditions consisted of an initial 5 min of denaturation at 94°C, and is followed by 40 cycles of denaturation at 94°C for 20 s each cycle, annealing (temperatures for different primers as listed in ) for 30 s and extending at 72°C for 20 s.
Table 2. Primers used for qPCR assay to target total bacteria, total protozoa, methanogens, R. albus, R. flavefaciens and F. succinogenes.
Statistical analyses
All of the experimental data obtained were analysed with General Linear Model (GLM) procedure by Statistical Analysis Software (SAS). The significant differences among treatment means were compared by using Duncan Multiple Range test.
Results and discussion
In vitro gas production, rumen fermentation characteristic and IVDMD
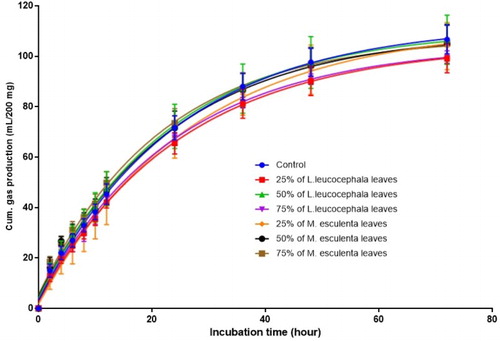
In this study, the cumulative gas production was not affected by the differences in the treatment diet. The increments in the gas production trend were shown in the . shows the effect of different levels of L. leucocephala and M. esculenta leaves on the in vitro fermentation profiles, which is rumen pH, ammonia-nitrogen and VFA production as well as IVDMD of the treatment diets. A significant difference was observed in the mean concentration of propionic acid when compared among treatment diets. T2 and T4 show a higher value of mean concentrations of propionic acid (20.148 and 19.907 mol/100 mol) while T5 and T6 show a lower value (18.646 and 18.416 mol/100 mol) when compared among treatment means, respectively. Meanwhile, supplementation of L. leucocephala and M. esculenta leaves showed that the IVDMD of the diet were found to significantly differ (P < .05), where T1 showed the highest value among the treatment diets. The variability of fermentation product in this study may be due to the differences in the diet protein content as the concentrate level in the diets was constant. The rumen pH was found not to be affected by the inclusion level of treatment diets when compared to the control treatment. Nevertheless, the range of rumen pH observed in present study was between 6.60 and 6.77 and incongruent with the data reported by Kang et al. (Citation2012) in a study of buffalo fed with leucaena-based diet. Acetic acid production, albeit insignificant, was the dominant VFA among the other individual VFA, which indicates that the contents of treatment diets were high in structural carbohydrate (NRC Citation2007). In an in vitro study, the substrate balance between carbohydrate and protein was the key factor for in vitro fermentation (Zhou et al. Citation2011). Nevertheless, the decrease in IVDMD of the treatment diet in this study might be caused by the lignin content and the presence of tannin in the diet that could interrupt the feed digestibility (Hariadi & Santoso Citation2010).
Table 3. Diets’ effects on in vitro ruminal pH, rumen ammonia-nitrogen, VFA concentration and IVDMD (Mean ± SE).
In vivo rumen fermentation profiles and urinary PD
The effect of L. leucocephala and M. esculenta leaves supplemented diets on in vivo rumen fermentation characteristic were presented in . Supplementation of L. leucocephala and M. esculenta leaves in the diets has significantly increased (P < .01) the mean concentration of ammonia-N levels in the rumen fluids. The increased rumen NH₃-N content was understandable and may as a result lead to an increase in CP content in the diet. This is in agreement with the finding of Sahlu et al. (Citation1992), where an increase in rumen NH₃-N was found in the diet with higher CP contents. An increase in rumen NH₃-N would lead to an increase in rumen bacteria and protozoa as well as urinary PD (Wanapat Citation2000).
Table 4. Diets effects on in vivo ruminal pH, rumen NH₃-N and VFA concentration of the goats (Mean ± SE).
Meanwhile, significant differences were found in the mean concentration of propionic acid (P < .05), butyric acid (P < .01), total VFA (P < .01) and A: P ratio (P < .05). As for the mean concentration of propionic acid, T7 recorded the highest value and T2 are noted as the lowest value. In terms of butyric acid production, the highest mean concentration of butyric acid was found in T4 and the lowest value was found in T6 when the treatment diets were compared among each other. The mean concentration of total VFA was recorded the highest at T2 while the lowest are in T4, which is 84.83 and 68.35 mmol/L, respectively. In addition, it was found that A-to-P ratio was affected significantly with the treatment diets when comparison was made among the treatments. T2 showed the highest A to P value (5.06) while T7 showed the lowest value (3.92).
According to Estrada-Liévano et al. (Citation2009), the rate of VFA’s production is closely related to the rate of substrate fermentation, whereas the molar proportion is associated with the microbial species present in the rumen. In this study, propionic acid increment may suggest that microbial ecosystem involved in the propionic acid formation differs with dietary treatment. An increase in the population of cellulolytic bacteria F. succinogenes may also be one of the reasons why propionic acid production increases, as this bacteria is a major propionic-acid producer through succinate formation pathway (Moss et al. Citation2000).
The high production of propionate could also be attributed to high solubility of nitrogen and high substrate availability for VFA production. This was in agreement with the study by Phesatcha et al. (Citation2013), where an increase in total VFA and propionic acid was observed when dried leucaena leaf was supplemented to swamp buffalo.
High total VFA concentration was probably due to the high fermentation intensity as a result of high uptake of nitrogen for microbial synthesis (Osakwe & Steingass Citation2006; Liu et al. Citation2009). In the present study, T2 produced the highest total VFA concentration with slightly higher rumen pH than control. This shows the ability of saliva as a buffer medium to balance out the reduction in pH due to the increased production of VFA and the ability of the fibrous feed to promote saliva production during feed ingestion by animals. The variation on the acetic acid concentration depends greatly on the amount of structural carbohydrates in the rumen, where it interrelated with pH and the predominant species of microbial population in the rumen. In a study by Calsamiglia et al. (Citation2008), variations in true OM and fibre degradation, and acetate and butyrate concentrations are mostly correlated with the level of rumen pH. Increased in nutrient digestion in the current study was manifested by efficient fermentation of substrates by rumen microorganisms. This could be the reason why high amount of acetate was recorded in the rumen of goat fed with T2 than the other treatment group in this study. However, the observed molar proportion of acetate, propionate, butyrate, acetate to propionate and total VFA concentration in the present study are in agreement with a study done by Rira et al. (Citation2015) in sheep fed with a tannin-rich plant diet.
Diet with different levels of L. leucocephala and M. esculenta leaves show no effect on rumen pH and acetic acid production. In the present study, ruminal pH remains significantly unchanged regardless of the treatment. Although no significant differences was observed, pH values reported in this experiment were within the range (6.8–7.2) of previous studies of cattle fed with cassava by Wanapat (Citation2000) and Geihauser et al. (Citation2012).
The effects of L. leucocephala and M. esculenta leaves supplemented diets on urinary PD of goats were reported in . Urinary allantoin and xanthine was affected significantly (P < .05) by supplementation of L. leucocephala and M. esculenta leaves in the diets where T2 showed the highest value for both concentration at 227.15 and 16.23 μmol/kg BW⁰˙⁷⁵ d−1, respectively. Meanwhile, significant differences were found in the mean concentration of total PD (P < .05), where T6 showed the highest value (273.18 mmol/day). On the contrary, the lowest values for mean concentration of allantoin, xanthine and total PD were recorded at T7 when compared to the other treatment diets. However, there were no significant differences between all treatment diets in the mean concentration of uric acid and hypoxanthine.
Table 5. Effect of L. leucocephala and M. esculenta leaves supplemented diets on urinary PD of goats (Mean ± SE).
PDs have been commonly used as a predictor of the amount of microbial protein arriving at the duodenum. Allantoin being the highest in the proportion of total PD production is probably because of its final product of purine metabolism in the ruminant, as hypoxanthine and xanthine would be converted into uric acid and later converted to allantoin through enzyme reaction (Hernandez et al. Citation2014). In the present study, animals fed with a higher amount of L. leucocephala and M. esculenta leaves diets demonstrated a decrease in the production of urinary allantoin. The reduction in the urinary allantoin production may probably be due to the presence of anti-nutritive factors in the leaves. According to Jones (Citation1979), diets with high mimosine content would increase the amount of mimosine degraded and would disrupt the cell division process (e.g. growth and development). Likewise, a lower production of urinary allantoin was observed in the study as the L. leucocephala leaves in the diets increased. This might be due to the high cyanide content present in the diets. According to Broderick and Balthrop (Citation1979), cyanide would inhibit protein deamination processes by rumen microbes and hence affecting the rumen bacterial protein turnover. This corroborates with the findings of Jetana et al. (Citation2012), where high microbial yield was found as the production of urinary allantoin was increased as low supplementation of L. leucocephala leaf diet was fed to the animals.
Quantification of rumen microbial populations by qPCR
In this experiment, rumen microbial populations of goats were affected by feeding different levels of L. leucocephala and M. esculenta leaves. As presented in , the results showed that the population of R. flavefacien was altered significantly (P < .01) when compared with control treatment by feeding different levels of L. leucocephala and M. esculenta leaves to the goats. Increased populations of R. flavefacien was observed as higher inclusion levels of both leaves were offered to the goats compared to T1, with T7 recorded as the highest population of R. flavefaciens. In comparison with control diets, results show that the population of total protozoa was found to significantly increase (P < .05), while the population of R. albus and F. succinogens was reported to decrease significantly (P < .05).
Table 6. Effect of L. leucocephala and M. esculenta leaves supplemented diets on rumen microbial population in goats (Mean ± SE).
Supplementations of L. leucocephala and M. esculenta leaves in the diet have increased the population of total protozoa in the rumen. The effect was in line with the data reported by Vasta et al. (Citation2010), where an increase in the populations of protozoa was noted in the rumen of lamb fed with tannin-containing diet. However, previous studies proposed that the mode of action of tannin on protozoa was to alter the protozoa cell membrane permeability and thereby disintegrating the protozoa cell membrane (Francis et al. Citation2002). The changes in the rumen protozoa population found in the present study could be influenced by other factors such as diet composition, turnover rate, feeding frequency and feed level, as reported by Franzolin and Dehority (Citation1996).
R. albus, R. flavefacien and F. succinogenes are the main bacteria in the rumen responsible for the biodegradation of plant cell wall (Forsberg et al. Citation1997). In this study, it was found that the population of R. flavefacien was positively influenced by the supplementation of L. leucocephala and M. esculenta leaves, while the population of R. albus and F. succinogenes were reduced when comparing the treatment diets with the control diet. According to Michalet-Doreau et al. (Citation2001), F. succinogenes are the most dominant species among the three cellulolytic bacteria. This is consistent with the results in our study, where F. succinogenes population exhibits higher values than R. flavefacien and R. albus. The decrease in the populations of F. succinogenes and R. albus may probably be due to the presence of the anti-nutritive factors in the diets, hence reducing the activities of cellulolytic bacteria. This was supported by McSweeney et al. (Citation2000), where they show that rumen cellulolytic bacteria in sheep fed with tannin-containing diets were low without affecting its microbial protein synthesis. On the contrary, it has been found that the rumen bacteria would adapt to the challenged environment and improved the digestion considerably within a week (Ruskin Citation1977). R. flavefaciens population increments may be explained through the increase in the leaf fraction in the treatment diets as the level of leaves inclusion treatment diet increased. This is corroborated with the findings of an influorescence in situ hybridization study by Shinkai and Kobayashi (Citation2007), where R. flavefaciens colonization in the rumen of sheep were found to occur into the plant leaves rather than into the stem. This was substantiated through quantification of R. flavefaciens by real-time PCR within the same study, where the result shows R. flavefaciens attached to stems was less than 20% of that attached to leaf sheaths.
Conclusion
In conclusion, the results obtained from the present study demonstrated the effect of L. leucocephala and M. esculenta leaves supplemented diet on rumen fermentation characteristic, urinary PD and rumen microbial populations were divergent. However, the addition of 25% of L. leucocephala leaves in the diet and 50% of M. esculenta leaves in the diet shows a notable amount of VFA and PD production, with the population of total protozoa as well as cellulolytic bacteria being moderately present in the rumen. Thus, it can be concluded that 25% of L. leucocephala leaves supplemented diet and 50% of M. esculenta leaves supplemented diet were the appropriate supplementation level to be fed to goats without causing a detrimental effect towards the animal as a whole.
Acknowledgement
Liyana, N. A. H was a recipient of MyBrain15 Scholarship from the Malaysia Ministry of Higher Education. We would like to express our gratitude to Malaysian Agro-Biotechnological Institute (ABI), for providing access and permission to use their laboratory facilities, especially real-time PCR machine.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adejumo JO, Ademosun AA. 1991. Utilization of leucaena as supplement for growing dwarf sheep and goats in the humid zone of West Africa. Small Rum Res. 5:75–82. doi: 10.1016/0921-4488(91)90032-L
- Aganga AA, Tshwenyane SO. 2003. Feeding values and anti-nutritive factors of forage tree legumes. Pak J Nutr. 2:170–177. doi: 10.3923/pjn.2003.170.177
- Balcells J, Guada J, Peiro JM, Parker DS. 1992. Simultaneous determination of allantoin and oxypurines in biological fluids by high performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 575:153–157. doi: 10.1016/0378-4347(92)80517-T
- Broderick GA, Balthrop JE. 1979. Chemical inhibition of amino acid deamination by ruminal microbes in vitro. J Anim Sci. 49:1101–1111.
- Calsamiglia S, Cardozo PW, Ferret A, Bach A. 2008. Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J Anim Sci. 86(3):702–711. doi: 10.2527/jas.2007-0146
- Chen XB, Gomez MJ. 1995. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives – An overview of the technical details. Occasional Publication 1992, International Feed Resources Unit, Rowette Research Institute, Aberdeen, UK.
- Denman SE, McSweeney CS. 2006. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 58(3):572–582. doi: 10.1111/j.1574-6941.2006.00190.x
- Estrada-Liévano JM, Sandoval-Castro CA, Ramirez Avilés L, Capetillo-Leal CM. 2009. In vitro fermentation efficiency of mixtures of Cynodon nlemfuensis, Leucaena leucocephala and two energy sources (maize or sugar cane molasses). Trop subtrop agroecosyst. 10(3):497–503.
- Filípek J, Dvořák R. 2009. Determination of the volatile fatty acid content in the rumen liquid: comparison of gas chromatography and capillary isotachophoresis. J Acta Vet Brno. 78:627–633. doi: 10.2754/avb200978040627
- Forsberg CW, Cheng KJ, White BA. 1997. Polysaccharide degradation in the rumen and large intestine. In: Mackie RI, White BA, editors. Gastrointestinal Microbiology. Springer US; p. 319–379.
- Francis G, Kerem Z, Makkar HP, Becker K. 2002. The biological action of Saponins in animal systems: a review. Br J Nutr. 88(06):587–605. doi: 10.1079/BJN2002725
- Franzolin R, Dehority BA. 1996. Effect of prolonged high-concentrate feeding on ruminal protozoa concentrations. J Anim Sci. 74(11):2803–2809.
- Geihauser T, Linhart N, Neidl A, Reimann A. 2012. Factors associated with ruminal pH at herd level. J Dairy Sci. 95(8):4556–4567. doi: 10.3168/jds.2012-5380
- Harborne JB. 1989. Biosynthesis and function of anti-nutritional factors in plants. Asp Appl Biol. 19:21–28.
- Hariadi BT, Santoso B. 2010. Evaluation of tropical plants containing tannin on in-vitro methanogenesis and fermentation parameters using rumen fluid. J Sci Food Agri. 90:456–461.
- Hernandez P, Salema AZM, López S, Sun XZ, Rojo R, Camachoe LM, Elghandour MMY, Gonzalez-Ronquillo M. 2014. Influence of Salix babylonica and Leucaena leucocephala leaf extracts on ruminal fermentation characteristics, urinary purine derivative excretion and microbial protein synthesis of lambs. Livest Sci. 163:80–84. doi: 10.1016/j.livsci.2014.01.030
- Hue KT, Van DTT, Ledin I. 2008. Effect of supplementing urea treated rice straw and molasses with different forage species on the performance of lambs. Small Rum Res. 78:134–143. doi: 10.1016/j.smallrumres.2008.05.010
- Jetana T, Thongruay S, Uswang S, Hengtrakulsin R. 2012. A comparative study on mimosine, 3, 4-dihydroxy pyridone (3, 4-DHP) and 2, 3-dihydroxy pyridone (2, 3-DHP), purine derivatives (PD) excretion in the urine, thyroid hormone and blood metabolites profiles of Thai swamp buffalo (Bubalus bubalis) and Murrah buffalo (Bubalus bubalis). Trop Anim Health Prod. 44(4):887–897. doi: 10.1007/s11250-011-9983-1
- Jones RJ. 1979. The value of Leucaena leucocephala as a feed for ruminants in the tropics. World Anim Rev. 31(1):3–2.
- Kang S, Wanapat M, Pakdee P, Pilajun R, Cherdthong A. 2012. Effects of energy level and Leucaena leucocephala leaf meal as a protein source on rumen fermentation efficiency and digestibility in swamp buffalo. J Anim Feed Sci Tech. 174(3–4):131–139. doi: 10.1016/j.anifeedsci.2012.03.007
- Koike S, Kobayashi Y. 2001. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett. 204(2):361–366. doi: 10.1111/j.1574-6968.2001.tb10911.x
- Lane DJ. 1991. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester: John Wiley and Sons; p. 115–175.
- Liu Q, Wang C, Huang YX, Dong KH, Yang WZ, Zhang SL, Wang H. 2009. Effects of isovalerate on ruminal fermentation, urinary excretion of purine derivatives and digestibility in steers. J Anim Physiol Anim Nutr. 93(6):716–725. doi: 10.1111/j.1439-0396.2008.00861.x
- McSweeney CS, Palmer B, Krause DO. 2000. Rumen microbial ecology and physiology in sheep and goats fed a tannin-containing diet. In: Brooker JD, editor. Proc Intern. Workshop on Tannins in Livestock and Human Nutrition. ACIAR Proc. No. 92. 171 p.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in-vitro gas production using rumen fluid. Anim Res Dev. 28:7–55.
- Michalet-Doreau B, Fernandez I, Peyron C, Millet L, Fonty G. 2001. Fibrolytic activities and cellulolytic bacterial community structure in the solid and liquid phases of rumen contents. Reprod Nutr Dev. 41(2):187–194. doi: 10.1051/rnd:2001122
- Moss AR, Jouany JP, Newbold J. 2000. Methane production by ruminants: its contribution to global warming. Ann Zootech. 49:231–253. doi: 10.1051/animres:2000119
- [NRC] National Research Council. 2007. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Committee on the Nutrient Requirements of Small Ruminants. Washington, DC: The National Academies Press.
- Osakwe II, Steingass H. 2006. Ruminal fermentation and nutrient digestion in West African Dwarf (WAD) sheep fed Leucaena leucocephala supplemental diets. Agroforest Sys. 67:129–133. doi: 10.1007/s10457-005-7474-y
- Parsons TR, Maita Y, Lalli CM. 1984. A manual of chemical and biological methods for seawater analysis. New York, NY: Pergamon Press; p. 173.
- Phengvichith V, Ledin I. 2007. Effects of supplementing gamba grass (Andropogon gayanus) with cassava (Manihot esculenta Crantz) hay and cassava root chips on feed intake, digestibility and growth in goats. Asian Australas J Anim Sci. 20(5):725–732. doi: 10.5713/ajas.2007.725
- Phesatcha K, Wanapat M, Mcsweeney C. 2013. Effect of dried leucaena leaf supplementation on rumen ecology, nutrient digestibility and urinary excretion of 2,3-dihydroxy pyridone (2,3-DHP) and 3,4-dihydroxy pyridone (3,4-DHP) in swamp buffaloes. Buffalo Bull. 32(2):975–979.
- Rira M, Morgavi DP, Archimède H, Marie-Magdeleine C, Popova M, Bousseboua H, Doreau M. 2015. Potential of tannin-rich plants for modulating ruminal microbes and ruminal fermentation in sheep. J Anim Sci. 93(1):334–347. doi: 10.2527/jas.2014-7961
- Ruskin FR. 1977. Leucaena: Promising forage and tree crop for the tropics. 1st ed. Washington, DC: National Academy Press and National Research Council.
- Sahlu T, Fernandez JM, Lu CD, Manning R. 1992. Dietary protein level and ruminal degradability for mohair production in Angora goats. J Anim Sci. 70:1526–1533.
- Shinkai T, Kobayashi Y. 2007. Localization of ruminal cellulolytic bacteria on plant fibrous materials as determined by fluorescence in-situ hybridization and real-time PCR. Appl Environ Microbiol. 73(5):1646–1652. doi: 10.1128/AEM.01896-06
- Srivastava SNL, Sharma K. 1998. Response of goats to pelleted diets containing different proportions of sun-dried Leucaena leucocephala. Small Rum Res. 28:139–148. doi: 10.1016/S0921-4488(97)00069-2
- Sylvester JT, Karnati SK, Yu Z, Morrison M, Firkins JL. 2004. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J Nutr. 134(12):3378–3384.
- Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, Ho YW. 2011. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim Feed Sci Technol. 169:185–193. doi: 10.1016/j.anifeedsci.2011.07.004
- Vasta V, Yáñez-Ruiz DR, Mele M, Serra A, Luciano G, Lanza M, Priolo A. 2010. Bacterial and protozoal communities and fatty acid profile in the rumen of sheep fed a diet containing added tannins. Appl Environ Microbiol. 76(8):2549–2555. doi: 10.1128/AEM.02583-09
- Wanapat M. 2000. Rumen manipulation to increase the efficient use of local feed resources and productivity of ruminants in the tropics. Asian Australas J Anim Sci. 13:59–67.
- Zhou H, Li M, Zi X, Xu T, Hou G. 2011. Nutritive Value of Several Tropical Legume Shrubs in Hainan Province of China. J Anim Vet Adv. 10(13):1640–1648. doi: 10.3923/javaa.2011.1640.1648