ABSTRACT
The effects of consumption of β2-agonist zilpaterol hydrochloride (ZH) were evaluated on hormonal blood metabolites (insulin, cortisol and thyroids), haemoglobin, haematocrit, plasma volume, heart rate and respiratory changes in male goats in the finishing phase. For this, 16 Mahabadi castrated male goats were used in a completely random design to evaluate the effects of treatments. Goats which were fed a finishing basal diet for 60 days prior to start the experiment were subsequently supplemented during 30 days with ZH at a dosage of 0.0 or with 20 mg ZH/kg LW. Haemoglobin concentration and haematocrit levels were recorded at 10-day intervals of experiment. Plasma volume was calculated using the haematocrit value. Heart and respiratory rates were recorded at 10-day intervals of experiment. Rates were recorded at 1-h intervals between 06:00 and 18:00 h. Hormonal profiles were measured at days 1 and 30. Plasma volume, heart and respiratory rates increased (P < .001) in the ZH group. Zilpaterol supplementation increased (P < .04) plasma insulin, and thyroids hormones, but decreased (P < .01) haemoglobin, haematocrit value and cortisol hormones. It can be concluded that zilpaterol supplementation of 0.20 mg ZH/kg LW significantly influences blood metabolic hormones concentrations, plasma volume, and cardiovascular and respiration rates.
1. Introduction
Zilpaterol hydrochloride (ZH) (Zilmax, Intervet©, South Africa) is a type 2 β-agonist approved in some countries (Mexico, Canada, USA and South Africa) for continuous feeding to feedlot cattle at the rate of 6.0–8.33 mg zilpaterol/kg of dietary dry matter (DM) [considering the daily intake (as percentage of live weight [LW]) for finishing cattle these dosages represent approximately 0.15–16.5 mg zilpaterol/kg LW, respectively; CitationMerck, Animal Health] during the final 20–40 days followed by a 3-day withdrawal period before harvest. Under these conditions, ZH markedly enhances daily gain, feed efficiency, hot carcass weight, dressing percentage, LM area, and boneless closely trimmed carcass yield (Avendaño-Reyes et al. Citation2006; Plascencia et al. Citation2008). Although there is no label dosage for use of zilpaterol in ruminants other than cattle, maximal responses to ZH supplementation have been observed in feedlot lambs when were supplemented with a similar concentration (6.0 mg of ZH/kg of dietary DM) than in feedlot cattle (Estrada-Angulo et al. Citation2008; Ríos Rincón et al. Citation2010; López-Carlos et al. Citation2014). However, due to the level of feed intake of finishing lambs, this concentration of ZH in diet represents from 18 to 22 mg ZH/kg LW. This becomes more relevant if we consider that β2-agonist receptors have a physiological effect on the cardiovascular, respiratory and endocrine system independently of muscle accretion and lipolysis (Mersmann Citation1998). Even when the dosage for ZH that targets muscle growth is much lower than that of other agonists routinely used in human medicine for other purposes, there is scarce or null information available on the administration during 30-day of ZH on cardiovascular (heart rate), respiratory, some blood constituents and metabolic hormonal changes in ruminants fed high-energy diet. Thus, the objective of this study was the evaluation of the physiological changes in castrated male goats supplemented with 20 mg ZH/kg LW.
2. Material and methods
The study was conducted at the University of Tehran Agricultural Research with trained personnel of this centre according to procedures approved by the Institutional Animal Care and Use Committee at Department of Animal Science of the University in Karaj, Iran (35.80 N, 50.95E).
2.1. Animals and basal diet
Sixteen Mahabadi castrated male kid goats (6 months old and averaging 29.93 ± 1.84 kg LW in weight) were used to determine the effects of consumption of ZH on heart and respiratory rates, haematocrit percentage, plasma volume, haemoglobin concentration during the last 30 days of finishing. Sixty days prior to start the experiment goats were dewormed (Albendazole 2.5%®, Damloran Razak Pharma©, Borujerd, Iran), vaccinated against Clostridium spp. (Enterotoxemia polyvalent®, Razi Vaccine and Serum research institute, Karaj, Iran) and were placed, to start the finishing phase, in 16 boxes (0.8 × 1.2 m) with roof and cement floor and equipped with individual automatic water taps and metallic feed bunks. Lambs were fed ad libitum with a finishing diet formulated as follows (g/kg DM basis): alfalfa hay, 250; silage corn, 50; soybean meal, 60; barley grain, 450; wheat bran, 50; canola meal, 72; milled corn, 50, and mineral supplement, 18. The calculated nutrient composition of the diet was: crude protein (CP), 152 g/kg; neutral detergent fiber (NDF), 352 g/kg; ether extract, 20 g/kg; calcium, 81 g/kg and phosphorus, 41 g/kg (NRC Citation2007; DM basis). The calculated ME of basal diet was 9.80 MJ/kg.
2.2. Treatments
Even when the dosage of ZH for goats has not been established, in lambs, maximal responses were observed with a dosage of 20 mg ZH kg LW (Estrada-Angulo et al. Citation2008; Ríos Rincón et al. Citation2010; López-Carlos et al. Citation2014). Thus, this is the rationale of the concentration used in the present experiment. Therefore, treatments consisted of the same basal finishing diet without ZH (control) or supplemented with ZH 20 mg ZH kg LW (Zilmax®, Intervet, South Africa). The experiment lasted 30 days (which correspond to the last 30 days of the 90 days of feedlot period). Daily feed allotments to each pen were adjusted to allow minimal (<5%) feed refusals in the feed bunk. The dose of ZH was hand-weighed using a precision balance (Ohaus, mod AS612, Pine Brook, NJ). To ensure the consumption of the planned dose, the total daily dose of ZH was mixed with 10 g of grain and was provided in the morning feeding using individual feeders. Daily dosage of ZH was adjusted weekly based on body weight and feed consumed in the previous week.
2.3. Sampling, records and laboratory analyses
Individual blood samples (7 mL) were obtained from each goat through the jugular vein by venipuncture (Venojet, Terumo Europe, Belgium) prior to the morning meal on days 1 and 30 of the ZH supplementing period. Samples were immediately centrifuged for 15 min at 3000 × g at a temperature of 5°C and plasma was stored at −20°C until assayed for metabolic hormones. Plasma insulin was measured by Radioimmunoassay (RIA) using ovine insulin and antibody against insulin provided by Tabeshyarnoor Co. (Industrial City of Bu-Ali, Hamadan, Iran). Sensitivity of the assay was 15 nmol/L and intra- and inter-assay variations were 8% and 5%, respectively. Plasma cortisol was determined by RIA using cortisol and antibody against cortisol (Tabeshyarnoor Co.; Industrial City of Bu-Ali, Hamadan, Iran). Both insulin and cortisol samples were read at 450 nm (MPR4, PerkinElmer Inc., Life Sciences, Germany). Assay sensitivity was 5.5 nmol/L and intra- and inter-assay variations were 9.4% and 8.1%, respectively. Triiodothyronine (T3) and Thyroxine (T4) were determined according to the ELISA procedure, using the commercial diagnostic human thyroid hormones (Diagnostic Automation/Cortez Diagnostics Inc, USA). Assay sensitivities for T3 and T4 were 0.20 ng/mL and 0.05 µg/mL, respectively, and coefficient of variation (inter- and intra-assay) for thyroid hormones was less than 10%. The ELISA samples were read at 450 nm using a Bio-Rad model 550 ELISA plate reader (ELX800, Bio Tek, USA). Haematocrit levels were determined at days 1, 10, 20 and 30 of the ZH supplementing period before morning feeding. A small amount of jugular vein samples of each individual was measured by graduated microhaematocrit tubes for centrifuging (XF800, Sysmex, japan) for 10 min at 3000 × g. Haematocrit was used for calculating estimated plasma volume (mL) and blood haemoglobin concentration utilizing the equations described by Hopfer et al. (Citation2004) as follows: plasma volume, mL = body weight, g × 0.07 × (1-haematocrit), and blood haemoglobin (g/dL) = haematocrit (decimal fraction) × 34.
Heart (beat/min) and respiratory rates (breaths/min) were recorded at 10-day intervals of experiment. Rates were recorded at 1-h intervals between 06:00 and 18:00 h measured using a stethoscope for heart rate and visual determination for respiration rate.
2.4. Statistical analysis
The trial was analysed as a completely randomized design using the MIXED procedure of SAS software (CitationSAS 9.1 Inst. Inc. Cary, NC). Treatments effects on insulin, cortisol and thyroid hormones were tested by means of orthogonal contrasts as control vs. supplemental ZH. F-test (numerator = 1 df, denominator = error df) was utilized to test contrasts. Haemoglobin concentration, plasma volume, haematocrit level, and heart and respiratory rates, which were recorded at 10-day intervals of experiment, were analysed with a linear mixed model for repeated measures in a completely randomized design according to SAS (SAS Inst., Inc., Cary, NC; Version 9.1) with evaluation of the covariance structures: A, CS, AR1 and the animal as a random component. The analysis was carried out with the MIXED procedure of SAS software. In all cases, least squares means and standard error are reported and evaluated significantly at P ≤ .05 and main trends at P > .05 and ≤ 0.10.
3. Results and discussion
The growth performance and carcass responses to the treatments were previously reported by Hatefi et al. (Citation2015) in which ZH increased total weight gain (5.82 vs. 3.93 kg, P < .01) and ADG (176 vs. 119 g, P = .02.) and reduced DMI (10.9%, P < .01); therefore, supplementation of ZH increased (P < .01) G:F by 65.2%.(0.152 vs. 0.092). In the USA, the label dosage is 0.165 mg/kg BW for a 20-day feeding period; for that, they recommend 8.33 mg ZH/kg of diet, assuming that during finishing, the cattle in USA have an average of daily intake of 2.0% of LW; while in Mexico, the dosage is 0.15 mg/kg BW for a 30-day feeding period and to reach this dosage they recommend 6 mg ZH/kg of diet, assuming that during finishing, the cattle in Mexico have an average of daily intake of 2.5% of BW. Thus, the dosage used in our experiment represents 22–33% more than the dose recommended to feedlot cattle. Basal metabolites and hormonal concentrations in plasma and heart and respiratory rates were not different from the controls in the ZH group before they were fed ZH.
3.1. Blood metabolites and hormonal profile
Effects of treatments on plasma levels of insulin, cortisol, Triiodothyronine (T3) and Thyroxine (T4) recorded at day 1 and day 30 are given in . There were no differences (P > .18) in plasma concentration of insulin, cortisol, T3 and T4 between groups at day 1, but ZH supplementation increased (P < .04) plasma insulin, plasma concentration of T3 (13.5%, P < .01) and T4 (13.3%, P < .01) at the end of experimental period. Conversely, total serum cortisol concentrations decreased (25.46%, P < .01) in goats that were fed ZH during 30 days of supplementation period.
Table 1. Effect ZH on plasmatic metabolic hormones during 30 days of supplementation period on castrated male goats.
The reports of the effects of β2-agonist administration on metabolite and hormone concentrations in plasma are inconsistent. For example, similar to our results, Zimmerli and Blum (Citation1990) reported that β2-agonists T-3660 increased (P > .05) the plasma insulin level in calves. Increases in insulin obtained in our study are consistent with results obtained when salbutamol (a β2-agonists, Vandenberg et al. Citation1998) and ractopamine (a β1-agonist, Collomp et al. Citation2000) were administered. ZH has an action mediated via stimulation of cAMP production by activation of the enzyme adenyl cyclase (Mersmann Citation1998; Hossner Citation2005). cAMP leads a sequence of intracellular events that lead to the pharmacological effects that include stimulation of insulin secretion (Rizza et al. Citation1980; Schiffelers et al. Citation1999). The repartitioning effects of the β-agonists such as ZH may be due in part to double behaviour (moreover its direct effect on protein metabolism and MHC type) on insulin receptor sensitivity in adipose tissue and skeletal muscle (Anderson et al. Citation1991; Beermann Citation2002). It has proved an increased sensitivity to insulin in muscles that received chronic treatment with β-agonists (Budohoski et al. Citation1998) that can improve the insulin’s role in the muscle protein synthesis (Beermann et al. Citation1987). In addition, it has been demonstrated that most of β-agonists (β1- and β2-agonists) reduce the sensitivity to insulin of adipocytes in rats [ractopamine (β1-agonist); Hausman et al. Citation1989], in pigs [ractopamine, clenbuterol (a potent β2 agonist); Liu and Mills Citation1990] and lambs [cimaterol (which stimulates β1, β2 and β3 receptors); O'Connor et al. Citation1991]. However, some studies show an absence of effects, or even decreases, on plasma insulin levels as a consequence of long-term administration of β2-agonists. In an early study (Beermann et al. Citation1987), the plasma insulin level decreased when was measured after 7 weeks in finishing sheep that were fed cimaterol (cimaterol stimulates β1, β2 and β3 receptors. That it strongly stimulates β1 receptors at a dosage level necessary to stimulate β2). Similarly, decreases in insulin plasma levels in cows that received ZH for 15 days have been reported (Guzman et al. Citation2012). In opposite, Fuller et al. (Citation2014) did not detected changes on blood metabolites in steers supplemented daily for 21 days with 15.7 mg ZH/kg LW. However, it is well known that responses to β-agonist on plasma metabolites are inconsistent and could be greatly influenced by dosage level, duration of treatment, feeding, gender and age (Wilcox Citation2005).
Consistent with our results, increases on Thyroxine (T4) and Triiodothyronine (T3) secretions as a result of chronic administration of β-agonist have been previously reported in sheep that were supplemented with cimaterol (Beermann et al. Citation1987). Beta-adrenergic agonists can stimulate lipolysis in ruminants (Blum & Flueckiger et al. Citation1988) and increase metabolic rate by the route of thyroid hormones enhancement (Beermann et al. Citation1987). Ahrin and Rerup (Citation1983) conclude that β-agonists act in the regulation of thyroid hormone secretion mainly through β2-receptors. In addition, these researchers showed that β-agonists and TSH exert their thyroid hormone secretory effects through different mechanisms. Increases on plasma T3 and T4 can be directed by β-agonist effects on insulin secretion and glucose metabolism. Scheidegger et al. (Citation1983) indicated that the increases in production of thyroid hormones after β2-agonist treatment is a result of an improvement in insulin-mediated glucose metabolism during β-receptor stimulation. Similar effects on T3 and T4 plasma concentrations were detected when isopropylnoradrenaline (β1 and β2 adrenoceptors) and terbutaline (β2 adrenoceptor) were administered (Ahrin and Rerup Citation1983; Collomp et al. Citation2000) in humans. Increases on thyroid plasma concentration were observed in calves with administration of β2-agonist T-3660 and P-5369 (Zimmerli and Blum Citation1990) and in lambs when were treated with cimaterol (O’Connor et al. Citation1991). In the present experiment, the administration of ZH had a potent reducer effect on serum cortisol concentration after 30 days of ZH supplementation. Nonetheless, we did not observe significant differences about general behaviour or visible signs of stress between these treatments groups. The effects on reduction on plasmatic cortisol observed here is in agreement with those obtained by Hayden et al. (Citation1992) when administered β2 agonist terbutaline in growing cattle. Similarly, Carroll et al. (Citation2014) reported a reduction on plasmatic cortisol when finishing heifers were supplemented for 20 days with 0.18 mg ZH/kg LW. In general, glucocorticoids have shown to decrease protein synthesis by affecting rates of translation (Sharpe et al. Citation1986). Nonetheless, Collomp et al. (Citation2000) did not explain the possible ways by which cortisol is decreased (P < .05) by administration of β2-agonist salbutamol. In contrast to our findings, a few researchers did not observe significant differences on cortisol concentrations between β2-agonist and control groups in humans (salmeterol; Kips and Pauwels Citation2001), pigs (salbutamol; Marchant-Forde et al. Citation2008; Moloney et al. Citation1995) and lambs (cimaterol; Beermann et al. Citation1987).
It is clear from the results of this study and from other researches that supplementary investigations are needed to gain a more complete description of the mechanisms underlying the nutrient repartitioning effects of β-agonists (ZH and other similar synthetic compounds) on described metabolic hormones.
3.2. Haematocrit, plasma volume and blood haemoglobin
Effects of treatments on haematocrit, plasma volume and blood haemoglobin on days 1, 10, 20 and 30 are given in . Significant differences (P < .01) on haematocrit, plasma volume and blood haemoglobin values were detected from day 10 in goats that were fed ZH. Even when these parameters increased with ZH supplementation, according to Anosa and Isoun (Citation1976) and Rajaratne et al. (Citation1996), they were within the normal range (haematocrit = 22–38%, plasma volume = 45–70 mL/kg BW and haemoglobin = 8–12 g/dL).
Table 2. Effect of ZH on heart and respiratory rates during 30 days of supplementation period on castrated male goats.
Zilpaterol supplementation decreased the haematocrit. Conversely, it could be stated that zilpaterol supplementation increased plasma volume [Plasma volume, mL = body weight, g × 0.07 × (1-haematocrit), Hopfer et al. Citation2004]. Increases on plasma volume as a response to β2-agonists supplementation has been reported previously in ruminants (Byrem et al. Citation1998) and non-ruminant species (Mersmann Citation1998), and this effect is mainly mediated by renal and extra-renal effects of several β2-agonists (Schrier et al. Citation1972; Pratt and McAteer Citation1989; Nakamura et al. Citation2005). In a surveying report of the effect of ZH on humans, mice, horse and donkeys, it is indicated that ZH administration significantly decreases haemoglobin concentration and haematocrit percent (Intervet Citation2006). Contrary to the latter, Schrier et al. (Citation1972) reported that haematocrit percentage was not affected by β2-agonist salbutamol administered in drinking water in both old and young rats.
3.3. Cardiovascular (heart rate)
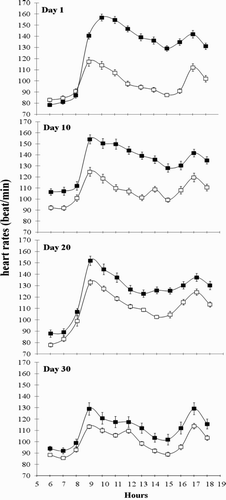
Changes in heart rate recorded at 1-h intervals between 06:00 and 18:00 h of each of days 1, 10, 20 and 30 of experiment are schematized in , while the heart rate average recorded on each day is presented in . The ingestion of ZH increased significantly (P < .001) the heart rate 4 h post-ingestion from the first day of experiment and this effect was maintained until day 10. However, from day 20 the difference decreased (P = .01) to not be significant (P = .07) at the end of the experiment.
Figure 1. Heart rate on days 1, 10, 20, and 30 in group ZH (▪) and group control (□). Animals of group ZH were orally administered 0.2 mg/kg live body ZH (Zilmax, Intervet©, South Africa).
Similar to our results, Frese et al. (Citation2014) observed an increase in heart rate in feedlot steers when were daily supplemented with 8.3 mg ZH/kg of DM. Previous reports confirm the effect of β2-agonist T-3660 and P-5369 (Zimmerli and Blum Citation1990) and clenbuterol (Brockway et al. Citation1987; Hoey et al. Citation1995) on increases in heart rate. However, Bruckmaier and Blum (Citation1992) did not detect changes in heart rate in calves supplemented with clenbuterol after 14 days. Beta-agonists influence on heart rate by stimulation of cardiac β1- and β2-adrenoceptors localized in the sinoatrial node (Bruckmaier and Blum Citation1992). In this regard, Gabriela et al. (Citation2007) indicated that clenbuterol chiefly stimulates the heart rate of guinea-pig by acting on β2-adrenoceptor, although heart responses to clenbuterol are apparently mediated by an inter-play between β1- and β2-adrenoceptors (Gabriela et al. Citation2007). On the other hand, authors emphasize that β-agonists effect on stimulation of β2-adrenoceptors existing on vascular smooth muscle to produce vasodilation and hypotension (Hoey et al. Citation1995), resulting in the activation of pressure-sensitive receptors (known as baroreceptors). This activation leads to a reflex increase in heart rate known as the baroreceptor reflex (Guyton and Hall Citation2006). Cardiovascular effects of ZH can also be the result of metabolic hormonal changes, especially thyroid hormone. It has been confirmed that the thyroid hormone effect on the heart and peripheral vasculature includes decreased systemic vascular resistance and increased resting heart rate, left ventricular contractility and blood volume. Thyroid hormones cause decreased resistance in peripheral arterioles through a direct effect on vascular smooth muscle cells and decreased mean arterial pressure, which, when sensed in the kidneys, activates the renin–angiotensin–aldosterone system and increases renal sodium absorption (Klein and Danzi Citation2007). Furthermore, it has been proved that the hormones also regulate cardiovascular function indirectly through the central nervous system (Mittag et al. Citation2013).
As evident from the results, initial supplementation of ZH has had more effect on heart rate (averagely by 30.18% on day 1 compare to control) and only elevated almost by 14 and 11% seen at the days 20 and 30 of ZH supplementation, respectively. In this regard, Hoey et al. (Citation1995) explained that after a few days, baroreceptors will be adapted to hypotension, and therefore input from the baroreceptors returns to the medulla as approximately normal impulses (Guyton and Hall Citation2006). This adaption returns the heart rate to the near-control level and prevents from resulting in a continuously enhanced heart rate.
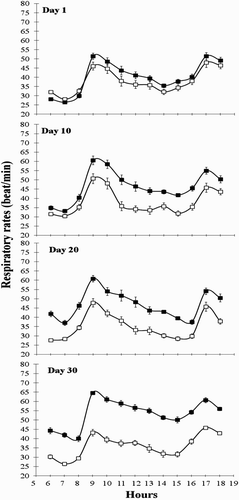
3.4. Respiratory rate
The effects of ZH supplementation on respiratory rate are given in and . The respiratory rate was affected (P < .01) by ZH supplementation from day 10 and this effect was exacerbated conforming to the time of consumption of ZH was prolonging. Based on the behaviour of cardiac rate, the absence of effect of ZH on respiratory rate during first hour of administration is surprising. The way in which the respiratory rate was measured (visually) could be a factor limited the possibility to detect differences during first hours of goats that were fed ZH. Zimmerli and Blum (Citation1990) reported that administration of β2-agonist P-5369 (25 µ/kg LW) and T-3660 (2700 µ/kg LW) in milk replacer caused increases in respiratory rate in calves at days 1, 14 and 28. Likewise, Bruckmaier and Blum (Citation1992) showed the significant effects of a β2-agonist clenbuterol administration on respiratory rate of calves since day 1, and its effects were maintained at day 14 and day 28. In addition, AVMA (Citation2014) announced that cattle receiving ZH may exhibit increased respiratory rate.
4. Conclusion
It can be concluded that zilpaterol supplementation of 0.20 mg ZH/kg LW did not provoke stress, as indicated by plasma cortisol levels. However, the concentration of metabolic blood hormones, and cardiovascular and respiration rates were significantly affected. Nevertheless, during phases of ZH administration the differences in respiration rates between controls and zilpaterol-supplemented goats increased with time on feed; on contrary, differences in heart rates decreased.
Acknowledgements
The authors are grateful for the assistance rendered by following persons: Salman Nasrollahi and Adel Moftakharzadeh aiding in the rearing, slaughtering and preparing laboratory reports of experimental animals, Zeinab Shamani and Seyed Mohammad Taghi Gharibzahedi for their assistance in writing the technical report.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahrin B, Rerup C. 1983. Effects of β-adrenoceptor agonists and antagonists on thyroid hormone secretion. Eur J Pharmacol. 88:383–387. doi: 10.1016/0014-2999(83)90590-3
- American Veterinary Medical Association (AVMA). 2014. Literature review on the welfare implications of the use of β-adrenoreceptor agonists. [Cited 2016 May]. Available from: https://www.avma.org/KB/Resources/LiteratureReviews/Documents/Welfare%20Implications%20of%20the%20Use%20of%20B-Adrenoreceptor%20Agonists.pdf
- Anderson DB, Veenhuizen EL, Schroeder AL, Jones DJ, Hancock DL. 1991. The use of phenethanolamines to reduce fat and increase leanness in meat animals. In: Proceedings of Symposium on fat and cholesterol reduced foods—advances in applied biotechnology series. New Orleans, LA: Portfolio Publishing Company; p. 43–73.
- Anosa VO, Isoun TT. 1976. Serum proteins, blood and plasma volumes in experimental Trypanosoma vivax infections of sheep and goats. Trop Anim Health Prod. 8:14–19. doi: 10.1007/BF02383360
- Avendaño-Reyes L, Torres-Rodríguez V, Meraz-Murillo FJ, Pérez-Linares C, Figueroa-Saavedra F, Robinson PH. 2006. J Anim Sci. 84:3259–3265. doi: 10.2527/jas.2006-173
- Beermann DH. 2002. Beta-Adrenergic receptor agonist modulation of skeletal muscle growth. J Anim Sci. 80:18–23.
- Beermann DH, Hogue DE, Fishell VK, Dalrymple RH, Ricks CA, Scanes CG. 1987. Cimaterol-induced muscle hypertrophy and altered endocrine status in lambs. J Anim Sci. 65:1514–1524.
- Blum JW, Flueckiger N. 1988. Early metabolic and endocrine effects of perorally administered ß-adrenoceptor agonists in calves. Eur J Pharmacol. 151:177–187. doi: 10.1016/0014-2999(88)90798-4
- Brockway JM, MacRae JC, Williams PE. 1987. Side effects of clenbuterol as a repartitioning agent. Vet Rec. 120:381–383. doi: 10.1136/vr.120.16.381
- Bruckmaier RM, Blum JW. 1992. Responses of calves to treadmill exercise during beta-adrenergic agonist administration. J Anim Sci. 70:2809–2821.
- Budohoski L, Challiss RAJ, Dubaniewicz A, Kaciuba-Uscilko H, Leighton B, Lozeman FJ, Nazar K, Byrem TM, Beermann DH, Robinson TF. 1998. The beta-agonist cimaterol directly enhances chronic protein accretion in skeletal muscle. J Anim Sci. 76:988–998.
- Byrem TM, Beermann DH, Robinson TF. 1998. The beta-agonist cimaterol directly enhances chronic protein accretion in skeletal muscle. J Anim Sci. 76:988–998.
- Carroll J, Sanchez N, Buntyn J, Sieren S, Jones S, Schmidt T. 2014. Supplementation of zilpaterol hydrochloride to crossbred Angus heifers does not increase stress responsiveness or homeostatic metabolic parameters after a combined corticoptropin releasing hormone and vasopressin challenge. J Anim Sci. 92E;Suppl 2:76 (Abstract).
- Collomp K, Candau R, Lasne F, Labsy Z, Préfaut C, De Ceaurriz J. 2000. Effects of short-term oral salbutamol administration on exercise endurance and metabolism. J Appl Physiol. 89:430–436.
- Estrada-Angulo A, Barreras-Serrano A, Contreras G, Obregon JF, Robles-Estrada JC, Plascencia A, Zinn RA. 2008. Influence of level of zilpaterol chlorhydrate supplementation on growth performance and carcass characteristics of feedlot lambs. Small Rum Res. 80:107–110. doi: 10.1016/j.smallrumres.2008.09.006
- Frese DA, Reinhardt C, Bartle SJ, Rethorst DN, Bawa BS, Thomason JD, Loneragan GH, Thomson D. 2014. Effect of ractopamine hydrochloride and zilpaterol hydrochloride on the electrocardiogram and blood lactate in finishing steers. J Anim Sci. 92E;Suppl 2:248 (Abstract).
- Fuller AL, Covey TL, Lawrence TE, Richeson JT. 2014. Effects of ractopamine or zilpaterol on physiologic and metabolic parameters in feedlot steers. J Anim Sci. 92;Suppl 2:23 (Abstract).
- Gabriela M, Di Sotto A, Daniele C, Battinelli L, Brambilla G, Fiori M, Loizzo S, Loizzo A. 2007. A pharmacodynamic study on clenbuterol-induced toxicity: β1- and β2-adrenoceptors involvement in guinea-pig tachycardia in an in vitro model. Food Chem Toxicol. 45:1694–1699. doi: 10.1016/j.fct.2007.03.002
- Guyton A, Hall J. 2006. Textbook of medical physiology. Philadelphia (PA): Elsevier.
- Guzman A, Gonzalez-Padilla E, Garcés-Yépez P, Rosete-Fernandez JV, Calderón-Robles RC, Murcia C, Gutiérrez CG. 2012. Reduced response to an estrous induction program in postpartum beef cows treated with zilpaterol and gaining body weight. Anim Reprod Sci. 130:1–8. doi: 10.1016/j.anireprosci.2011.12.001
- Hatefi A, Towhidi A, Zali A, Zeinoaldini S, Ganjkhanlou M, Masoudi R, Plascencia A. 2015. Influence of dietary zilpaterol hydrochloride on feedlot performance, carcass traits, chemical composition of longissimus muscle, and plasma metabolites of castrated male goats. Turk J Vet Anim Sci. 39:195–202. doi: 10.3906/vet-1405-14
- Hausman DB, Martin RJ, Veenhuizen EL, Anderson DB. 1989. Effect of ractopamine on insulin sensitivity and response of isolated rat adipocytes. J Anim Sci. 67:1455–1464.
- Hayden JM, Bergen WG, Merkel RA. 1992. Skeletal muscle protein metabolism and serum growth hormone, insulin, and cortisol concentrations in growing steers implanted with estradiol-17β, trenbolone acetate, or estradiol-17β plus trenbolone acetate. J Anim Sci. 70:2109–2119.
- Hoey AJ, Matthews ML, Badran TW, Pegg GG, Sillence MN. 1995. Cardiovascular effects of clenbuterol are beta 2-adrenoceptor-mediated in steers. J Anim Sci. 73:1754–1765.
- Hopfer SM, Nadeau FL, Sundra M, Makowski GS. 2004. Effect of protein on hemoglobin and hematocrit assays with a conductivity-based point-of-care testing device: comparison with optical methods. Ann Clin Lab Sci. 34:75–82.
- Hossner KL. 2005. Hormoral regulation of farm animal growth. Wallingford: CABI Publishing; p. 191–201.
- Intervet Inc. 2006. Freedom of information summary original. New animal application. p. 141–258 [Cited 2016 may]. Available from http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/ucm051412.pdf.
- Kips J, Pauwels R. 2001. Long-acting inhaled β2-agonist therapy in asthma. Am J Respir Crit Care Med. 164:923–932. doi: 10.1164/ajrccm.164.6.2010107
- Klein I, Danzi S. 2007. Thyroid disease and the heart. Circulation. 116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326
- Liu CY, Mills SE. 1990. Decreased insulin binding to porcine adipocytes in vitro by beta-adrenergic agonists. J Anim Sci. 68:1603–1608.
- López-Carlos MA, Aguilera-Soto JI, Ramírez RG, Rodríguez H, Carrillo-Muro O, Méndez-Llorente F. 2014. Effect of zilpaterol hydrochloride on growth performance and carcass characteristics of wether goats. Small Rum Res. 117:142–150. doi: 10.1016/j.smallrumres.2013.12.035
- Marchant-Forde JN, Lay DC Jr, Marchant-Forde RM, McMunn KA, Richert BT. 2008. The effects of R-salbutamol on behavior and physiology of finishing pigs. J Anim Sci. 86:3110–3124. doi: 10.2527/jas.2008-1075
- Merck, Animal Health. [Cited 2016 Jun 17]. Available from http://www.merck-animal-health.com/binaries/Zilmax_Type_A_Medicated_Article_Label__FINAL__tcm95-165067.pdf.
- Mersmann HJ. 1998. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci. 76:160–172.
- Mittag J, Lyons DJ, Sällström J, Vujovic M, Dudazy-Gralla S, Warner A, Wallis K, Alkemade A, Nords K, Monyer H, et al. 2013. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest. 123:509–516. doi: 10.1172/JCI65252
- Moloney AP, Allenb P, McHugh TV, Quirke JF. 1995. Effects of cimaterol on Finnish-Landrace wether lambs. 1. Feed conversion efficiency, body composition and selected plasma hormone and metabolite concentrations. Liv Prod Sci. 42:23–33. doi: 10.1016/0301-6226(94)00071-E
- Nakamura A, Imaizumi A, Yanagawa Y. 2005. Beta 2-adrenoceptor function in the kidney. Folia Pharmacol JPN. 24:427–434.
- NRC. 2007. Nutrient requirement of small ruminant. Sheep, goats, cervids, and new world camelids. Washington, DC: National Academy Press.
- O’Connor RM, Butler WR, Finnerty KD, Hogue DE, Beermann DH. 1991. Temporal pattern of skeletal muscle changes in lambs fed cimaterol. Domest J Anim Endocrinol. 8:549–554. doi: 10.1016/0739-7240(91)90024-E
- Plascencia A, Torrentera NG, Zinn RA. 2008. Influence of the ß-agonist, zilpaterol, on growth performance and carcass characteristics of feedlot steers. J Anim Vet Adv. 7:1257–1260.
- Pratt JH, McAteer JA. 1989. Beta-adrenergic enhancement of angiotensin II-stimulated aldosterone secretion. Life Sci. 44:2089–2095. doi: 10.1016/0024-3205(89)90356-1
- Rajaratne AAJ, Hydbring E, Dhalborn K, Olsson K. 1996. Indocyanine green dye as a marker for measurement of plasma volume in goats. Small Rum Res. 19:83–86. doi: 10.1016/0921-4488(95)00730-X
- Ríos Rincón FG, Barreras-Serrano A, Estrada-Angulo A, Obregón JF, Plascencia-Jorquera A, Portillo-Loera JJ, Zinn RA. 2010. Effect of level of dietary zilpaterol hydrochloride (β2-agonist) on performance, carcass characteristics and visceral organ mass in hairy lambs fed all-concentrate diets. J Appl Anim Res. 38:33–38. doi: 10.1080/09712119.2010.9707150
- Rizza RA, Cryer PE, Haymond MW, Gerich JE. 1980. Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metabolism. 29:1155–1163. doi: 10.1016/0026-0495(80)90025-6
- SAS 2004. SAS/STAT user’s guide: Version 9.1. Cary, NC: SAS Institute Inc.
- Scheidegger K, Robbins DC, Danforth E. 1983. Effects of chronic beta-agonist on glucose metabolism (abstract). Diabetes. 32:73A.
- Schiffelers SL, van Harmelin VJ, de Grauw HA, Saris WH, van Baak MA. 1999. Dobutamine as selective b1-adrenoceptor agonist in in vivo studies on human thermogenesis and lipid utilization. J Appl Physiol. 87:977–981.
- Schrier R, Lieberman R, Ufferman R. 1972. Mechanism of antidiuretic effect of beta adrenergic stimulation. J Clin Invest. 51:97–111. doi: 10.1172/JCI106803
- Sharpe PM, Haynes NB, Buttery PJ. 1986. Glucocorticoid status and growth. In: Buttery PJ, Lindsay DB, Haynes NB, editors. Control and manipulation of animal growth. London: Butterworths; p. 207–222.
- Vandenberg GW, Leatherland JF, Moccia RD. 1998. The effects of the beta-agonist ractopamine on growth hormone and intermediary metabolite concentrations in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture Res. 29:79–87. doi: 10.1111/j.1365-2109.1998.tb01112.x
- Wilcox G. 2005. Insulin and insulin resistance. Clin Biochem Rev. 26:19–39.
- Zimmerli UV, Blum JW. 1990. Acute and longterm metabolic, endocrine, respiratory, cardiac and skeletal muscle activity changes in response to perorally administered β-adrenoceptor agonists in calves. J Anim Physiol Anim Nutr. 63:157–172. doi: 10.1111/j.1439-0396.1990.tb00131.x