ABSTRACT
The purpose of this study was to evaluate the effects of inoculation of Lactobacillus buchneri (L. buchneri) alone or with Bacillus subtilis (B. subtilis) on gas and effluent losses, chemical composition, in vitro digestibility of nutrients, aerobic stability, and microbiological quality of sunflower silage. A randomized experimental design was used, which contained 3 treatments, each one included 15 mini-silos. Mini-silos were distributed to the following treatments: (1) Control (CON), without inoculants; (2) L. buchneri alone (LB), inoculation at 2.6 × 1010 cfu/g; and (3) L. buchneri with B. subtilis (LB + BS), inoculation at 2.6 × 1010 cfu/g and 1 × 109 cfu/g with L. buchneri and B. subtilis, respectively. Treatments were applied at 2 g/t of fresh forage ensiled. Silages with microbial inoculants had lower DM content, and higher in vitro digestibility of DM and neutral detergent fibre than CON. Inoculants decreased the number of aerobic bacteria, mould, and yeast, and increased the counts of lactic acid bacteria in sunflower silage. L. buchneri exhibited positive effects on aerobic stability, in vitro digestibility of nutrients and decreased the counts of mould and yeast, but did not show a synergistic effect with B. subtilis on sunflower silage.
1. Introduction
The whole sunflower plant (Helianthus annuus L.) is an annual dicotyledonous (legume) of Compositae family native from North America and has been highlighted as an energy and protein dietary source for ruminants (Domingues et al. Citation2010). Sunflower plant is tolerant to water scarcity, may be used as a plant to crop rotation in all regions of Brazil, and it can be ensiled (Eltz et al. Citation2010; Goes et al. Citation2012). Comparing with corn silage, the sunflower silage has higher levels of crude protein (CP) and ether extract (EE; Bueno et al. Citation2004; Goes et al. Citation2012).
In general, legume silages have a higher pH than corn or grass silages and take longer to ensile because of their high buffering capacity, especially because of their moderate levels of ammonia nitrogen (Rodrigues et al. Citation2001). Bacillus subtilis (B. subtilis) are lactic acid producers by the reduction of pyruvate under anaerobic conditions (Ramos et al. Citation2000). Efficient lactic acid bacteria may increase acidification rate, decreasing the final pH and reducing ammonia production from proteolysis in silages (Weinberg & Muck Citation1996; Driehuis et al. Citation1997). On the other hand, studies have shown that silage aerobic stability can be jeopardized by high concentrations of lactic acid (Weinberg et al. Citation1993; Hu et al. Citation2009).
Although the major objective of adding microbial additives in silage is to ensure the fermentation process to produce well-preserved silages, minimize losses due to aerobic exposition and silage deterioration should be also taken into consideration. Lactobacillus buchneri (L. buchneri) is one of the most studied bacterial inoculant to silage, and since 1990 it has been evaluated in order to metabolize the excess of lactic acid to acetic acid and 1,2-propanediol, under anaerobic conditions promoting forage stability (Weinberg & Muck Citation1996; Mayrhuber et al. Citation1999).
Combining lactic-acid-bacteria producers with L. buchneri has been proposed in order to accelerate fermentation and improve aerobic stability (Driehuis et al. Citation2001; Adesogan & Salawu Citation2004), but there is no data to our knowledge that examined both types of bacterial inoculants on sunflower silage. The objective of this study was to evaluate the effects of inoculation of L. buchneri alone or with B. subtilis on gas and effluent losses, chemical composition, in vitro digestibility of nutrients, aerobic stability, and microbiological quality of sunflower silage. Our hypothesis was that inoculants would reduce silage pH and improve its aerobic stability.
2. Materials and methods
2.1. Treatments and ensiling
The experiment was conducted at Animal Science Department – School of Agrarian Sciences of Federal University of Grande Dourados, Dourados, Mato Grosso do Sul, Brazil. This study was performed from January to September 2015, 22°14′S latitude, 54°49′W longitude and 450 m altitude. The sunflower plant variety BRS 321 was manually harvested 105 days after planting (at the beginning of vegetative stage - R9) from 10 locations within one 0.35-ha plot. Approximately 70 kg of sunflower tillers from each location was separately chopped in a stationary cutter to a theoretical cut length of 10 mm.
Prior to ensiling, samples (1000 g) were stored at −20°C for further chemical analyses of DM (method 950.15), ash (method (942.05), organic matter (OM, DM – ash), crude protein (CP, N × 6.25; method 984.13), and ether extract (EE, method 920.39) according to AOAC (Citation2000). Neutral detergent fibre (aNDF, without sodium sulphite), acid detergent fibre (ADF) and lignin (sulphuric acid method) were determined according to Van Soest et al. (Citation1991). Net energy of lactation was estimated according to NRC (Citation2001). The chemical composition of sunflower plant after harvesting is shown in .
Table 1. Chemical composition of sunflower plant (g/kg DM, otherwise stated).
A randomized experimental design was used, which contained 3 treatments, each one included 15 mini-silos. Ensiling process was carried out in plastic buckets (30 cm of height and 30 cm of diameter) containing Bunsen valves to avoid gas penetration and allow gas scape. Two kilograms of sand were placed at the bottom of mini-silos and were separated from forage by a nylon screen. Silos were packed to a density of 650 kg/m3 (fresh forage), then silos were sealed, weighed, and stored at room temperature (28.5 ± 2.3°C) for 60 days. The silo’s density was achieved after silo volume calculation, as follows: V = πr2 × h, where r is the radius and h is the height of the plastic bucket. The calculated volume of mini-silos was approximately 0.022 m3, thus by adding 13.5 kg of fresh forage of sunflower plant in each bucket the desired density is achieved. Before the silos opening, they were weighed to record DM and gas losses.
Mini-silos were distributed to the following treatments: (1) Control (CON), without inoculants; (2) L. buchneri alone (LB), inoculation at 2.6 × 1010 cfu/g; and (3) L. buchneri with B. subtilis (LB + BS), inoculation at 9 × 109 cfu/g and 1 × 109 cfu/g with L. buchneri and B. subtilis, respectively. Bacterial inoculants were obtained from Biocampo Nutricao Animal Importação e Exportacao Animal Ltda. (Presidente Prudente, Brazil). Treatments were applied at 2 g/t of fresh forage ensiled. All inoculants were diluted in water (2 g/L), sprayed into the forage from each bucket, and same amounts of water were added to CON mini-silos.
2.2. Fermentative and effluent losses
On days 15, 30, 45, and 60 relative to ensiling, mini-silos were weighed to determine gas losses. Mini-silos were opened on day 60 and then silage content, silage assembly, sand layer, and nylon screen to determine effluent production. Gas losses were calculated as follows:
where GL is the gas losses (% DM), SWE is the silo weight at the ensiling (kg), SWO is the silo weight at the opening (kg), and DME is the dry matter ensiled (amounts of forage in kg × % DM).
Effluent production was calculated according to the equation:
where EP is the effluent production (kg of effluent by ton of fresh forage ensiled), WSAO is the weight of the silo assembly after the opening (kg), WSAE is the weight of the silo before the ensiling, and DME is the dry matter ensiled (kg).
Dry matter recovery (DMR) was calculated as
where FDM is the dry matter after the silos opening (kg) and IDM is the dry matter before the ensiling (kg).
Changes in the DM content were calculated as the difference in module of DM percentage at the ensiling moment and the DM percentage at the opening.
2.3. Chemical composition
After the silos opening, the sunflower silage was homogenized and one sample (500 g) of each bucket was collected to extract silage juice using a hydraulic press and pH was measured using a digital potentiometer (MB-10, Marte, Santa Rita do Sapucaí, Brazil). Aliquots (2 ml) of silage juice were transferred to test tubes containing sulphuric acid (1 ml at 1N) and analysed for ammonia nitrogen by a colorimetric method as described by Kulasek (Citation1972) and adapted by Foldager (Citation1977). Titratable acidity was determined according to AOAC (Citation2000, method 942.15).
At the silos opening, one sample (500 g) of each mini-silo was also collected to determine the DM, OM, CP, EE, NDF, ADF, lignin, ash, and net energy of lactation, as previously described. Non-fibre carbohydrate (NFC) was calculated according to Hall (Citation2003): NFC = 100 – (NDF + CP + EE + ash). Total digestible nutrient (TDN) was calculated as: TDN = (digestible NFE + digestible CF + digestible CP + (digestible EE × 2.25) – 7) (NRC Citation2001). The in vitro digestibility of DM, NDF, and ADF was performed using filter bags method using an artificial rumen incubator (TE-150, Tecnal, Piracicaba, Brazil) as described by Tilley and Terry (Citation1963) and adapted by Holden (Citation1999).
2.4. Aerobic stability
After opening the silos, silage temperature was measured every eight hours during five days using an infrared digital thermometer (MS6530, Wiltronics Research Pty. Ltd., Alfredton Victoria, Australia). Aerobic stability was defined as the period (hours) in which the silage temperature remained stable before rising more than 1°C above the room temperature (Driehuis et al. Citation2001). During the aerobic stability determination, one bucket was randomly selected to collect samples (200 g) every 24 h to determine DM and pH after oxygen exposure (Kung et al. Citation1984).
2.5. Microbiological quality
On days 60, three mini-silos of each treatment were opened to collect samples (100 g) for microbiological determination. Samples were collected from different locations of all mini-silos, homogenized and composited into one sample for each mini-silo. Sub-samples (10 g) from the composite samples were diluted in sterilized sodium chloride solution (90 ml at 0.9%) and then a serial dilution (10−1 until 10−6) was performed in test tubes. Microbial counts were performed in triplicate using decimal dilution series on plates containing: MRS agar (De Man, Rogosa and Sharpe) for lactic acid bacteria as described in Briceño and Martinez (Citation1995) nutrient agar for aerobic and anaerobic bacteria (48 h of incubation at 30°C) and PDA (potato dextrose agar, 120 h of incubation at 26°C) for mould and yeast as described by Rabie et al. (Citation1997). The absolute values were obtained from colony-forming units and then log transformed.
2.6. Statistical analyses
The data were submitted to analysis of variance, after verifying residuals normality and variance homogeneity, using the MIXED procedure of SAS (9.3 version) following the model below:
where Yi is the dependent variable, µ is the overall mean, and Ti is the fixed effect of treatment. Degrees of freedom were calculated according to Satterthwaite method (ddfm = satterth). Microbiology data were log transformed.
Data of pH and DM losses during the aerobic stability evaluation were submitted to the MIXED procedure as repeated measures following the model below:
where Yij is the dependent variable, µ is the overall mean, Ti is thefixed effect of treatment, hj is the random effect of time (hours), Ti*hj is the treatment by time interaction random effect, and eij is the residual error.
Differences among treatments were defined by Tukey test, and significance level was set at P ≤ .05.
3. Results
3.1. Fermentative and effluent losses
Sunflower silage inoculated with LB had the lowest gas losses (%), whereas CON showed intermediate and LB + BS the highest value of gas losses (P = .016, ). On the other hand, LB exhibited the highest gas losses (% DM), LB + BS showed intermediate values and CON the lowest values of gas losses (P = .040).
Table 2. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on fermentative and effluent losses of sunflower silage.
The lowest effluent loss (kg/t) was observed in silages inoculated with LB, and intermediate and high losses were observed for LB + BS and CON, respectively (P = .043). Inoculants (LB or LB + BS) decreased (P = .001) the effluent losses (% DM) compared to CON. The silages-treated LB had the highest value of total losses (% DM), those treated with LB + BS exhibited intermediate values, and CON, the lowest value of total losses (P = .033). Of all treatments, inoculants decreased (P = .001) the DM recovery (% DM).
3.2. Chemical composition and in vitro digestibility
Treatments did not affect pH and ammonia nitrogen concentration of sunflower silage (P ≥ .114, ). LB + BS decreased (P = .031) the titratable acidity of silages when compared to LB or CON. Both microbial inoculants decreased (P = .001) the DM content of sunflower silage. The highest level of EE was observed when silages were treated LB, whereas intermediate levels were observed for CON, and lowest level of EE was observed for those silage treated LB + BS (P = .004). Inoculants showed positive effects on in vitro digestibility of nutrients, promoting greater (P ≤ .05) DM, OM, and NDF digestibility compared to CON.
Table 3. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on chemical composition and in vitro digestibility of sunflower silage at the silo opening.
3.3. Aerobic stability
Treatments did not (P = .516) affect the maximum temperature of silage. Inoculants increased (P = .001) the accumulated temperature over the aerobic stability evaluation, in which LB + BS had the highest temperature, LB showed intermediate values, and CON exhibited the lowest value of accumulated temperature in silages (). Treatments did not (P = .743) affect the temperature of sunflower silage stability.
Table 4. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on 5-d aerobic stability of sunflower silage.
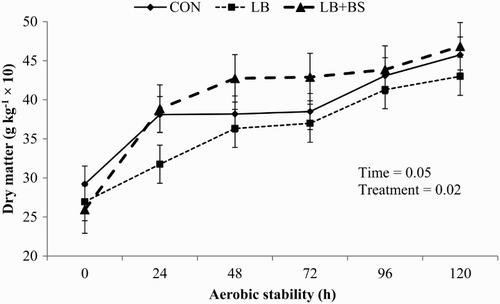
Inoculants increased (P = .001) the period (hours) of which silage maintained stable. Bacterial inoculants diminished (P = .034) silage juice pH, whereas LB alone had the lowest pH, LB with BS exhibited intermediate values, and CON showed the highest pH of treatments. Interestingly, LB + BS had the highest DM losses, CON showed intermediate values, and LB the lowest values of DM losses (P = .006). No interaction effects were observed for pH and DM losses, just a progressive increase of values according to the time ( and ).
Figure 1. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on sunflower silage pH during 5-d of oxygen exposure (mean ± SD). Note: Control (CON), without inoculants; L. buchneri alone (LB), inoculation at 2.6 × 1010 cfu/g; and L. buchneri with B. subtilis (LB + BS), inoculation at 9 × 109 cfu/g and 1 × 109 cfu/g with L. buchneri and B. subtilis, respectively. Treatments were applied at 2 g/t of fresh forage ensiled.
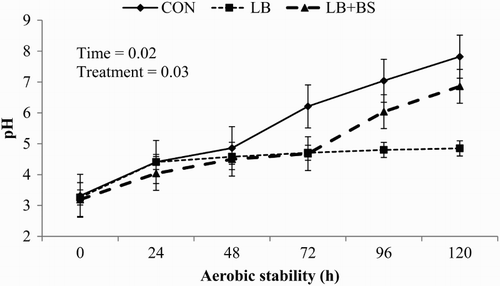
Figure 2. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on dry matter losses of sunflower silage during 5-d of oxygen exposure (mean ± SD). Note: Control (CON), without inoculants; L. buchneri alone (LB), inoculation at 2.6 × 1010 cfu/g; and L. buchneri with B. subtilis (LB + BS), inoculation at 9 × 109 cfu/g and 1 × 109 cfu/g with L. buchneri and B. subtilis, respectively. Treatments were applied at 2 g/t of fresh forage ensiled.
3.4. Microbiological quality
Treatments did not affect total bacteria (P = .316) and anaerobic bacteria (P = .0743) counting (). Silages-treated LB or LB + BS had lower (P = .001) number of aerobic bacteria, mould, and yeast. Silages inoculated LB or LB + BS also had higher number of lactic bacteria than CON. Finally, silage treated with LB had lower count of mould and yeast than those treated with LB + BS.
Table 5. Influence of inoculation of Lactobacillus buchneri alone or with Bacillus subtilis on microbiology of sunflower silage at the silo opening.
4. Discussion
The sunflower silage treated with LB exhibited higher values of gas losses (% DM) and total losses than CON and LB + BS, which may be attributed to the CO2 production by heterolactic fermentation of L. buchneri and secondary fermentation (Bernardes et al. Citation2008). Heterelolactic fermentation is considered undesirable in relation to homolactic fermentation since it promotes a higher DM loss due to production of ethanol and CO2, which does not occur in homolactic fermentation (McDonald et al. Citation1991). Although treated silages had lower effluent losses compared to CON, they showed lower DM recovery, which is related to the high gas production and total losses (% DM) of inoculated silages. In addition, the lower DM content of treated silages compared to CON directly influenced DM recovery calculation.
Titratable acidity is an assay that has a minimal value when total acid levels are not available. Although titratable acidity is highly correlated with acid levels in corn silage, that correlation is not applied for legumes because of their greater buffering capacity (Ward and Ondarza, private communication, October, 2008). The DM content ranged from 259.4 to 292.1 g/kg for LB and CON, respectively, whereas microbial inoculants decreased sunflower silage DM content. Silages produced with high humidity level are more likely to compression, which triggers the cell wall rupture, releasing cellular content and consequently nutrient losses (Hu et al. Citation2009; Rabelo et al. Citation2012). Data evaluating bacterial inoculant on chemical characteristics of sunflower silage are scarce in literature. Agreeing with our results, authors found a decrease in DM content when producing sunflower silage treated with lactic acid bacteria mixture, and associated this result with lactic acid bacteria growth and consumption of nutrients (Konca et al. Citation2015). The current study exhibited an increase in lactic acid colony-forming units when adding bacterial inoculants, partially explaining the lower DM content of silages. However, Ozduven et al. (Citation2009) added a mixture of lactic acid bacteria (L. plantarum and Enterococcus faecium) in sunflower silage and did not report differences on DM content.
Silages treated with LB + BS had lower EE content compared to LB. It was not expected to find differences on silage EE content between those treatments since they showed similar DM content and bacteria do not use EE for growth. The majority of studies evaluating microbial additives did not find differences in sunflower silage EE content (Ozduven et al. Citation2009; Konca et al. Citation2015). Microbially inoculated silages had lower DM content, and higher in vitro digestibility of DM, organic matter, and NDF than CON. Aksu et al. (Citation2004) evaluated a mixture of homofermentative and heterofermentative lactic acid bacteria on corn silages and reported no differences on nutrient composition, but found increased DM and NDF digestibility in sheep. Muck (Citation1993) suggested that rapid pH drop of microbial inoculated silages causes additional acid hydrolysis of cell-wall components, promoting a more rapid and extensive digestion.
The pH values of CON and LB + BS silages increased from the first day until the fifth day of aerobic exposure, but the silages treated with LB showed an increase in pH values during the first 24 hours and stabilized until 5 days of aerobic exposure. The CON showed higher pH values during the 120 hours of aerobic exposure than other treatments. After the silo opening, aerobic microorganisms start to develop and may use lactic acid as an energy source, which determines the increase in pH values when silages are exposed to the aerobic environment (Weinberg et al. Citation1993). Kleinschmit and Kung (Citation2006) provided a summary of 43 experiments evaluating the influence of L. buchneri on silage quality; these authors reported that L. buchneri reduced the amounts of acetic acid in silage and consequently increased silage pH. Kleinschmit and Kung (Citation2006) also reported reduced yeast counts and improved aerobic stability when adding L. buchneri to corn silage. L. buchneri ferments lactic acid to acetic acid and 1,2 propanediol (Holzer et al. Citation2003). Danner et al. (Citation2003) stated that acetic acid acts as an inhibitor of the growth of spoilage organisms while 1,2-propanediol have no effect on aerobic stability of corn silage. Although B. subtilis produces high amounts of lactic acid, the combination of LB + BS seems to not impair aerobic stability of sunflower silage.
The maximum temperature of silages may be considered stable (mean of 26.04°C) and did not exceed more than 1.5°C in relation to the environment temperature during the 5 days of aerobic exposure. However, the accumulated temperature during the 5 days of aerobic exposure was higher for silages treated with LB + BS than CON or LB.
Hill and Leaver (Citation2002) reported that the increase in silage temperature occurs according to the balance between the rate of heat production of microbial activity and the heat losses by CO2, which is directly related with the DM oxidation. Nevertheless, the increase in accumulated temperature observed in silage treated LB + BS was not sufficient to increase the DM losses compared to the other treatments.
Bernardes et al. (Citation2003) reported that silages of tropical forages with low DM content (<30%) are more prone to bacterial deterioration due to fermentation instability, high moisture and low substrate for yeast. In the present study, despite the average DM content of silage maintained below of 30% and pH values lower than 3.31, the CON silage had higher concentrations of aerobic bacteria and yeast and moulds when compared to the treated silages, suggesting deterioration process. On the other hand, silages with DM content higher than 35% may provide favourable conditions for the appearance of fungi, yeast and aerobic microorganisms because of the difficulty of compaction, which does not allow the air expulsion, leading to decrease in the nutritional value of silages (Gurbuz & Kaplan Citation2008). According to Muck (Citation2004), the opposite occurs in high-quality silages, including corn and sorghum silages, which are deteriorated mainly by filamentous fungi and yeast, increasing the temperature of silos (McDonald et al. Citation1991). The presence of enterobacter makes the silage more stable during aerobic conditions, because of production of compounds which inhibit the yeast.
5. Conclusion
Microbial inoculants (L. buchneri alone or in combination with B. subtilis) decreased DMR, but they increased in vitro digestibility of nutrients of sunflower silage. In addition, both inoculants improved silage pH after oxygen exposure and also enhanced microbiological quality of sunflower silage by decreasing aerobic bacteria and spoilage microorganism count and increased lactic acid bacteria count. The combination of L. buchneri with B. subtilis did not show synergetic effects.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adesogan AT, Salawu MB. 2004. Effect of applying formic acid, heterolactic bacteria or homolactic and heterolactic bacteria on the fermentation of bi-crops of peas and wheat. J Sci Food Agric. 84:983–992.
- Aksu T, Baytok E, Bolat D. 2004. Technical note: effects of a bacterial silage inoculant on corn silage fermentation and nutrient digestibility. Small Rum Res. 55:249–252.
- [AOAC] Association of Official Analytical Chemistry. 2000. Official methods of analysis, 17th ed. Arlington, VA: AOAC.
- Bernardes TF, Reis RA, Amaral RC, Siqueira GR, Roth APTP, Roth MTP, Berchielli TT. 2008. Perfil fermentativo, estabilidade aeróbica e valor nutritivo de silagens de capim-marandu ensilado com aditivos. R Bras Zootec. 37:1728–1736.
- Bernardes TF, Reis RA, Shocken-Iturrino RP. 2003. Dinâmica microbiológica e alterações químicas das silagens de capim-Marandu (Brachiariabrizanthacv. Marandu) após a abertura dos silos. In: Reunião Anual da Sociedade Brasileira de Zootecnia; 2003; 40, Santa Maria. Proceedings … Santa Maria: Sociedade Brasileira de Zootecnia.
- Briceño AG, Martínez R. 1995. Comparison of methods for the detection and enumeration of lactic acid bacteria. Arch Latinoam Nutr. 45:207–212.
- Bueno MS, Ferrari J, únior E, Possenti RA, Bianchini D, Leinz FF, Rodrigues CFC. 2004. Desempenho de cordeiros alimentados com silagem de girassol ou de milho com proporções crescentes de ração concentrada. R Bras Zootec. 33:1941–1948.
- Danner H, Holzer M, Mayrhuber E, Braun R. 2003. Acetic acid increases stability of silage under aerobic conditions. Appl Environ Microbiol. 69:562–567.
- Domingues AR, Silva LDF, Ribeiro ELA, Castro VS, Barbosa MAAF, Mori RM, Vieira MTL, Silva JAO. 2010. Consumo, parâmetros ruminais e concentração de ureia plasmática em novilhos alimentados com diferentes níveis de torta de girassol em substituição ao farelo de algodão. Semina: Anim Sci. 31:1059–1070.
- Driehuis F, Oude Elferink WH, Van Wikselaar PG. 2001. Fermentation characteristics and aerobic stability of grass silage inoculant with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci. 56:330–343.
- Driehuis F, van Wikselaar PG, van Vuuren AM, Spoelstra SF. 1997. Effect of a bacterial inoculant on rate of fermentation and chemical composition of high dry matter grass silages. J Agric Sci. 128:323–329.
- Eltz FLF, Villalba EH, Lovato T. 2010. Phosphorus fertilization for sunflower under no-tillage system in Paraguay. Bragantia. 69:899–904.
- Foldager J. 1977. Protein requirement and non-protein nitrogen for high producing cow in early lactation [PhD dissertation]. East Lasing, MI: Michigan State University.
- Goes RHTB, Cerilo SLN, Lima HL, Fernandes ARM, Oliveira ER, Souza KA, Patussi RA, Brabes KCS, Gressler MGM. 2012. Torta de girassol em substituição ao farelo de soja nos suplementos de novilhas: desempenho e características de carcaça. R Bras Saúde Prod Anim. 13:396–409.
- Gurbuz Y, Kaplan M. 2008. Chemical composition, organic matter digestibility, in vitro gas production characteristics and ensiling of sugar beet leaves as alternative feed resource. J Anim Vet Sci. 7:1568–1574.
- Hall MB. 2003. Challenges with non fiber carbohydrate methods. J Anim Sci. 81:3226–3232.
- Hill J, Leaver JD. 2002. Changes in chemical composition and nutritive value of urea treated whole crop wheat during exposure to air. Anim Feed Sci Technol. 102:181–195.
- Holden LA. 1999. Comparison of methods of in vitro matter digestibility for ten feeds. J Dairy Sci. 2:1791–1794.
- Holzer M, Mayrhuber E, Danner H, Braun R. 2003. The role of Lactobacillus buchneri in forage preservation. Trends Biotechnol. 21:282–287.
- Hu W, Schimdt RJ, McDonell EE, Klingerman CM, Kung Jr. K. 2009. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J Dairy Sci. 92:3907–3914.
- Kleinschmit DH, Kung Jr. LA. 2006. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J Dairy Sci. 89:4005–4013.
- Konca Y, Buyukkiliç Beyzi S, Kaliber M, Ulger I. 2015. Chemical and nutritional changes in sunflower silage associated with molasses, lactic acid bacteria and enzyme supplementation. Harran Tarim ve Gida Bilimleri Dergisi. 19:223–231.
- Kulasek GA. 1972. A micromethod for determination of urea in plasm, whole blood and blood cells using urease and phenol reagent. Polskie Archiwum Wetterinaryjne. 15:801–810.
- Kung Jr. L, Grieve DB, Thomas JW, Huber JT. 1984. Added ammonia or microbial inocula for fermentation and nitrogenous compounds of alfalfa ensiled at various percents of dry matter. J Dairy Sci. 67:299–306.
- Mayrhuber E, Holzer M, Danner H, Madzingaidzo L, Braun R. 1999. Comparison of homofermentative and heterofermentative Lactobacillus strains as silage inoculum, to improve aerobic stability. The XIIth International Silage Conference – Conference Proceedings. Uppsala. 276–277.
- McDonald P, Henderson AR, Heron S. 1991. The biochemistry of silage, 2nd ed. Marlow: Chalcombe.
- Muck RE. 1993. The role of silage additives in making high quality silage. In: Silage production from seed to Animal, NRAES–67, pp. 106–116. Ithaca, NY: Northeast Regional Agric. Engineering Service.
- Muck RE. 2004. Effects of corn silage inoculants on aerobic stability. Transactions of the ASAE. 47:1011–1016.
- [NRC] National Research Council. 2001. Nutrient requirements of dairy cattle, 7th revised ed. Washington, DC: National Academy Science.
- Ozduven ML, Koc F, Polat C, Coskuntuna L. 2009. The effects of lactic acid bacteria and enzyme mixture inoculants on fermentation and nutrient digestibility of sunflower silage. KafkasUniv Vet FakDerg. 15:195–199.
- Rabelo CHS, Rezende AV, Nogueira DA, Rabelo FHS, Senedese SS, Vieira PF, Barbosa LA, Carvalho A. 2012. Perdas fermentativas e estabilidade aeróbica de silagens de milho inoculadas com bactérias ácido-láticas em diferentes estádios de maturidade. R Bras Saúde Prod Anim. 13:656–668.
- Rabie CJ, Lubben A, Marais GJ, Jansen Van Vuuren H. 1997. Enumeration of fungi in barley. Int J Food Microbiol. 35:117–127.
- Ramos HC, Hoffman T, Marino M, Nedjari H, Presecan-Siedel E, Dreesen O, Glasser P, Jahn D. 2000. Fermentative metabolism of bacillus subtilis: physiology and regulation of gene expression. J Bacteriol. 182:3072–3080.
- Rodrigues PHM, Andrade SJT, Fernandes T, Lima FR, Melotti L, Lucci CS. 2001. Valor nutritivo da silagem de capim-elefante cultivar Napier (Pennisetumpurpureum, Schum) inoculada com bactérias ácido-láticas. Acta Scientiarum. 23:809–813.
- Tilley JMA, Terry RA. 1963. A two-stage technique for the in vitro digestion of forage crops. J BrGrassl Soc. 18:104–111.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Weinberg ZG, Ashbell G, Hen Y, Azrieli A. 1993. The effect of applying lactic acid bacteria at ensiling on the aerobic stability of silages. J Appl Bacteriol. 75:512–518.
- Weinberg ZG, Muck RE. 1996. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol Rec. 19:53–68.
