ABSTRACT
The importance of polyunsaturated fatty acids (PUFAs) within the biological functions of animals has been widely recognized. In this study, exercise and PUFAs’ supplementation effects on serum triglycerides (Try), total cholesterol (Chol), and nonesterified fatty acids (NEFAs) concentration were evaluated in athletic horses. Ten regularly trained thoroughbred horses were randomly divided in two groups, control group (CG; n = 5) and experimental group (EG; n = 5). EG received a 4-week PUFA supplementation; CG received no dietary supplement. Blood samples were collected from the animals every 10 days before (PreD10, PreD20, PreD30) and after (PostD10, PostD20, PostD30) 1700 metres of race and were tested for selected parameters. Two-way analysis of variance for repeated measures showed lower Chol levels at PreD10, PreD20, and PreD30 in EG with respect to control CG (P < .05). EG showed lower NEFA levels than CG at PreD20 and PostD30 (P < .05). Increase of Try levels was found in EG at PreD20 and PostD30 (P < .05). The results of this study highlighted both exercise and PUFA supplementation diet’s effect on selected lipid parameters in thoroughbred horses. In addition PUFA supplementation diet seems to improve metabolic adaptation to physical exercise probably by increasing animal capacity for fatty acids’ utilization in muscle.
1. Introduction
Lipids represent one of the most important substances involved in many metabolic pathways in mammals (Pagan Citation1998). They play multiple roles and are an essential element in the composition of cellular membrane, in the transmission of cellular signals and, furthermore, they provide an important metabolic intermediate in the skeletal muscle metabolism representing the most important energy stock in the body (Hinchcliff et al. Citation2004). Concerning equine athlete, lipid metabolism is important in aerobic and in anaerobic exercise (Hodgson & Rose Citation1994; Piccione et al. Citation2009). Physical exercise induces various stress responses and metabolic adaptations. Similarly to others stressors, including delivery, transport, and environmental conditions (Budzynska Citation2014; Casella et al. Citation2012; Piccione et al. Citation2015), physical exercise needs adequate response to re-establish homeostatic equilibrium (Arfuso et al. Citation2016). Exercise has great influences on lipid metabolism through its action on some particular hormones (Pösö & Hyyppa Citation1999). In particular, catecholamines (norepinephrine and epinephrine) influence respiratory and cardiovascular functions and enhance the possibility of the body to react to exercise, optimizing and increasing the use of energy substrates through their action on metabolism in adipose, muscular, and hepatic tissue. Catecholamines determine an increase of the plasma-free fatty acids through their action on the hormone-responsive lipases of adipose tissue (Hinchcliff et al. Citation2004; Pösö et al. Citation1989; Sen & Packer Citation2000).
The influence of physical exercise on oxidative stress, inflammatory response, and on haematological and biochemical parameters makes dietary supplementation with polyunsaturated fatty acids (PUFAs) (6–8) a popular matter in athletic horses’ nutrition studies (O’Connor et al. Citation2004; Orme et al. Citation1997). The most biologically active PUFAs are Omega-3 and Omega-6 fatty acids. They have shown positive effects on both physiological and pathologic conditions (Holub & Holub Citation2004; Piccione et al. Citation2014). Fatty acids could promote the athletic performance, allowing the utilization of energetic substrates and improving the stability and permeability of cellular membranes (Gibney & Bolton-Smith Citation1988; Piccione et al. Citation2013). Furthermore, they show an important influence on aerobic and anaerobic metabolism, muscle glycogen synthesis and use, and peripheral oxygen transport (Harking et al. Citation1992; Watson et al. Citation1993). A fatty acid supplementation in horse diet must include omega-3 and omega-6 fatty acids both in a proper ratio (1:1, 1:3, 1:6) depending on the diet. In fact, natural diets, thanks to pasture grass, possess significant amounts of omega-3 fatty acids, while the ratio of omega-3 to omega-6 fatty acids shows important changes in animals fed with traditional equine diet without pasture grass, which causes a decrease in the supply of omega-3 fatty acids and exceed in omega-6 fatty acids. Fatty acids represent an important source of energy and their metabolic effects on lipids were already taken into consideration in athletic horses (O’Connor et al. Citation2004; O’Connor et al. Citation2007). The aim of this study was to evaluate the effect of PUFAs’ dietary supplementation on fat metabolism in thoroughbred horses in order to improve the knowledge of the effect of PUFAs on metabolic processes occurring during exercise in horses undergoing specific training programmes and give useful information for better management of athletic horses.
2. Materials and methods
2.1. Animals
Ten regularly trained thoroughbred horses (6 geldings and 4 mares, 4–5 years old, mean body weight 500 ± 25 Kg) were enrolled in this study with the owner’s informed consent. Before starting the study, the horses were subjected to clinical examination, routine haematology, and haematochemical analyses at rest, and only healthy subjects were used. All animals were housed in individual boxes (3.50 × 3.50 m) under natural winter photoperiod (sunrise at 07:28 am, sunset at 17:38 pm) and 18–21°C indoor temperatures. The horses were fed twice a day a diet consisting of hay (first cut meadow hay, sun cured, and late cut; 7 ± 1 kg/d) and total mixed ration (Bernunzo Feeds Factory, Enna, Italy) composed of the following: crude protein 16%, ash 10.09%, crude fat 6%, crude fibre 7.35%, sodium 0.46%, lysine 0.85%, methionine 0.35%, omega-3 0.65%, at a total of 10 ± 2 kg/day distributed in two meals (at 7:00, 19:00). Water was available ad libitum. All horses were randomly divided into two groups: Control group (CG) and experimental group (EG). EG received 70 ml of Omega Horse orally by means of a syringe. The composition of the supplement was: crude fat 99.4% (total omega 3: 22.5 g, Eicosapentaenoic acid (EPA) 11.5 g, Docasaenoid acid (DHA) 7.7 g, VitE: 2 g), crude protein 0.09%, crude fibre 0.09%, crude ash 0.04% (NBF Lanes, Milan, Italy) (), once a day for 30 days.
Table 1. Nutritional composition of supplementation: fatty acids as a percentage of fat.
2.2. Blood sampling and analysis
Blood samples were collected by means of jugular venipuncture in tubes without anticoagulant agent (Terumo Co., Tokyo, Japan) every 10 days before (PreD10, PreD20, PreD30) and within 10 min after (PostD10, PostD20, PostD30) 1700 metres’ harness race performed at the ‘La Favorita’ race track () Palermo, Italy. Blood samples were placed on ice and transferred to the laboratory within 2 h after collection. After standing at room temperature for 20 min, the tubes were centrifuged at 1300 ×g for 10 min and the obtained serum was stored at −25°C until analysis. Only sera, not lipemic or haemolysed, were analysed to test triglycerides (Try), total cholesterol (Chol), and nonesterified fatty acids (NEFAs) concentration with commercially available kits (Biosystems, Reagents and Instruments, Barcelona, Spain; Giesse Diagnostics, Rome, Italy; Randox, Crumlin, UK) by means of an automated analyzer ultraviolet-visible spectrophotometer (SEAC, Florence, Italy).
Table 2. ‘La Favorita’ horse race track characteristics.
2.3. Statistical analysis
A Barlett test was applied to verify the homoscedasticity of data and all passed the test. P < .05 was considered statistically significant, with an alpha level of 90%. Two-way analysis of variance (ANOVA) for repeated measures and Bonferroni’s post hoc were used to assess the statistical significant effect of exercise and PUFA supplementation diet at different time points on serum Try, Chol, and NEFAs. The data were analysed with Stats package of R: R Core Team (Citation2013) (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, 2013, URL: http://www.R-project.org/).
3. Result and discussion
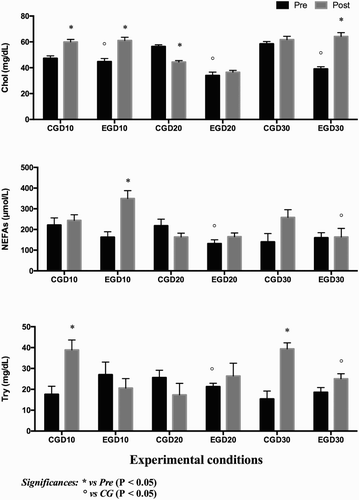
Two-way ANOVA showed significant effect of exercise (Pre-race respect to Post-race) in all studied parameters (P < .05). Moreover, a significant effect of PUFA supplementation (CG with respect to EG) was found on Chol, Try, and NEFAs values (P < .05). During the experimental period of this study, Chol and Try showed normal mean levels within the physiological range for the horses (Kaneko Citation1997). Since other researchers studied the effect of omega 3 supplementation in horses (Bowen et al. Citation2013; De Moffarts et al. Citation2007; Orme et al. Citation1997) we actually have limited knowledge about the normal ranges of NEFAs in physiological conditions in horses; however the values obtained in this research were in accordance with the values already observed in Piccione et al. (Citation2009). The analysis of our results showed a significant effect of exercise on selected parameters. In fact, significantly higher values of Chol, NEFAs, and Try were found in the Post-race. In particular, Chol showed higher levels at PostD10 with respect to PreD10 in both groups and at PostD30 with respect to PreD30 in EG; whereas lower Chol levels were found at PostD20 compared to PreD20 in CG. NEFAs showed a statistically significant increase at D10 in EG and Try levels’ increase at D10 and D30 in CG (P < .05). The trend of these parameters represents a possible metabolic and hormonal response of horses to physical exercise. During physical exercise an increased sympathetic-adrenal activity and a reduction in insulin concentration are likely to occur. The increased beta-adrenergic sensitivity of the adipose tissue induces lipomobilization, enhancing the use of fatty acids as an energy source. The fatty acids used in muscular metabolism are provided not only by the adipose tissue, but also by circulating lipoproteins and by the triglycerides stored in the muscle cells themselves. The aerobic metabolism leads to a solicitation and to an activation of type I and IIA muscle fibres (Assenza et al. Citation2012), enhancing lipolysis and increasing the ability to use lipids as energy substrate increases. However, because of the known low ability of thoroughbred horses to use lipids as energy substrate, the released lipids cannot be used in a proper way, as the ability to burn lipids by the muscle fibres is still low (Assenza et al. Citation2012). As a consequence, the levels of NEFAs increased and the surplus of unused NEFAs returned to the adipose tissue to be re-esterified to triglycerides (Magkos Citation2009; Pösö et al. Citation1989). In agreement with Pösö et al. (Citation1989) our results showed higher Try and Chol levels in CG with respect to EG (). The higher Try and Chol values in CG showed the effect of PUFA supplementation in athletic horses during exercise. In this study after 30 days, NEFAs reach levels lower than first days probably for a metabolic response to the stress exercise-induced in fact, it is note that a proper training could improve the recovery capacity and the energetic substrates use through NEFAs mobilization (Christensen et al. Citation1999; Piccione et al. Citation2009). The results obtained in the present study suggest that this peculiar metabolic response could be stimulated due to PUFA supplementation in athletic horse diet. In particular, following omega-3 supplementation in the EG it was possible to observe a significant decrease in Try and NEFA levels compared to the CGs during rest and after the race. Several studies have shown a significant decrease in serum triglycerides due to fish oil supplementation in human, horses, and rats (Christensen et al. Citation1999; Fickova et al. Citation1998; Hinchcliff et al. Citation2004). This event could be attributed to an increasing of lipoprotein lipase action and in fatty acid oxidation (Geleen et al. Citation1999; Orme et al. Citation1997) or to a down-regulation effect on triglyceride synthesis enzymes performed by fatty acids (Surette et al. Citation1992). Triglycerides in horses are transported mainly by very low density lipoprotein (VLDL). In fact, this lipoprotein contains about 57% of triglycerides while, for example, in low density lipoprotein (LDL) they are transported at a rate of 5.5% (Watson et al. Citation1993). If the lipoprotein lipase activity and the clearance of LDL increase it could result in a decrease of triglycerides circulating in bloodstream. The influence of fish oil supplementation on the enzymes involved in triglyceride catabolism could suggest an increase of fatty acid utilization in muscular tissue. The EG showed a significant decrease in NEFA concentration. NEFA values express the ratio between fatty acid mobilization from adipose tissue and fatty acid utilization in muscular tissue. The lower serum NEFA values found in the EG with respect to the CG could be due to an increase of free fatty acid utilization in order to produce energy or to decrease the fatty acid mobilization (O’Connor et al. Citation2004).
Figure 1. Mean ± standard deviation (SD) with related statistical significances of serum total cholesterol (Chol) triglyceride (Try) and nonesterified fatty acid (NEFAs) values measured before exercise (Pred10, pred20, pred30) and after exercise (Postd10, postd20, postd30) conditions in control (CG) and experimental (EG) thoroughbred horses.
4. Conclusion
The interaction and the importance of PUFAs within functions and biological systems of animals have been widely recognized. The results obtained in the present study highlighted the effect both of exercise and PUFA supplementation diet on selected lipid parameters in thoroughbred horses. In addition, PUFA supplementation diet seems to improve metabolic adaptation to physical exercise by athletic horses, probably by increasing animal capacity for fatty acid utilization in muscle. However, further studies should be conducted to better understand the effect and the benefice of omega-3 supplementation in athletic horses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arfuso F, Giannetto C, Giudice E, Fazio F, Piccione G. 2016. Dynamic modulation of platelet aggregation, albumin and nonesterified fatty acids during physical exercise in thoroughbred horses. Res Vet Sci. 104:86–91. doi: 10.1016/j.rvsc.2015.11.013
- Assenza A, Tosto F, Piccione G, Fazio F, Nery J, Valle E, Bergero D. 2012. Lipid utilization pathways induced by early training in standardbred trotters and thoroughbreds. J Equine Vet Sci. 32(11):704–710. doi: 10.1016/j.jevs.2012.02.015
- Bowen LE, Spooner HS, Zambito JL, Barnes KM. 2013. Comparison of krill oil and fish oil supplementation on serum and tissue fatty acid profiles in horses. J Equine Vet Sci. 33(5):321–399. doi: 10.1016/j.jevs.2013.03.054
- Budzyńska M. 2014. Stress reactivity and coping in horse adaptation to environment. J Equine Vet Sci. 34(8):935–941. doi: 10.1016/j.jevs.2014.05.010
- Casella S, Fazio F, Giannetto C, Giudice E, Piccione G. 2012. Influence of transportation on serum concentration of acute phase proteins in horse. Res Vet Sci. 93:914–917. doi: 10.1016/j.rvsc.2012.01.004
- Christensen JH, Christenson MS, Dyerberg J, Schmidt EB. 1999. Heart rate variability and fatty acid content of blood cell membranes: a dose-dependent study with n-3 fatty acids. Am J Clin Nutr. 70(3):331–337.
- De Moffarts B, Portier K, Kirschvink N, Coudert J, Fellmann N, Van Erck E, Letellier C, Motta C, Pincemail J, Art T, Lekeux P. 2007. Effects of exercise and oral antioxidant supplementation enriched in (n−3) fatty acids on blood oxidant markers and erythrocyte membrane fluidity in horses original. Vet J. 174(1):113–121. doi: 10.1016/j.tvjl.2006.06.001
- Fickova M, Hubert P, Cremel G, Leray C. 1998. Dietary n-3 and n-6 polyunsaturated fatty acids rapidly modify fatty acid composition and insulin effects in rat adipocytes. J Nutr. 128(3):512–519.
- Geelen SNJ, Sloet Van Oldruitenborgh-Oosterbaan MM, Beynen AC. 1999. Dietary fat supplementation and equine plasma lipid metabolism. Equine Vet J. 30(S30):475–478.
- Gibney MJ, Bolton-Smith C. 1988. The effect of dietary supplement of n-3 polyunsaturated fat on platelet lipid composition, platelet function and platelet plasma membrane fluidity in healthy volunteers. Brit J Nutr. 60(1):91–95. doi: 10.1079/BJN19880080
- Harking JD, Morris GS, Tulley RT, Nelson AG, Kamerling SG. 1992. Effect of added dietary fat on racing performance in thoroughbred horses. J Vet Sci. 12(2):123–129.
- Hinchcliff KW, Kaneps AJ, Geor RJ. 2004. Equine sports medicine and surgery. In: Basic and clinical sciences of the equine athlete. 1st ed. Edinburgh: Saunders Company; p.775–784.
- Hodgson DR, Rose RJ. 2014. Nutrition of the performance horse. In: The athletic horse. 2nd ed. Philadelphia, PA: Saunders WB Company; p. 50–61.
- Holub DJ, Holub BJ. 2004. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 263(1–2):217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d
- Kaneko JJ. 1997. Appendix VIII: blood analyte reference values in large animals. In: Kaneko JJ, Harvey JW, Bruss ML editors. Clinical biochemistry of domestic animals. 5th ed. San Diego, CA: Academic Press Inc; 1997. p. 890–894.
- Magkos F. 2009. Basal very low-density lipoprotein metabolism in response to exercise: mechanism of hypotriacylglycerolemia. Prog Lipid Res. 48(3-4):171–190. doi: 10.1016/j.plipres.2009.02.003
- O’Connor CI, Lawrence LM, Hayes SH. 2007. Dietary fish oil supplementation affects serum fatty acid concentrations in horses. J Anim Sci. 85(9):2183–2189. doi: 10.2527/jas.2006-528
- O’Connor CI, Lawrence LM, Lawrence AC, Janicki KM, Warren LK, Hayes S. 2004. The effect of dietary fish oil supplementation on exercising horses. J Anim Sci. 82(10):2978–2984. doi: 10.2527/2004.82102978x
- Orme CE, Harris RC, Marlin DJ, Hurley J. 1997. Metabolic adaptation to a fat-supplemented diet by the thoroughbred horse. Brit Vet J. 78:443–458.
- Pagan JD. 1998. Energy and the performance horse. In: Pagan JD, Geor RJ editors. Advances in equine nutrition, Vol. II. Nottingham: Nottingham University Press; p. 141–147.
- Piccione G, Assenza A, Borruso M, Fazio F, Caola G. 2009. Daily pattern of some fatty acids in the athletic horse. J Anim Physiol Anim Nutr. 93(1):7–14. doi: 10.1111/j.1439-0396.2007.00790.x
- Piccione G, Marafioti S, Bazzano M, Rizzo M, Arfuso F, Assenza A. 2013. Integrazione alimentare della razione alimentare con acidi grassi della serie omega 3. Pratica Vet Equina. 2(1):42–48.
- Piccione G, Marafioti S, Giannetto C, Panzera M, Fazio F. 2014. Effect of dietary supplementation with omega 3 on clotting time, fibrinogen concentration and platelet aggregation in the athletic horse. Livest Sci. 161(complete):109–113. doi: 10.1016/j.livsci.2013.12.032
- Piccione G, Rizzo M, Arfuso F, Giannetto C, Di Pietro S, Bazzano M, Quartuccio M. 2015. Leukocyte modifications during the first month after foaling in mares and their newborn foals. Pol J Vet Sci. 18(3):621–625.
- Pösö AR, Hyyppa S. 1999. Metabolic and hormonal changes after exercise in relation to muscle glycogen concentrations. Equine Vet J. 30(S30):332–336. doi: 10.1111/j.2042-3306.1999.tb05244.x
- Pösö AR, Viljanen-Tarifa E, Soveri T, Oksanem HE. 1989. Exercise-induced transient hyperlipidemia in the racehorse. J Vet Med Series A. 36(1–10): 603–611. doi: 10.1111/j.1439-0442.1989.tb00771.x
- R Core Team. 2013. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/.
- Sen CK, Packer L. 2000. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 72(2):653S–669S.
- Surette ME, Whelan J, Broughton KS, Kinsella JE. 1992. Evidence for mechanisms of the hypotriglyceridemic effect of n-3 polyunsaturated fatty acids. Biochimica et Biophysica Acta. 1126(2):199–205. doi: 10.1016/0005-2760(92)90291-3
- Watson TD, Burns GL, Freeman DJ, Packard CJ, Shepherd J. 1993. High density lipoprotein metabolism in the horse (Equus caballus). Comp Biochemestry Physiol Part B. 104(1):45–53. doi: 10.1016/0305-0491(93)90336-4
