ABSTRACT
The aim of this research was to gather preliminary data about the potential inhibitory effects of commercial diets on the protease activities of meagre larvae using in vitro techniques. Four commercial diets (Gemma Micro 150 (100–200 µm), Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm)) were tested in this study. The differences in the protease activities of meagre larvae during the sampling period were statistically significant (p < .05). The highest and lowest protease activities of meagre larvae were 393.97 ± 7.9 U/mg protein (7 DAH) and 9.64 ± 1.25 U/mg protein (20 DAH), respectively. The digestive proteases of meagre larvae showed the greatest sensitivity to protease inhibitors present in Gemma Micro 150 (100–200 µm). In conclusion, Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm) are moderately advisable as the commercial diets in the feeding protocol of meagre larvae from 15 to 32 DAH except for more than 50% inhibitions, but not Gemma Micro 150 (100–200 µm).
1. Introduction
In general, marine aquaculture production is based on gilthead seabream (Sparus aurata) and the European seabass (Dicentrarchus labrax). The prices of cultured species, such as seabass and seabream, have decreased due to their over production. Therefore, aquaculture needs to focus on the introduction of new candidate species. Meagre appears to be one of the potential species for diversification in aquaculture (El-Shebly et al. Citation2007; Monfort Citation2010). Quemener (Citation2002) reported that the most important advantage of meagre, suggested as the candidate species in aquaculture, was its high growth rates.
The weaning stage has been known as the transition from live food to microdiet in aquaculture. It has a critical importance in the feeding of marine fish larvae. Therefore, studies have focused on the production of microdiets used in the weaning stage (Yúfera et al. Citation1999; Yúfera et al. Citation2000; Kolkovski Citation2013). The survival and growth rate of fish larvae fed solely on microdiet during the weaning stage are known to be poor, but supplementation with live foods usually results in a marked improvement (Cahu & Zambonino Infante Citation1994). To explain the success of live food over microdiets, researchers mentioned that fish larvae had insufficient digestive enzyme capacity for exogenous food (Munilla-Moran et al. Citation1990; Kolkovski et al. Citation1993; Kolkovski et al. Citation1996). Therefore, attention has been focused on the contribution of digestive enzymes from Artemia nauplii and rotifers commonly used in the feeding of marine fish larvae (García-Ortega et al. Citation1998; Kurokawa et al. Citation1998; García-Ortega et al. Citation2000). García-Ortega et al. (Citation2000) showed that the digestive enzymes from Artemia contributed ∼1% to the total digestion of food by the catfish larvae.
On the other hand, studies on determining the inhibitory effects on the protease activity of widely used feed ingredients in commercial diets are the key tools to understand and solve nutritional problems. Fish meal is commonly used as the main dietary ingredient in commercial diets. The shortage observed in fish meal production together with the increased demand has caused increases in fish meal prices. For this reason, studies were carried out to evaluate cheap and sustainable vegetable protein sources. However, the main obstacles to the use of high amounts of vegetable protein sources in fish diets are low protein quality due to the amino acid imbalances and the presence of antinutritional factors reducing the activity of fish digestive enzymes (Huisman & Tolman Citation1992; Tacon Citation1997; Krogdahl et al. Citation2003).
A study showed a different sensitivity of fish proteases to the inhibitors present in feeds, suggesting the need of a preliminary evaluation of such effects when feed ingredients such as vegetable protein source in formulations are used (Moyano et al. Citation1998; Alarcón et al. Citation1999). In this sense, the effects of feed ingredients used in the production of microdiets on the protease activities of seabream larvae and shrimps were studied (Alarcón et al. Citation1997; Alarcón et al. Citation1999).
Until now, researchers have focused on growth, survival and larval rearing of meagre (Pastor et al. Citation2013; Vallés & Estévez Citation2013; Vallés & Estévez Citation2015), the ontogeny of digestive system of meagre (Papadakis et al. Citation2013) and the effects of different levels of vegetable proteins on juvenile meagre (Estévez et al. Citation2011). In addition, the digestive enzymes of marine fish larvae such as D. labrax, S. aurata, Solea senegalensis, Diplodus sargus, Pagrus auriga and A. regius were investigated by some authors (Zambonino Infante & Cahu Citation1994; Moyano et al. Citation1996; Ribeiro et al. Citation1999; Cara et al. Citation2003; Moyano et al. Citation2005; Süzer et al. Citation2013). We could only find studies on digestive enzymes (Süzer et al. Citation2013) but no study was found on the inhibitory effects of commercial diets on the protease activities of meagre larvae. At this point, inhibitory effects of the commercial diets in the larvae rearing must be investigated to solve the nutritional problem. Therefore, the aim of this research was to collect preliminary data on the potential inhibitory effects of commercial diets, such as Gemma Micro 150 (100–200 µm), Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm) on the protease activities of meagre larvae using in vitro techniques.
2. Materials and methods
2.1. Larvae rearing and sampling
The sampling stage of the present study was carried out at the EGEMAR Aquaculture Food Industry and Commercial Incorporated Company. Eggs were obtained with hormone injection from meagre broodstocks (GnRH; 20 µg/kg ♀ and 10 µg/kg ♂). The fertilized eggs of meagre were collected from the broodstock tanks and incubated in conical fibreglass tanks at a temperature of 22.0 ± 0.2°C. Newly hatched larvae were transferred from the incubators to 7 m3 ellipsoidal fibreglass tanks with black walls until 15 days after hatching (DAH). From 15 to 32 DAH, larvae were stocked in concrete raceway 15 m3 tanks (stocking density; at 0–15 DAH, 75–80 larvae/L and at 16–32 DAH, 10–12 larvae/L). The rearing tanks were supplied with running sea water that had been filtered through sand, bag and UV filters. Temperature, salinity, oxygen levels and pH were 20.8–22.2°C, 27.0–40.0 g/L, 7.8–14.7 mg/L and 7.7–8.1, respectively. Air and fresh seawater were introduced into the surface of the tanks to prevent water stratification until 15 DAH. Rearing tanks were exposed to a photoperiod of 18:6.
Nannochloropsis occulata was used for the green water technique from 3 to 15 DAH. Rotifer (Brachionus plicatilis) was cultured with Algamac Protein Plus (Aquafaune Bio-Marine Inc., Hawthorne, USA) and Sparkle (Inve Aquaculture). The average water temperature and salinity during the culture were 25°C and 25 g/L, respectively. Rotifer was enriched with Spresso (INVE Aquaculture) prior to its transfer to the larval feeding tanks. The average water temperature and salinity during the enrichment were 26°C and 28 g/L, respectively.
Artemia nauplii (Artemia Cysts; Vinh Chau-Bac Lieu Artemia Co.O) were cultured at 29°C and 28 g/L. Artemia metanauplii (Artemia EG; Artemia SepArt EG > 250,000 npl/g INVE Aquaculture, Salt Lake City, Utah, USA) were cultured at 29°C and 28 g/L. Artemia metanauplii were enriched with enrichments (Spresso-INVE Aquaculture) for 24 h at 26°C and 28 g/L.
The feeding regime consisted of B. plicatilis from 3 to 8 DAH reaching a maximum concentration of 10–15 prey/mL, Artemia nauplii from 7 to 11 DAH with a maximum density of 4–6 prey/mL, Artemia metanauplii from 10 to 15 DAH with a maximum density of 2–4 prey/mL; from 16 to 32 DAH with a maximum density of 1.5–5 prey/mL; from 16 to 26 DAH with a maximum density of 2 prey/mL and from 27 to 32 DAH with a maximum density of 4.5 prey/mL. Commercial diets such as Gemma Micro 150 (100–200 µm; Skretting AS) from 17 to 22 DAH, Caviar (200–300 µm; BernAqua) from 21 to 24 DAH, Caviar (300–500 µm; BernAqua) from 24 to 29 DAH and Perla Larva Proactive 4.0 (300–500 µm; Skretting AS) from 28 to 32 DAH were used in the commercial feeding procedure of meagre larvae. The proximate compositions of commercial diets used in the present study are given in . Also, Nannochloropsis occulata was added into the growth tanks from 16 to 26 DAH. The samples of meagre larvae fed on commercial feeding procedure were collected in triplicates from 3 to 32 DAH. Larvae were taken before the morning feeding and immediately stored in liquid nitrogen (−196°C) to prevent protein autolysis.
Table 1. Proximate compositions of commercial diets used in the present study.
2.2. Extracts of larvae
Argyrosomus regius larvae fed on commercial feeding procedure were sampled 13 times, during the sampling period (from 3 to 32 DAH). Larvae were taken before the morning feeding and immediately stored in liquid nitrogen (−196°C) to prevent protein autolysis. The larvae sampled according to the above procedure were rinsed in distilled water after thawing and then the extracts of larvae were prepared by homogenization of the whole larvae followed by centrifugation (16,000g, 30 min, 4°C).
2.3. Extracts of commercial diets
Four commercial diets (Gemma Micro 150 (100–200 µm), Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm)) were tested with in vitro techniques. The extracts of commercial diets prepared by homogenization (100 mg/mL in distilled water) followed by centrifugation (15,000g, 10 min) were used in protease inhibition analyses.
2.4. Determination of protease activities of larvae
Total protease activities of meagre larvae were measured, as described by Walter (Citation1984), using casein (10 mg/mL) in 50 mM Tris–HCl buffer at pH 8.5 as the substrate. The mixtures containing extracts of larvae and substrate were incubated and then the reaction was stopped by the addition of 500 μL trichloroaceticacid (TCA) (120 g/L). One unit of enzyme activity was defined as 1 μg of tyrosine release per minute. The soluble protein concentrations of meagre larvae were determined according to Bradford (Citation1976).
2.5. Effects of commercial diets on the protease activities of larvae
The inhibitory effects of commercial diets on the protease activities of meagre larvae were determined by measuring the reduction in protease activity of extracts using a modification of the method described by García-Carreno (Citation1996). The mixtures containing commercial diets and larval extracts were incubated for 90 min at 37°C and the reaction was stopped by the addition of 500 μL TCA (120 g/L). One unit of enzyme activity was defined as 1 μg of tyrosine release per minute. The method is based on the measurement of residual protease activity remaining after pre-incubation with different commercial diets, namely Gemma Micro 150 (100–200 µm), Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm).
2.6. Statistical methods
All measurements were carried out in triplicates. The experimental data were subjected to one-way ANOVA, and mean ± standard error (SE) differences were measured by Duncan test at the p = .05 content level by using SPSS 15.0 statistical package (SPSS Citation2006).
3. Results
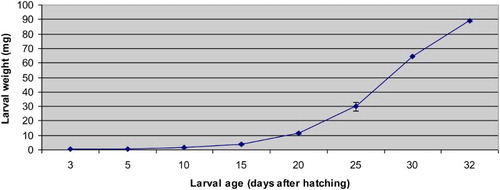
The growth of meagre larvae is shown in and . The differences determined in the total lengths and weights from 3 to 32 DAH were statistically significant (p < .05). The lowest and highest weights were 0.54 ± 0.02 mg (3 DAH) and 89.21 ± 0.91 mg (32 DAH), respectively. Larval weight remained relatively constant until 10 DAH and followed by a sharp increase that continued until 32 DAH (p < .05). The lowest and highest total lengths were 3.22 ± 0.02 mm (3 DAH) and 20.95 ± 0.3 mm (32 DAH), respectively. The total length of meagre larvae remained relatively stable up to 5 DAH. After 5 DAH, the total length tended to increase until 32 DAH (p < .05).
Figure 1. The weight values of meagre (Argyrosomus regius) larvae (mg) observed during the study. Results are expressed as mean ± SE (a pool of 30 larvae).
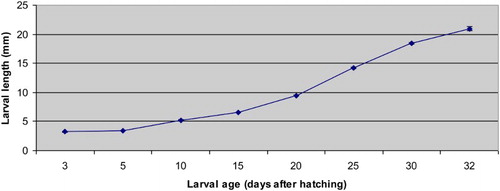
Figure 2. The total length values of meagre (Argyrosomus regius) larvae (mm) observed during the study. Results are expressed as mean ± SE (a pool of 30 larvae).
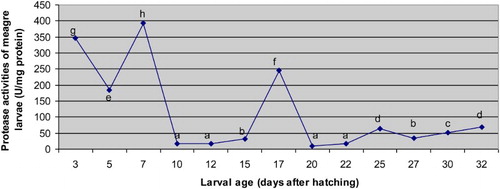
The changes measured in protease activities of meagre larvae are given in . The differences observed in protease activities from 3 to 32 DAH were statistically significant (p < .05). The highest and lowest protease activities of meagre larvae were 393.97 ± 7.9 U/mg protein (7 DAH) and 9.64 ± 1.25 U/mg protein (20 DAH), respectively. Protease activities of larvae tended to decrease from 3 to 5 DAH. After 5 DAH, a sharp increase until 7 DAH and then, a sharp decrease from 7 to 10 DAH were observed. Protease activities of larvae tended to increase from 10 to 17 DAH and then, followed by a sharp decrease up to 20 DAH. After 20 DAH, the protease activities of meagre larvae tended to increase until 25 DAH and followed by a decrease at 27 DAH and then, increased from 27 to 32 DAH.
Figure 3. The changes determined in protease activities of meagre (Argyrosomus regius) larvae during the study (U/mg protein). Results are expressed as mean ± SE.
The inhibitory effects of commercial diets on the protease activities of meagre larvae are shown in . The high inhibitions of the protease activities of meagre larvae were obtained when extracts were incubated in the presence of solutions prepared with commercial diets used in the present study. The lowest inhibitions of commercial diets were observed at 20 DAH except for Perla LP 4.0. The highest and lowest inhibitions of commercial diets on the protease activities of meagre larvae were observed in Gemma Micro 150 (100–200 µm) at 22 DAH (70.42%) and Caviar (200–300 µm) at 20 DAH (24.24%), respectively.
Figure 4. The inhibitory effects of different diets such as Gemma Micro 150, Caviar (200–300 μ), Caviar (300–500 μ) and Perla LP 4.0 on protease activities of meagre (Argyrosomus regius) larvae (%).
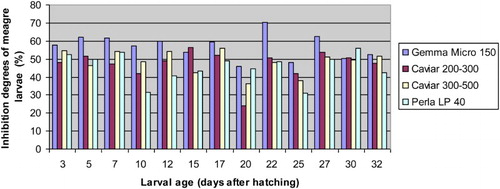
The inhibitory effects of Gemma Micro 150 (100–200 µm) on the protease activities of meagre larvae from 3 to 32 DAH were higher than those of other commercial diets used in the present study. Gemma Micro 150 (100–200 µm) showed more than 50% inhibitions except for 20 DAH (45.82%) and 25 DAH (48.22%). Caviar (200–300 µm) exhibited more than 50% inhibitions at 5, 15, 17 and 27 DAH while Caviar (300–500 µm) showed more than 50% inhibitions at 3, 7, 12, 17 and 32 DAH. However, Perla Larva Proactive 4.0 (300–500 µm) exhibited more than 50% inhibitions at 3, 7 and 30 DAH.
Gemma Micro 150 (100–200 µm) had more than 50% inhibitions in the critical larval stage (from 3 to 15 DAH). Perla Larva Proactive 4.0 (300–500 µm) had showed better performance than those of Caviar (200–300 µm) and Caviar (300–500 µm) between 10 and 12 DAH known as the critical larval stage but not 3, 5, 7 and 15 DAH. On the other hand, the inhibitions of Caviar (200–300 µm) on protease activities of larvae during the weaning stage were lower than those of Caviar (300–500 µm) except for 22, 25, 27 and 30 DAH. However, Perla Larva Proactive 4.0 (300–500 µm) had lower inhibitions than those of Caviar (200–300 µm) except for 20 and 30 DAH and Caviar (300–500 µm) except for 20, 22 and 30 DAH. Results showed that Perla Larva Proactive 4.0 (300–500 µm) exhibited better performance during the weaning period (from 15 to 32 DAH) except for 20 and 30 DAH.
4. Discussion
Market prices of live foods commonly used in the critical stages of marine fish larvae have increased due to the shortage observed in live food stocks together with the increased demand of, especially Artemia nauplii. For this reason, there has been a growing interest in developing commercial diets for sustainable aquaculture. In the present study, the potential inhibitory effects of commercial diets, namely Gemma Micro 150 (100–200 µm), Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm), on protease activities of meagre larvae were investigated using in vitro techniques. Also, growth parameters (total length and weight) of larvae were determined. The results of the study showed that larvae had high growth rates. Quemener (Citation2002) supported that the most important advantage of meagre larvae was high growth rates.
Currently, we could only found studies on the digestive enzymes of meagre larvae (Süzer et al. Citation2013) and none about protease activities and the inhibition effects of commercial diets on protease activities of meagre larvae. In the present study, the fluctuations observed in protease activities of meagre larvae were high until 10 DAH. Protease activities of larvae tended to increase from 10 to 15 DAH and, then, followed by a sharp increase up to 17 DAH. The lowest level of protease activities of meagre larvae was observed at 20 DAH. Then, protease activity values remained relatively constant from 20 to 32 DAH. Zambonino Infante and Cahu (Citation2001) indicated that the fluctuations observed in specific activities of enzymes are not due to a diminution in enzyme synthesis but is the result of an increase in tissue proteins. In addition, Zambonino Infante and Cahu (Citation2001, p. 482) reported that ‘the decline in amylase expression is transcriptionally regulated during larval development’. It has also been shown that the dietary starch content can modulate the decrease in amylase-specific activity. It means that the observed ontogenetic changes could be also depended on genetic programme of studied species and also on its diets. On the other hand, Solovyev et al. (Citation2016) strongly advised using histology methods for detection of acid digestion at the beginning. In addition, the rearing conditions, such as mesocosm technique, larval density, water temperature and feeding sequence, could affect the functional development of the digestive system in meagre larvae, and also the activity of alkaline and acid proteases (because of the variations in the body size and larval age) (Solovyev et al. Citation2016).
Our results showed that commercial diets used in the study caused an important inhibition on the protease activities of meagre larvae. Cahu and Zambonino Infante (Citation1994) showed that the survival and growth of marine fish larvae fed solely on microdiet through the weaning period are known to be very poor, but supplementation with live foods usually results in a marked improvement. Naz (Citation2008) indicated that the highest contribution of the digestive enzymes derived from live food commonly used in marine fish culture appears to be provided by Artemia metanauplii. For the reduction of the inhibitions of commercial diets observed on the protease activities of meagre larvae, microdiets exhibiting more than 50% inhibitions may be advised to use together with live food due to the highest enzyme contribution of Artemia metanauplii, as mentioned by Naz (Citation2008).
The highest resistances to protease inhibitors in Gemma Micro 150 (100–200 µm), Caviar (200–300 µm) and Caviar (300–500 µm) were found at 20 DAH. However, the resistance of Perla Larva Proactive 4.0 (300–500 µm) was observed at 10 and 25 DAH. Results obtained from the in vitro inhibition assays also revealed to the negative effects of feed ingredients used in commercial diets on the protease activities of meagre larvae, which could affect whole digestibility of diets.
Moyano et al. (Citation1999) indicated that the negative effects of using protease inhibitor-containing diets on fish growth may be related to dietary factors, such as the type of meal and the sensitivity of a given fish species to the antinutritional compounds. Alarcon et al. (Citation1999) showed that ovalbumin significantly reduced (60%) the activity of proteases in 8-day-old seabream larvae. Similar results were found when commercially produced microcapsules containing ovalbumin were tested using shrimp proteases (Alarcon et al. Citation1997). Reductions in the nutritional value of commercial diets are the results of the presence of antinutritional compounds found in feed ingredients commonly used in the formulation of aquaculture feeds. The present study indicates a different sensitivity of meagre proteases to inhibitors present in commercial diets, especially Gemma Micro 150 (100–200 µm) exhibiting more than 50% inhibitions from 3 to 32 DAH except for 20 and 25 DAH. For this reason, Gemma Micro 150 (100–200 µm) should not be recommended as the sole diet for the weaning and critical larval periods of meagre. The high inhibitions observed in commercial diets may be overcome by a careful combination of the most suitable ingredients in the formulation after the individually inhibitory effects of feed ingredients on the protease activities of marine fish larvae were determined. Results suggested that the use of Gemma Micro 150 (100–200 µm) together with live foods due to the meagre larvae exhibits better performance with the contributions of exogenous enzymes.
Also the present study indicated that Perla Larva Proactive 4.0 (300–500 µm) in the critical larval stage is not suggested until 7 DAH. Perla Larva Proactive 4.0 (300–500 µm) can be used in the critical larval stage from 7 to 15 DAH but not Gemma Micro 150 (100–200 µm), Caviar (200–300 µm) and Caviar (300–500 µm). Caviar (200–300 µm) and Caviar (300–500 µm) had the similar inhibitions on the protease activities of meagre larvae in both the weaning and critical larval stages.
Results suggest that the need of a preliminary evaluation of both positive and negative effects of the feed ingredients used in commercial diets in future. Also, the present paper reveals the usefulness of using in vitro assays for a preliminary assessment of the effects of commercial diets used in the feeding of fish larvae. In conclusion, results obtained from the study confirm the existence of protease inhibitors in feed ingredients used. In addition, the results of the study provide important contributions to determine the most suitable commercial diet for the use of meagre larvae.
Caviar (200–300 µm), Caviar (300–500 µm) and Perla Larva Proactive 4.0 (300–500 µm) are moderately advisable as the commercial diets in the feeding protocol of meagre larvae from 15 to 32 DAH except for more than 50% inhibitions, but not Gemma Micro 150 (100–200 µm). When such data become available, they will serve the regulation of feeding protocol of cultured marine fish larvae. For this reason, the inhibitory effects of commercial diets used through both the weaning and critical larval stages as well as feed ingredients on the protease activities of marine fish larvae to sustainable aquaculture should be investigated in the future studies.
Acknowledgements
I would like to thank Metin Neke and Doğan Neke with the hatchery staff from EGEMAR who support the research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alarcón FJ, Díaz M, Moyano FJ. 1997. Studies of digestive enzymes in characterization and practical applications. In: Tacon AGJ, Basurco B, editors. Feeding tomorrow’s fish. Zaragoza: CIHEAM Cahiers Options Meditérranéennes, n: 22; p. 113–121.
- Alarcón FJ, Moyan FJM, Díaz M, Fernández-Díaz C, Yúfera M. 1999. Optimization of the protein fraction of microcapsules used in feeding of marine fish larvae using in vitro digestibility techniques. Aquacult Nutr. 5:107–113. doi: 10.1046/j.1365-2095.1999.00093.x
- Bradford MM. 1976. A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Cahu CL, Zambonino Infante JL. 1994. Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: effect on digestive enzymes. Comp Biochem Physiol A. 109:213–222. doi: 10.1016/0300-9629(94)90123-6
- Cara JB, Moyano FJ, Cárdenas S, Fernández-Díaz C, Yúfera M. 2003. Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol. 63:48–58. doi: 10.1046/j.1095-8649.2003.00120.x
- El-Shebly AA, El-Kady MAH, Hussin AB, Hossain MdY. 2007. Preliminary observations on the pond culture of meagre Argyrosomus regius (Asso, 1801) (Sciaenidae) in Egypt. J Fish Aquat Sci. 2:345–352. doi: 10.3923/jfas.2007.345.352
- Estevéz A, Treviño L, Kotzamanis Y, Karacostas I, Tort L, Gisbert E. 2011. Effects of different levels of plant proteins on the ongrowing of meagre (Argyrosomus regius) juveniles at low temperatures. Aquacult Nutr. 17:572–582. doi: 10.1111/j.1365-2095.2010.00798.x
- García-Carreño FL. 1996. Proteinase inhibitors. Trends Food Sci Technol. 7:197–204. doi: 10.1016/0924-2244(96)10023-6
- García-Ortega A, Verreth J, Coutteau P, Segner H, Huisman EA, Sorgeloos P. 1998. Biochemical and enzymatic characterization of decapsulated cysts and nauplii of the brine shrimp Artemia at different developmental stages. Aquaculture. 161:501–514. doi: 10.1016/S0044-8486(97)00297-4
- García-Ortega A, Verreth J, Segner H. 2000. Post-prandial protease activity in the digestive tract of African catfish Clarias gariepinus larvae fed decapsulated cysts of Artemia. Fish Physiol Biochem. 22:237–244. doi: 10.1023/A:1007893223006
- Huisman J, Tolman GH. 1992. Antinutritional factors in the plant proteins of diets for non ruminants. In: Garnsworty PC, Haresing W, Cole DJA, editors. Recent advances in animal nutrition. Oxford: Butterworth-Heinemann Ltd; p. 3–31.
- Kolkovski S. 2013. Microdiets as alternatives to live feeds for fish larvae in aquaculture: improving the efficiency of feed particle utilization. In: Allan G, Burnell G, editors. Advances in aquaculture hatchery technology. Cambridge: Woodhead Publishing Limited; p. 203–222.
- Kolkovski S, Tandler A, Izquierdo MS. 1996. The effects of live food and dietary digestive enzymes on the efficiency of microdiets for seabass (Dicentrarchus labrax) larvae. Aquaculture. 148:313–322. doi: 10.1016/S0044-8486(96)01366-X
- Kolkovski S, Tandler A, Kissil Wm, Gertler A. 1993. The effect of dietary exogenous digestive enzymes on ingestion, assimilation, growth and survival of gilthead seabream (Sparus aurata, Sparidae, Linnaeus) larvae. Fish Physiol Biochem. 12:203–209. doi: 10.1007/BF00004368
- Krogdahl Å, Bakke-McKellep AM, Baevefjordi G. 2003. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquacult Nutr. 9:361–371. doi: 10.1046/j.1365-2095.2003.00264.x
- Kurokawa T, Shiraishi M, Suzuki T. 1998. Quantification of exogenous protease derived from zooplankton in the intestine of Japanese sardine Sardinops melanotictus larvae. Aquaculture. 161:491–499.
- Monfort MC. 2010. Present market situation and prospects of meagre (Argyrosomus regius), as an emerging species in mediterranean aquaculture. In: Studies and reviews general fisheries commission for the mediterranean No 89. Rome: FAO; p. 28.
- Moyano FJ, Alarcón FJ, Díaz M. 1998. Comparative biochemistry of fish digestive proteases applied to the development of in vitro digestibility assays. Comp Biochem Physiol. 5:136–143.
- Moyano FJ, Barros AM, Prieto A, Cañavate JP, Cárdenas S. 2005. Evaluación de la ontogenia de enzimas digestivas en larvas de hurta, Pagrus auriga (Pisces: Sparidae). Revista AquaTIC. 22:39–47.
- Moyano FJ, Díaz M, Alarcón FJ, Sarasquete MC. 1996. Characterisation of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem. 15:121–130. doi: 10.1007/BF01875591
- Moyano López FJ, Martínez Díaz I, Díaz López M, Alarcón López FJ. 1999. Inhibition of digestive proteases by vegetable meals in three species (Sparus aurata), tilapia (Oreochromis niloticus) and African sole (Solea senegalensis). Comp Biochem Physiol B. 122:327–332. doi: 10.1016/S0305-0491(99)00024-3
- Munilla-Moran R, Starch JR, Barbout A. 1990. The role of exogenous enzymes in digestion in cultured turbot larvae (Scophthalmus maximus). Aquaculture. 88:337–350. doi: 10.1016/0044-8486(90)90159-K
- Naz M. 2008. The changes in the biochemical compositions and enzymatic activities of rotifer (Branchionus plicatilis, Müller) and Artemia during the enrichment and starvation period. Fish Physiol Biochem. 34:391–404. doi: 10.1007/s10695-007-9199-5
- Papadakis IE, Kentouri M, Divanach P, Mylonas CC. 2013. Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture. 388–391:76–88. doi: 10.1016/j.aquaculture.2013.01.012
- Pastor E, Rodríguez-Rúa A, Grau A, Jiménez MT, Durán J, Gil MM, Cárdenas C. 2013. Hormonal spawning induction and larval rearing of meagre, Argyrosomus regius (Pisces: Sciaenidae). Bol Soc Hist Nat Balear. 56:111–127.
- Quéméner L. 2002. Meagre (Argyrosomus regius): biology, fisheries, market and rearing potential. Resources-de-La-mer-Ifremer, Plouzane, France.
- Ribeiro L, Zambonino-Infante JL, Cahu C, Dinis MT. 1999. Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture. 179:465–473. doi: 10.1016/S0044-8486(99)00180-5
- Solovyev MM, Campoverde C, Öztürk S, Moreira C, Diaz M, Moyano FJ, Estévez A, Gisbert E. 2016. Morphological and functional description of the development of the digestive system in meagre (Argyrosomus regius): an integrative approach. Aquaculture. 464:381–391. doi: 10.1016/j.aquaculture.2016.07.008
- SPSS. 2006. SPSS Base 15.0 user’s guide for Windows. Chicago: SPSS; 591 p.
- Süzer C, Kamacı HO, Çoban D, Yıldırım Ş, Fırat K, Saka S. 2013. Functional changes in digestive enzyme activities of meagre (Argyrosomus regius; Asso, 1801) during early ontogeny. Fish Physiol Biochem. 39:967–977. doi: 10.1007/s10695-012-9755-5
- Tacon AGJ. 1997. Fishmeal replacers: review of antinutrients within oilseeds and pulses. A limiting factor for the aquafeed green revolution? In: Tacon AGJ, Basurco B, editors. CIHEAM cahiers options Meditérranéennes n:22. Zaragoza: Feeding Tomorrow’s Fish; p. 153–182.
- Vallés R, Estévez A. 2013. Light conditions for larval rearing of meagre (Argyrosomus regius). Aquaculture. 376–379:15–19. doi: 10.1016/j.aquaculture.2012.11.011
- Vallés R, Estévez A. 2015. Effect of different enrichment products rich in docosahexaenoic acid on growth and survival of meagre, Argyrosomus regius (Asso, 1801). J World Aquacult Soc. 46(2):191–200. doi: 10.1111/jwas.12175
- Walter HE. 1984. Proteinases: methods with haemoglobin, casein and azocoll as substrates. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; p. 270–277.
- Yúfera M, Fernandez-Diaz C, Pascual E. 1999. A highly efficient microencapsulated food for rearing early larvae of marine fish. Aquaculture. 177:249–256. doi: 10.1016/S0044-8486(99)00088-5
- Yúfera M, Fernandez-Díaz C, Pascual E, Sarasquete MC, Moyano FJ, Díaz M, Alarcón FJ, García-Gallego M, Parra G. 2000. Towards an inert diet for first-feeding gilthead seabream (Sparus aurata) larvae. Aquacult Nutr. 6:143–152. doi: 10.1046/j.1365-2095.2000.00110.x
- Zambonino Infante JL, Cahu CL. 1994. Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem. 12:399–408. doi: 10.1007/BF00004304
- Zambonino Infante JL, Cahu CL. 2001. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol C. 130:477–487.
