ABSTRACT
This study was conducted to determine the goodness of fit of Gompertz, Logistic, Von Bertalanffy, Richards, Levakovich and Janoschek growth models in Japanese quail. Therefore, weekly live-weight data obtained from 372 females and 339 males were fitted. Females’ live weights were found to be higher than that of males, and the first divergence in the growth of female and male birds occurred in 21–28 days, and it survived until the experiment (P < .001). The coefficient of determination (R2), adjusted coefficient of determination (adj. R2), mean square error (MSE), Akaike’s information criteria (AIC) and Bayesian information criterion (BIC) were used to determine the best growth model. R2 and adjusted R2 values of the growth models were similar and close to 1, indicating that all models perform well in describing age-related changes in live weight in quail. Based on the MSE, AIC and BIC values, Richards model was determined to be the best fitting model to the growth data of both sexes. Consequently, it has been demonstrated that Richards function which has a flexible structure in terms of inflection point is the most appropriate growth function for both female and male birds.
1. Introduction
Growth in an animal is a whole of complex physiological and morphological processes from hatching to maturity which is defined as the increases in the weight and volume measurements of the organs or body for a given time (Topal et al. Citation2003; Citation2004; Topal & Bölükbaşı Citation2008). Numerous growth models have been used considering the growth of poultry species. There are differences between species, lines or individuals in terms of growth (Akbaş & Yaylak Citation2000; Narinç, Karaman et al. Citation2010). Live weights of birds at certain time points are related to both genetic factors and environmental conditions. Growth modelling in poultry species gives information on suitable slaughter age, general management and health conditions, age of sexual maturity and the effects of genetic improvement studies. Determining the deviation on the standard growth curve of the production flock in the feeding period is carried out to eliminate the negative effects (Akbaş & Oğuz Citation1998; Narinç, Üçkardeş et al. Citation2014). Scientists have been working on the expression of growth with different mathematical functions for a long time. In the case of birds, the observed growth curve is a sigmoidal (S-shape) structure (Akbaş & Oğuz Citation1998). Generally, semi-empirical non-linear regression functions have been used to model growth. These functions have a varying number of parameters, among which at least one has a biological meaning (Akbaş & Oğuz Citation1998; Tzeng & Becker Citation1981). The most common growth models used in poultry animals are Gompertz, Richards, Von Bertalanffy, Brody, Logistic, Negative Exponential, Morgan–Mercer–Flodin and recently the Hyperbolastic models (Ahmadi & Mottaghitalab Citation2007; Narinç, Aksoy, Karaman Citation2010).
Japanese quail (Coturnix coturnix japonica) have high meat and egg production capability. Having a short generation interval of three to four months, Japanese quail is used in genetic improvement studies, animal production treatments, and health and behavioural sciences as a model for poultry species (Akbaş & Oğuz Citation1998; Karabağ et al. Citation2010; Alkan et al. Citation2012). Recently, quail commercial production has increased, especially in South America, the Middle East and some African countries. In many studies conducted for the modelling of Japanese quail growth data, it has been reported that Gompertz model is the best model in terms of goodness-of-fit criteria (Tzeng & Becker Citation1981; Akbaş & Oğuz Citation1998; Narinç, Aksoy, Karaman Citation2010, Alkan et al. Citation2009; Alkan et al. Citation2012). In addition, Logistic and Von Bertalanffy growth models were used extensively in many studies (Narinç, Aksoy, et al. 2010). A common characteristic of these functions is the fixed model inflection point. Inflection point weight is identified as 37% of the asymptotic weight in the Gompertz model, 50% of the Logistic growth function and 37% of the Von Bertalanffy. This situation comes with some drawbacks. In fixed growth models, the genetic variations of asymptotic weight and point of inflection weight are equal and this situation is a problem for genetic improvement studies (Porter et al. Citation2010).
Recently, some researchers emphasized the use of flexible alternative models. Ahmadi and Mottaghitalab (Citation2007) applied a flexible hyperplastic model in evaluating broiler growth data and compared it with Gompertz and Richards models. Similarly, Porter et al. (Citation2010) used flexible structures of Richards, Von Bertalanffy and Morgan models alternatively to the Gompertz model in order to model the growth in turkeys. The aim of this study was to compare non-linear models for best fitting which are used to determine the age-related changes in the live weight of female and male quails. The growth was followed and modelled with commonly used models such as Gompertz, Logistic and Von Bertalanffy. Moreover, flexible functions such as Richards, Levakovich and Janoschek were used to model the growth. Differences between the female and male quails were tested with profile analysis. This study aimed to compare male and female quail growth with the most appropriate function according to the goodness-of-fit criteria.
2. Material and methods
2.1. Animal material and husbandry
This study was performed in the Poultry Research Unit of Namık Kemal University, Turkey. Japanese quail (Coturnix coturnix japonica) were used as animal material. Approximately 1200 eggs were obtained from non-selected 40 males and 120 females. A total of 711 birds, including 372 females and 339 males were used in the study. All chicks were wing-banded and then weighted from hatching to six weeks of age. The chicks were housed in heated brooding cages (82.56 cm2/quail) for the first three weeks. Then, they were transferred to grower cages (150 cm2/quail). The diet supplied contained 24% CP (crude protein) and 2900 kcal of ME (metabolizable energy)/kg, and ad libitum feeding and a 23-h lighting programme were applied from hatching to the end of the experiment (Narinç et al. Citation2016). The study was approved by the Animal Experimentation Ethics Committee of Namık Kemal University (Protocol 2014/06).
2.2. Profile analysis
In determination of the difference between female and male quails in terms of body weight measurements at a time point, profile analysis method was utilized. Profile analysis is a special case of multivariate analysis of variance (MANOVA) (Alkan et al. Citation2012; Narinç, Aksoy, Karaman, Çürek İlaslan Citation2010). The method can be utilized to compare profiles of the levels of an independent variable either when different traits from the same experimental unit were considered, or when a single trait of the same unit was measured at several time points. Basically three hypotheses are tested by profile analysis. These tests are parallelism (H01), overlap (H02) and levels (H03) of profiles. The most emphasized test in profile analysis is the parallelism test, and other tests depend on the provision of parallelism condition. Profiles of the groups are parallel if the differences between successive measurements of the dependent variable are the same at all levels of the independent variable. Null hypothesis is related to the parallelism test.Here, ‘k’ and ‘p’ represent the number of groups in the independent variable and time points, respectively. The multivariate test statistics of the Hotelling–Lawley trace was used for testing parallelism (Srivastava Citation1987).
2.3. Non-linear regression
In this study, Richards, Janoschek and Levakovich that are known as flexible inflection point models and Gompertz, Logistic and Von Bertalanffy functions which exhibit fixed behaviour in terms of inflection point were used to determine the most consistent growth model for quails (Aggrey Citation2002; Korkmaz & Uckardeş Citation2013; Üçkardeş et al. Citation2013). Expression, growth rate and inflection point coordinates of these functions are presented in . parameter is the asymptotic (mature) weight,
and
are constants,
is the hatching weight in the Janoschek model and
is the age at the point of inflection in the Richards function. Model parameters were analysed utilizing the SAS 9.3 software NLIN (non-linear) procedure using the Levenberg–Marquardt iteration method (Karaman et al. Citation2013).
Table 1. Model expressions and parameters of studied growth functions.
2.4. Goodness-of-fit criteria
The goodness-of-fit criteria to compare the studied functions that explain the growth of Japanese quail are as follows:
• Determination Coefficient, R2 = 1−(SSE/SST), where SSE is the sum of square errors and SST the total sum of squares.
• Adjusted Determination Coefficient, adj. R2 = R2−((k−1/n−k)(1−R2)), where n is the number of observations and k the number of parameters.
• Mean Square Error, MSE = SSE/(n−k), where n is the number of observations, SSE sum square of errors and k the number of parameters.
• Akaike’s Information Criteria, AIC = n.ln(SSE/n) + 2k, where n is the number of observations, SSE sum square of errors and k the number of parameters.
• Schwarz Bayesian Information Criterion, BIC = n.ln(SSE/n) + k.ln(n), where n is the number of observations, SSE sum of square errors and k the number of parameters (Narinç, Üçkardeş et al. Citation2014).
3. Results
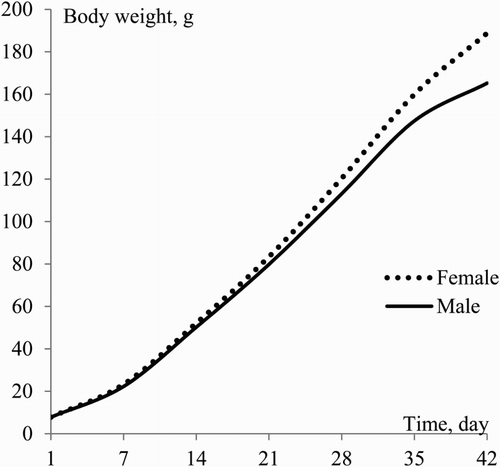
The results of the profile analyses, which were performed to determine the difference between consecutive time points for female and male quails’ growth, are presented in . Actual growth curves of female and male birds are presented in . The test statistic is significant (P < .001) and it was determined that there was no parallelism in the growth of female and male birds. Moreover, there was no difference between the sexes in terms of the first three weeks weight (P < .001).
Table 2. Differences between the gender groups for sequential weeks (Profile analysis results).
The goodness-of-fit criteria (R2, MSE, adj. R2, AIC and BIC) computed using Richards, Janoschek, Levakovich, Gompertz, Logistic and Von Bertalanffy growth models are shown in for both sexes. R2 and adj. R2 values of the growth models were similar and close to 1, indicating that all models perform well in describing age-related changes in live weight in quails. The values of MSE, AIC and BIC ranged between 3.62 and 23.02, −1044.97 and −499.76, and −488.37 and 1029.19, respectively. According to the lowest values of MSE, AIC, BIC, and high R2 and adj. R2, the Richards growth curve was determined to be the best fitting model to the growth data of both female and male quails.
Table 3. Goodness-of-fit criteria for the studied growth functions (female, n = 372; male, n = 339).
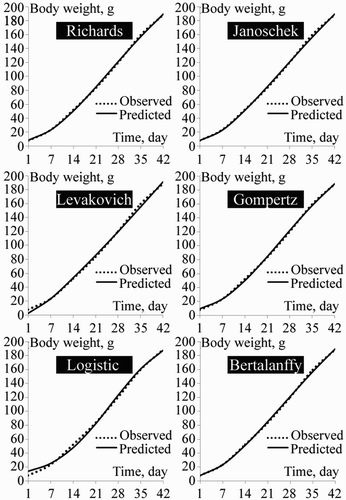
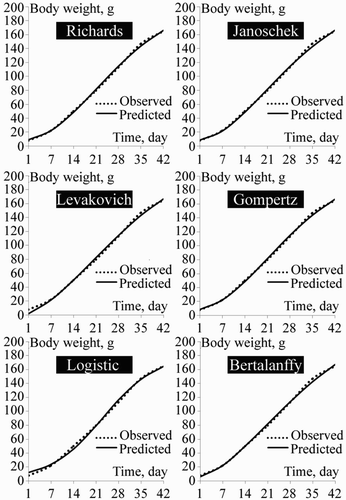
Non-linear regression parameters of Richards, Janoschek, Levakovich, Gompertz, Logistic and Von Bertalanffy functions are presented in . The actual and estimated growth curves of the different models are shown in and .
Table 4. Estimates of parameters for the studied growth functions.
4. Discussion
According to the results of the profile analyses, the first divergence in the growth of female and male birds occurred in 21–28 days (P < .001), and it remained until the end of the experiment (P < .001 for all successive time intervals). Similar findings were reported by many researchers (Alkan et al. Citation2012; Karaman et al. Citation2013). However, some researchers reported that the sexual dimorphism was not observed in quail (Oğuz et al. Citation1996; Beiki et al. Citation2013). This may be caused by environmental factors or empirical analysis. The comparison of the models according to the goodness-of-fit criteria was carried out seperately, as the growth samples of male and female birds were not parallel.
As seen in , R2 and adjusted R2 values of all models were found to be between 0.9987–0.9998 and 0.9901–0.9944, respectively. Many researchers (Balcıoğlu et al. Citation2005; Alkan et al. Citation2009; Narinç, Aksoy, Karaman Citation2010) have reported quite high values of the determination coefficients for growth models such as Richards, Logistic and Von Bertalanffy. In the current study, the best fitting growth model for female quail was determined to be the Richards growth function according to the lowest values of MSE, AIC and BIC (3.62, −1034.12 and −1018.94, respectively). Also, a similar result was found for male quail. MSE, AIC and BIC values of the Richards model were the smallest (6.01, −1044.97 and −1029.19, respectively). The Richards model, which also assesses the shape of a growth curve, has had limited use in quail (Hyankova et al. Citation2001; Aggrey et al. Citation2003; Beiki et al. Citation2013). Beiki et al. (Citation2013) investigated the growth patterns of quail using seven non-linear regression models (Hyperbolastic 1, Hyperbolastic 2, Hyperbolastic 3, Richards, Logistic, Gompertz and Von Bertalanffy). They reported that the Richards growth curve was the best fitting model for quail growth data, which is in agreement with the results of the current study. The Richards model is important not only due to having a flexible structure with respect to the point of inflection, but also due to having more interpretable parameters than others.
Our results are in disagreement with the previous reports putting forward that the Gompertz model was the best-fitting model for galliforms (Tzeng & Becker Citation1981; Akbaş & Oğuz Citation1998; Narinç, Aksoy, Karaman Citation2010). Growth is a phenomenon affected by both genetics and environmental conditions, and thus, it does not depend on species, strain, line or family (Narinç & Aksoy Citation2012; Üçkardeş & Narinç Citation2014). Therefore, it is necessary to determine the best-fitting model for every studied flock. Moreover, the Gompertz model was defined the second best fitting function in the current study. According to our knowledge, there is no study about the analyses of quail growth data using Janoschek and Levakovich functions. Both functions showed good fit to the quail growth data as indicated by the R2 and adj. R2 values. Especially the Janoschek function is the prominent one due to having more interpretable parameters (parameters of mature weight and hatching weight).
Asymptotic weight parameter values of the Richards model for female and male quail (324.0 and 216.4 g) are in agreement with the value reported by Beiki et al. (Citation2013) for their control group involving both sexes. In another study (Akbaş & Oğuz Citation1998), the estimated mature weight parameter (β0) of the Gompertz model for the selection line (239.5 g) was higher than that of the control line (208.3 g), and that of female quail (244.4 g) were higher than male ones (203.5 g). In most of the studies in which the growth of Japanese quail was examined by the Gompertz model, the mature weight parameter was found to be from 204 to 281 g (Akbaş & Oğuz Citation1998; Kızılkaya et al. Citation2005; Narinç et al. Citation2009; Alkan et al. Citation2009; Narinç, Aksoy, Karaman Citation2010). Alkan et al. (Citation2009) applied selection to increase the live weight in Japanese quail. They estimated β0 parameter values to be 295–306 g and 151–164 g for a selected and a non-selected line, respectively. In the other study, Alkan et al. (Citation2009) performed thermal manipulation in the embryonic period of quail, and they reported that the mature weight parameters were found to be between 203 and 241 g. It is expected that quail growth and growth curve parameters can be changed via breeding studies or environmental practices (Narinç & Aksoy Citation2014; Narinç, Aksoy et al. Citation2014).
In all models, β1 and β2 are constants related to the shape of the growth curves, and are not intended to be biological meaningful. β1 and β2 parameter values for both male and female quail were in the range of 0.002–957786 and 0.039–3.846, respectively. In the current study, β3 parameter of the Richards model representing age at the point of inflection was estimated to be 25.29 and 21.30 days for female and male quail, respectively. Age at the point of inflection of the Richards function for a non-selected control quail line was determined as 17.08 and 16.38 days for female and male quail, respectively (Aggrey et al. Citation2003). It is thought that these values are lower than that of the current study due to using lower weight quail. Similarly, Aggrey et al. (Citation2003) reported that the mature weight parameters (β0) of the Richards function were found to be 144.01 g and 104.42 g for female and male quail.
In the current study, age and weight at the point of inflection of the Gompertz model were determined to be 25.05 days and 105.84 g for female quail, 21.20 days and 81.96 g for male quail. However, Akbaş & Oğuz (Citation1998) reported lower values (19.75 days – 88.13 g and 20.20 days – 76.62 g, respectively) for age and weight at inflection point using the Gompertz model in a selected quail line and a randomly mated line. In other study, Kızılkaya et al. (Citation2005) reported that age and weight at the point of inflection of the Gompertz model were found to be between 16.19 and 17.05 days, and from 81.57 to 82.96 g, respectively. Alkan et al. (Citation2009) estimated age and weight at the point of inflection using the Gompertz model for selected and control lines. They reported that the mentioned parameters in the selection line were found to be 15.68 days and 113 g for female, and 17.64 days and 108 g for male quail. Also, 18.27 days and 82.3 g for female quail, and 17.99 days and 75 g for male quail were found for the control line. As shown here, growth curve parameters of quail can be affected by both the selection and environmental conditions.
As a result, it has been demonstrated that the Richards function, which has a flexible structure in terms of inflection point, is the most appropriate growth function for both female and male birds. In addition, β3 parameter which was estimated with the Janoschek function represents the hatching weight. This parameter was estimated to be 7.27 g and 8.07 g for female and male birds, respectively. Potential use of the β3 parameter of the Janoschek model in breeding programmes can be examined by revealing its genetic relationship with weekly body weights, adult weight parameter and point of inflection coordinates. In order to include the parameters of Richards and Janoschek models in breeding programmes, heritabilities of the parameters and their genetic relationships with production traits should be estimated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aggrey SE. 2002. Comparison of three nonlinear and spline regression models for describing chicken growth curves. Poult Sci. 81:1782–1788. doi: 10.1093/ps/81.12.1782
- Aggrey SE, Ankra-Badu BA, Marks HL. 2003. Dynamics of relative growth rate in Japanese quail lines divergently selected for growth and their control. Growth Develop Aging. 67:47–54.
- Ahmadi H, Mottaghitalab M. 2007. Hyperbolastic models as a new powerful tool to describe broiler growth kinetics. Poult Sci. 86:2461–2465. doi: 10.3382/ps.2007-00086
- Akbaş Y, Oğuz I. 1998. Growth curve parameters of line of Japanese quail (Coturnix coturnix japonica), unselected and selected for four-week body weight. Arch Geflugelkd. 62:104–109.
- Akbaş Y, Yaylak E. 2000. Heritability estimates of growth curve parameters and genetic correlations between the growth curve parameters and weights at different age of Japanese quail. Arch Geflugelkd. 64:141–146.
- Alkan S, Mendeş M, Karabağ K, Balcıoğlu MS. 2009. Effects short term divergent selection of 5-week body weight on growth characteristics in Japanese quail. Arch Geflugelkd. 73:124–131.
- Alkan S, Narinç D, Karslı T, Karabağ K, Balcıoğlu MS. 2012. Effects of thermal manipulations during early and late embryogenesis on growth characteristics in Japanese quails. Arch Geflugelkd. 76:184–190.
- Balcıoğlu MS, Kızılkaya K, Yolcu HI, Karabağ K. 2005. Analysis of growth characteristics in short-term divergently selected Japanese quail. S Afr J Anim Sci. 35:83–89.
- Beiki H, Pakdel A, Moradi-shahrbabak M, Mehrban H. 2013. Evaluation of growth functions on Japanese quail lines. J Poult Sci. 50:20–27. doi: 10.2141/jpsa.0110142
- Hyankova L, Knizetova H, Dedkova L, Hort J. 2001. Divergent selection for shape of growth curve in Japanese quail. 1. Responses in growth parameters and food conversion. Br Poult Sci. 42:583–589. doi: 10.1080/00071660120088371
- Karabağ K, Alkan S, Balcıoğlu MS. 2010. The differences in some production and clutch traits in divergently selected Japanese quails. Kafkas Univ Vet Fak Derg. 16:383–387.
- Karaman E, Narinc D, Fırat MZ, Aksoy T. 2013. Non-linear mixed effects modeling of growth in Japanese quail. Poult Sci. 92:1942–1948. doi: 10.3382/ps.2012-02896
- Kızılkaya K, Balcıoğlu MS, Yolcu Hİ, Karabağ K. 2005. The application of exponential method in the analysis of growth curve for Japanese quail. Arch Geflugelkd. 69:193–198.
- Korkmaz M, Uckardes F. 2013. Transformation to some growth models widely used in agriculture. J Anim Plant Sci. 23:840–844.
- Narinç D, Aksoy T. 2012. Effects of mass selection based on phenotype and early feed restriction on the performance and carcass characteristics in Japanese quails. Kafkas Univ Vet Fak Derg. 18:425–430.
- Narinç D, Aksoy T. 2014. Effects of multi-trait selection on phenotypic and genetic changes in a meat type dam line of Japanese quail. Kafkas Univ Vet Fak Derg. 20:231–238.
- Narinç D, Aksoy T, Kaplan S. 2016. Effects of multi-trait selection on phenotypic and genetic changes in Japanese quail (Coturnix coturnix Japonica). J Poult Sci. 53:103–110. doi: 10.2141/jpsa.0150068
- Narinç D, Aksoy T, Karaman E. 2010. Genetic parameters of growth curve parameters and weekly body weights in Japanese quails (Coturnix coturnix japonica). J Anim Vet Adv. 9:501–507. doi: 10.3923/javaa.2010.501.507
- Narinç D, Aksoy T, Karaman E, Çürek İlaslan D. 2010. Analysis of fitting growth models in medium growing chicken raised indoor system. Trends Anim Vet Sci. 1:12–18.
- Narinç D, Aksoy T, Karaman E, Fırat MZ. 2014. Genetic parameter estimates of growth curve and reproduction traits in Japanese quail. Poult Sci. 93:24–30. doi: 10.3382/ps.2013-03508
- Narinç D, Aksoy T, Karaman E, Karabağ K. 2009. Effect of selection applied in the direction of high live weight on growth parameters in Japanese quail. Akdeniz Univ Zir Fak Derg. 22:149–156.
- Narinç D, Karaman E, Fırat MZ, Aksoy T. 2010. Comparison of non-linear growth models to describe the growth in Japanese quail. J Anim Vet Adv. 9:1961–1966. doi: 10.3923/javaa.2010.1961.1966
- Narinç D, Üçkardeş F, Aslan E. 2014. Egg production curve analyses in poultry science. Worlds Poult Sci. 70:817–828. doi: 10.1017/S0043933914000877
- Oğuz I, Altan O, Kırkpınar F, Settar P. 1996. Body weights, carcase characteristics, organ weights, abdominal fat and lipid content of liver and carcase’ in two lines of Japanese quail (Coturnix coturnix Japonica), unselected and selected for four week body weight. Br Poult Sci. 37:579–588. doi: 10.1080/00071669608417888
- Porter T, Kebreab E, Darmani Kuhi H, Lopez S, Strathe AB, France J. 2010. Flexible alternatives to the Gompertz equation for describing growth with age in turkey hens. Poult Sci. 89:371–378. doi: 10.3382/ps.2009-00141
- Srivastava MS. 1987. Profile analysis of several groups. Comm in Statist A Theory and Methods. 16:909–926. doi: 10.1080/03610928708829411
- Topal M, Bölükbaşı Ş. 2008. Comparison of nonlinear growth curve models in broiler chickens. J Appl Anim Res. 34:149–152. doi: 10.1080/09712119.2008.9706960
- Topal M, Özdemir M, Aksakal V, Yıldız N, Doğru Ü. 2004. Determination of the best nonlinear function in order to estimate growth in Morkaraman and Awassi lambs. Small Ruminant Res. 55:229–232. doi: 10.1016/j.smallrumres.2004.01.007
- Topal M, Yıldız N, Esenbuğa N, Aksakal V, Macit M, Özdemir M. 2003. Determination of best fitted regression model for estimation of body weight in Awassi sheep. J Appl Anim Res. 23:201–208. doi: 10.1080/09712119.2003.9706422
- Tzeng RY, Becker WA. 1981. Growth patterns of body and abdominal fat weights in male broiler chickens. Poult Sci. 60:1101–1106. doi: 10.3382/ps.0601101
- Üçkardeş F, Korkmaz M, Ocal P. 2013. Comparison of models and estimation of missing parameters of some mathematical models related to in situ dry matter degradation. J Anim Plant Sci. 23:999–1007.
- Üçkardeş F, Narinç D. 2014. An application of modified Logistic and Gompertz growth models in Japanese quail. Indian J Anim Sci. 84:903–907.