ABSTRACT
To explore the molecular response of fish larvae to nutritional manipulation, the partial sequences of bone morphogenetic protein (BMP1, BMP2, BMP4, BMP5, and BMP10) genes were obtained, and their expressions were quantified on golden pompano Trachinotus ovatus at 28 days post hatching. Artemia nauplii were separately enriched with Nannochloropsis and Algamac 3080, and non-enriched Artemia nauplii were used as a control feed. The lowest jaw deformity rate occurred in fish fed Algamac 3080 enriched Artemia nauplii. The expression level of BMP4 of fish fed Algamac 3080 enriched Artemia nauplii was significantly lower than those fed Nannochloropsis enriched and non-enriched Artemia nauplii. The lowest expression level of BMP10 was found in fish fed non-enriched Artemia nauplii, and the highest expression of BMP10 was observed in fish fed Algamac 3080 enriched Artemia nauplii. Correlation analysis indicates that nutritional manipulations can significantly affect the expression level of BMP4 and BMP10 genes. The concentration of dietary docosahexaenoic acid (DHA) can significantly affect the expression level of BMP10. This study indicates that the use of Algamac 3080 enriched Artemia nauplii results in fast fish growth and low jaw deformity, and BMP4 and BMP10 gene expressions were closely related to jaw deformity in golden pompano larvae.
Introduction
Skeleton malformation is a major bottleneck continually hindering the production of marine fish fingerlings (Sandel et al. Citation2010; Cobcroft & Battaglene Citation2013; Ma et al. Citation2014c). Malformed fish are usually sold at a low price or are manually removed before sale to market (Ma et al. Citation2014d). Moreover, deformation in fish can negatively affect fish growth, survival, food conversion ratio, and susceptibility to stress and pathogens (Andrades et al. Citation1996; Koumoundourous et al. Citation1997; Boglione et al. Citation2013). Although genetic factors (Ferguson & Danzmann Citation1998; Gjerde et al. Citation2005; Castro et al. Citation2007; Ma et al. Citation2014c), environmental factors (Hattori et al. Citation2004; Sfakianakis et al. Citation2004; Georgakopoulou et al. Citation2010; Owen et al. Citation2012), parasites and pesticides (Liang et al. Citation2012; Liu et al. Citation2012) can affect fish bone development, increasingly evidence has showed that nutritional factors during larval fish rearing can directly cause skeleton malformation (Villeneuve et al. Citation2005a; Mazurais et al. Citation2009; Darias et al. Citation2011; Yang et al. Citation2015).
Skeletogenesis is an important developmental process in vertebrates through which the skeletal development is completed (Grünbaum et al. Citation2012). During this process, the differentiation and proliferation of different cell types (such as chondrocytes, osteoblasts, osteocytes, and osteoclasts) determine the shape, size and mineral composition of bones (Fernández et al. Citation2011). The expression of genes associated with skeletal development not only reflects cell proliferation and differentiation, but can be affected by individual genetic characteristics, biotic, and abiotic factors (Cloutier et al. Citation2010; Fernández et al. Citation2011; Grünbaum et al. Citation2012). Therefore, knowledge on gene expression of fish larvae during skeletogenesis is useful to identify the potential cause of skeleton malformation.
Bone morphogenetic proteins (BMPs) belonging to the super family of transforming growth factor-β (TGF-β) were originally identified as the molecules that induce ectopic bone formation after implantation into rodent muscles (Wozney et al. Citation1988; Chen et al. Citation2004). BMPs are functionally and structurally very conserved throughout animal kingdom, and their biological importance is reflected through functional and structural redundancy of different BMPs in a single species (Razdorov & Vukicevic Citation2012). Generally, BMP1 and BMP2 can stimulate osteoblast, and play an important role in bone fracture repair (Grgurevic et al. Citation2011). BMP2 and BMP4 are involved in skeletogenesis, especially in differentiation of chondrocytes to form cartilage, and both cell differentiation and maturation in the osteoblastie lineage lead to bone formation (Rickard et al. Citation1994; Minina et al. Citation2001; Canalis et al. Citation2003; Wan & Cao Citation2005).
BMPs play an important role in vertebrate biology, acting as morphogens during embryonic development and as bone inducers, being first recognized for their osteogenic properties (Urist Citation1965; Wozney et al. Citation1988). Although several studies have been conducted to analyse the expression of BMP genes in different fish species, most of these studies focused on fish at the embryonic developmental stage (Myers et al. Citation2002; Palomino et al. Citation2014). Knowledge on the expression of BMP genes after hatching and their possible biological significance are very limited in commercially cultured fish species. Up to present, such expression analyses have been only carried out in Atlantic salmon Salmo salar larvae (Ytteborg et al. Citation2010), and European sea bass Dicentrarchus labrax larvae (Villeneuve et al. Citation2005b, Citation2006).
Lipid is the main source of energy supply for larval fish (Sargent et al. Citation1999a, Citation1999b). The morphogenesis of marine fish larvae can be altered by changing dietary lipids (Cahu et al. Citation2003). Among different lipid components, fatty acids are indispensable to modulate the transcription of genes involved in metabolism (Kliewer et al. Citation1997). Although previous studies have demonstrated that feeding with a high level of dietary lipid can improve fish growth performance and reduce skeletal malformation (Cahu et al. Citation2003; Koven et al. Citation2003; Izquierdo et al. Citation2013), excessive dietary lipids and unbalanced fatty acid ratios can also lead to low survival (Fernández & Gisbert Citation2011; Hamre et al. Citation2013; Ma & Qin Citation2014) and skeleton malformation (Izquierdo et al. Citation2010; Haga et al. Citation2011; Izquierdo et al. Citation2013). Nutrient enrichment for Artemia nauplii has been used in larval fish culture for decades, but suitable enrichment formula is not available to lower skeleton malformation in larval fish culture.
Due to high flesh quality, fast growth, and suitability for cage culture, golden pompano has become a good candidate species for aquaculture (Guo et al. Citation2014). Although several aspects pertaining to hatchery rearing of golden pompano larvae have been well studied (Ma et al. Citation2014a, Citation2014b, Citation2014e), high malformation during early development stage of this species has continually affected its production efficiency in hatchery (Ma et al. Citation2014d; Zheng et al. Citation2014). Furthermore, factors causing malformations on this fish are still unclear. Our previous study has found that enriching formula for Artemia nauplii can affect the expression of retinoid X receptors (RXRs) and jaw malformation in golden pompano, but there was no clear correlation between retinoid X receptors (RXRs) expressions and malformation rates when golden pompano larvae were subjected to nutrient change (Yang et al. Citation2015). In order to further explore the relationships between BMPs gene expression and jaw malformation during the Artemia nauplii feeding phase, we cloned BMPs genes in golden pompano larvae and evaluated the correlation between gene expressions and jaw malformation. Such information would contribute to improvement of larval quality and production efficiency in the aquaculture of golden pompano and other related species.
Materials and methods
The experimental material in present study was collected from an early study on nutritional trial and fish growth measurement, fatty acid analysis, and jaw malformation have been reported in Yang et al. (Citation2015). Fertilized eggs of golden pompano hatched in 500-L fibreglass incubators at 26°C with a hatch rate of 97.1 ± 1.9%. On two days post hatch (DPH), larvae were stocked into four 1000-L larval rearing tanks at a density of 60 fish L−1. Larval rearing tanks were supplied with filtered seawater (5-µm pores) from the bottom of each tank through upwelling with a daily exchange rate of 200% tank volume. Water was discharged through an outlet screen (300 µm) at the top of each tank, and the screen was daily cleaned to reduce clogging. Two air stones were used in each tank to maintain dissolved oxygen close to saturation. Light intensity was maintained at 2400 lux, and the light regime was controlled at 14 h light and 10 h dark. Salinity was maintained at 33 ± 0.8‰ and temperature was at 26.5 ± 1.0°C throughout the experiment. Rotifers Brachionus rotundiformis at a density of 10–20 rotifers mL−1 were used to feed fish larvae from 2 to 12 DPH. On the morning of 11 DPH, fish larvae were restocked into 12 500-L larval rearing tanks at a density of 20 fish L−1.
The nutritional manipulation experiment included three dietary treatments with three replicates each. Artemia nauplii were treated in three methods (1) enriched with instant microalgal paste (Nannochloropsis sp., Qingdao Hong Bang Biological Technology Co., Ltd, Qingdao, China), (2) enriched with Algamac 3080® (Aquafauna, USA), and (3) without any enrichment as control. For each treatment, three replicate tanks were used in this study, and a total of nine tanks were used in this study. Artemia cysts were produced from the Great Salt Lake, UT, USA (INVE Aquaculture). Artemia nauplii were fed to fish from 11 to 27 DPH. On 11 DPH, Artemia nauplii were first introduced at 200 nauplii L−1, and then added with a daily increment of 90% by number. For the analysis of gene expression, samples were collected from each tank. For each dietary treatment, samples in three replicates were collected.
Fish growth was determined by specific growth rate (SGR) as %/day: SGR = 100 × (Ln(SLf) − Ln(SLi))/Δt, where SLf and SLi are the final and initial fish total length (mm), respectively, and Δt is the time interval (days) between samples. At the end of this experiment, 50 fish larvae from each tank were sampled for assessing growth and jaw malformation. The remaining fish in each rearing tank were harvested and counted for survival determination.
Total RNA extraction and reverse transcription
On 28 DPH, approximately 50 individuals were collected from each tank and dissected under a stereo microscope, and jaws were collected for total RNA extraction. Total RNA was extracted using TRIzol (Invitrogen, USA). RNA integrity was verified by electrophoresis on a formaldehyde-agarose gel (1.2%). The RNA concentration was measured by absorbance at 260 nm and the purity was determined at the OD 260/280 ratio and agarose gel electrophoresis. RNA was reverse-transcribed to cDNA with oligo (dT) primers using a PrimeScript first strand cDNA synthesis kit (TaKaRa Biotechnology, Dalian Co., Ltd). The cDNA was used as a template in subsequent PCR.
Cloning of the genes cDNA
Based on unpublished golden pompano transcriptome sequences (Illumina HiSeq2000, annotated by NR, KOG, KEGG, and Swissprot), the genes cloning primers were designed (). The PCR reactions systems were as follows: 1 μL of golden pompano larval cDNA, 1 μL of gene-specific forward primer (F), 1 μL of gene-specific reverse primer(R), 0.5 μL of ExTaq, 5 μL of PCR buffer, 4 μL of dNTP mixture (2.5 μM), 37.5 μL of ddH2O, in a total volume of 50 μL. The PCR conditions were as follows: denaturation at 94°C for 1 min, 35-cycles of 94°C for 30 s, annealing temperature of each genes for 30s, 72°C for 4 min, followed by a 10 min extension at 72°C. The PCR products were cloned into the PMD-19 T vector (TAKARA, Japan), and sequenced. Identities and positives alignment analysis of BMPs in golden pompano was conducted by using nucleotide BLAST (http://www.ncbi.nlm.nih.gov/).
Table 1. Summary of genes cloning primers used in this study.
Gene transcriptional analysis by quantitative real-time PCR
Quantitative real-time PCR (qPCR) was used to analyse the expression levels of BMP genes in golden pompano larvae. Gene-specific primer pair for BMP genes () were amplified in LightCycler480 II (Roche, Switzerland). The qPCR was performed using SYBR® Premix Ex TaqTM II (TaKaRa, Japan), and EF-1α (GenBank Accession No. KT727924) was used as the internal reference and amplified. The 10 μL reaction systems contained 5 μL of 2×SYBR® Premix Ex Taq™ II, 0.4 μL of each forward and reverse primer (10 μM), 1 μL of cDNA template, and 3.2 μL of sterile distilled water. The cycling conditions for BMP genes and EF1α were as follows: 1 min at 95°C, followed by 40-cycles 95°C for 15 s, and 60°C for 1 min. Dissociation curves were employed to ensure that only one single PCR product was amplified in each gene reaction. For each test, three replicates were performed in this study. The relative quantification (RQ) was calculated using the ΔΔCT (comparative threshold cycle) method (ΔCT = CT of target gene − CT of EF-1α, ΔΔCT = ΔCT of any sample − ΔCT of calibrator sample). The efficiencies of the primers (E) were EBMP2 = 0.998, EBMP4 = 1.004, EBMP5 = 0.923, EBMP10 = 1.004.
Table 2. Summary of qPCR primers used in this study.
Fatty acid and jaw malformation analysis
The nutritional content of Artemia nauplii was assessed when fish larvae were at 18 DPH. Fatty acids were analysed at South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China following the method described by Ma and Qin (Citation2014). Jaw malformation was examined under a stereo microscope (Olympus SZ, Tokyo, Japan) using the criteria described by Ma et al. (Citation2014d). Jaw malformation (%) was calculated as Jaw malformation = (malformed larvae/total larvae) × 100%.
Statistical analysis
The data in this paper were expressed as mean ± SD, and tested by one-way ANOVA (PASW Statistics 18.0, Chicago, SPSS Inc.). When a significant treatment effect was found, Tukey’s test was performed for multiple range comparisons with the level of significant difference set at P < .05. All the data were tested for normality, homogeneity, and independence to satisfy the assumptions of ANOVA.
Results
Fatty acid compositions of enriched and non-enriched Artemia nauplii are shown in , which were reported in our previous study (Yang et al. Citation2015). The highest SGR was obtained in fish fed Artemia nauplii enriched with Algamac 3080 and the lowest SGR was observed in fish fed non-enriched Artemia nauplii. The highest survival was achieved in fish fed non-enriched Artemia or Nanochloropsis enriched Artemia (P < .05, ), and the lowest survival was observed when fish were fed with Algamac 3080 enriched Artemia nauplii (P < .05). On 28 DPH, the jaw deformity of fish larvae fed Algamac 3080 enriched Artemia nauplii was significantly lower than fish fed non-enriched Artemia nauplii or Nannochloropsis enriched Artemia nauplii (P < .05). However, the jaw deformity was not significantly different between fish fed non-enriched Artemia nauplii and Nannochloropsis enriched Artemia nauplii (P > .05).
Table 3. Fatty acid composition (% of total fatty acids) of enriched and non-enriched Artemia nauplii (Yang et al. Citation2015). Different letters represent significant differences at P < 0.05.
Table 4. SGR, coefficients of variation, final survival rate, and jaw deformity rate of golden pompano larvae fed with enriched and non-enriched Artemia nauplii (Yang et al. Citation2015). Different letters represent significant differences at P < 0.05.
Cloning and expressions of the BMP genes
Partial sequences of BMP1, BMP2, BMP4, BMP5, and BMP10 genes were obtained after sequencing analysis (Appendix 1–5). The BMP1 gene exhibited high identities with other fish species such as Xiphophorus maculatus (96%), Fundulus heteroclitus (96%), and Maylandia zebra (96%, ), while BMP2 showed high identities with Paralichthys olivaceus (95%), Larimichthys crocea (93%), and Sparus aurata (93%, ). BMP4 showed high identities with Cynoglossus semilaevis (97%), Oreochromis niloticus (96%), Boulengerochromis microlepis (96%), and Steatocranus casuarius (96%, ). BMP5 displayed high identities with M. zebra (97%), L. crocea (96%), and F. heteroclitus (96%, ). BMP10 had high identities with O. niloticus (91%), and L. crocea (86%, ).
Table 5. Identities and positives alignment analysis of BMP1 in golden pompano using nucleotide BLAST.
Table 6. Identities and positives alignment analysis of BMP2 in golden pompano using nucleotide BLAST.
Table 7. Identities and positives alignment analysis of BMP4 in golden pompano using nucleotide BLAST.
Table 8. Identities and positives alignment analysis of BMP5 in golden pompano using nucleotide BLAST.
Table 9. Identities and positives alignment analysis of BMP10 in golden pompano using nucleotide BLAST.
Nutrient enhancements significantly affected the gene expressions of BMP4 and BMP10 (P < .05, ). The expression of BMP4 in fish fed non-enriched Artemia nauplii or Nannochloropsis enriched Artemia nauplii was significantly higher than fish fed Algamac 3080 enriched Artemia nauplii (P < .05, ). The expression of BMP4 was not significantly different between fish fed non-enriched Artemia nauplii and Nannochloropsis enriched Artemia nauplii (P > .05). The lowest level of BMP10 expression was observed when fish were fed with non-enriched Artemia nauplii, and the highest BMP10 expression was found in fish fed Algamac 3080 enriched Artemia nauplii ().
Figure 1. Relative expression levels of BMPs in golden pompano larvae fed enriched and non-enriched Artemia nauplii.
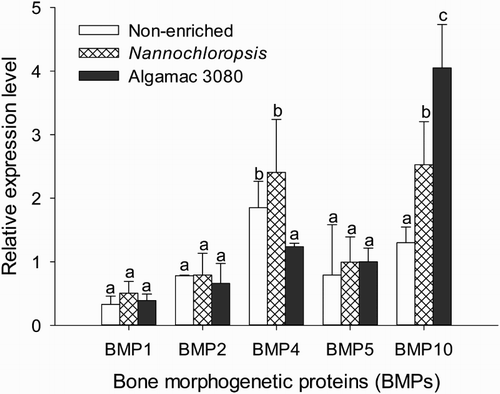
The correlation coefficients between nutrients, growth, jaw deformity, and different BMPs expressions are presented in . Nutrient enhancements were correlated to SGR, jaw deformity, and expressions of BMP4 and BMP10. Jaw deformity of golden pompano larvae was negatively correlated with docosahexaenoic acid (DHA) (r = −0.87, P < .01), EPA (r = −0.83, P < .01), BMP4 expression (r = 0.73, P < .05), and BMP10 (r = −0.86, P < .05, ).
Table 10. Spearman rank correlation coefficients among the response variables.
Discussion
Dietary n-3 highly unsaturated fatty acids such as DHA and EPA are essential to growth of fish larvae (Rezek et al. Citation2010), and their requirements are species-specific (Dantagnan et al. Citation2010). Fish growth rates are related to dietary DHA in many fish larvae such as gilthead seabream S. aurata (Koven et al. Citation1990), red porgy Pagrus pagrus (Roo et al. Citation2009), yellowtail Seiola quiqueradiata (Furuita et al. Citation1996), and striped jack Caranx vinctus (Takeuchi et al. Citation1996). Furthermore, the growth response of fish larvae to different enrichment products is also varied among species. For example, the growth rates of striped bass Morone saxatilis and gilthead seabream S. aurata larvae are not affected by feeding the Artemia nauplii enriched with Algamac 2000 or PL-Cr (DHA-rich phospholipid extract of Crypthecodinium sp.), but the growth rate of halibut Hippoglossus hippoglossus larvae fed Artemia nauplii enriched with DHA Seleco is lower than the larvae fed PL-Cr (Harel et al. Citation2002). In this study, fish growth was improved when fish larvae were fed with Artemia nauplii enriched with Algamac 3080 or Nannochloropsis. The best fish SGR was achieved in the treatment of Algamac 3080, which is consistent with the high dietary DHA levels in Artemia nauplii. Nevertheless, the low survival and high coefficients of variation of fish length in the treatment of Algamac 3080 could also contribute to the high SGR due to the death of small larvae in this treatment.
Although a high content of dietary lipid can improve fish survival, overdosed dietary lipid or unbalanced lipid class composition can also lead to poor growth and abnormal development in fish larvae (Salhi et al. Citation1999; Olsen et al. Citation2000; Kjørsvik et al. Citation2009). For instance, Ma and Qin (Citation2014) reported that a high DHA/EPA ratio in live feed could lead to low survival in yellowtail kingfish Seriola lalandi. In the present study, a higher DHA/EPA ratio (0.36:1) was achieved by enriching Artemia nauplii with Algamac 3080. The high DHA/EPA ratio in the Algamac 3080 treatment led to fast fish growth but low survival. In contrast, better survival was obtained in the non-enriched and Nannochloropsis treatments where the DHA/EPA ratio was 0.07:1–0.22:1. Low fish survival in the Algamac 3080 treatment supports the claim that a high DHA content and a high DHA/EPA ratio may reduce larval fish survival (Planas & Cunha Citation1999) as unbalanced lipid classes in the diet affect digestion and absorption of fatty acids in fish larvae (Salhi et al. Citation1997; Salhi et al. Citation1999).
Jaw malformation has been frequently observed in both artificially reared and wild-caught marine fish (Boglione et al. Citation2013; Ma et al. Citation2014c). Previous studies have indicated that poly unsaturated fatty acids play an important role in bone formation of fish (Izquierdo et al. Citation2010, Citation2013), and dietary fatty acids can alter the composition of bone and cartilage (Kokkinos et al. Citation1993; Watkins et al. Citation1997; Liu et al. Citation2004). Abnormal development of fish larvae may be caused by insufficient dietary n-3 highly unsaturated fatty acids (HUFA) in live food (Hamre et al. Citation2002). A 50% reduction of deformed fish was observed when fish larvae were fed with higher levels of dietary DHA (Izquierdo et al. Citation2010). In our study, fish fed Artemia nauplii enriched with Algamac 3080 showed twofold lower jaw malformation than those fed non-enriched Artemia nauplii or Artemia nauplii enriched with Nannochloropsis. Skeletal malformation was reduced in fish fed Artemia enriched with Algamac 3080, which is coincident with the high DHA content in the feed. This indicates that a dietary DHA level of 2.56% may be suitable for skeletal development in golden pompano larvae.
BMP1 is an astacin metalloprotease with important cellular functions and diverse substrates (Bond & Beynon Citation1995; Sterchi et al. Citation2008). BMP1 plays an essential role in osteogenesis and extracellular matrix, and it can exert influence over the dorsal-ventral structure through an indirect activation of some TGF-β-like proteins (Ge & Greenspan Citation2006a, Citation2006b). In zebrafish, BMP1 is a key portion of the chordin processing activity necessary to the formation of the dorsoventral axis (Jasuja et al. Citation2006). BMP2 and BMP4 are closely related proteins involved in key embryonic processes such as dorsal-ventral axis specification (Graff Citation1997), epithelio-mesenchymal interactions (Vainio et al. Citation1993), and apoptosis (Graham et al. Citation1994; Glozak & Rogers Citation1996; Zou & Niswander Citation1996). The BMP2 in zebrafish is responsible for induction and maintenance of ventro-lateral cell formation during early development, while a missense mutation in the BMP2b gene can lead to an early dorsalized phenotype in the zebrafish swirl mutant, resulting in the lack of cardiogenic mesoderm (Kishimoto et al. Citation1997). Rafael et al. (Citation2006) suggests that the role of BMP2 during vertebrate development is likely to be part of an ancient mechanism. According to our previous study, the ossification process of golden pompano larvae occured around 7 DPH, and most structures were completely formed and mineralized by 18 DPH (Zheng et al. Citation2014). In the present study, the expressions of BMP1 and BMP2 were not significantly affected by the nutrient enrichment by 28 DPH. Reuslts from the present study suggest that the expression of BMP1 and BMP2 in golden pompano may be less senstive to nutrient enrichment after the bone structure is formed and minerlized.
BMP4 plays diverse roles during vertebrate development, and it is involved not only in the formation of embryonic axis and germ layer induction, but also regulates the formation of tissues and organs (e.g. brain, neural crest, muscle, bone, and cartilage) (Hogan Citation1996; Mehler et al. Citation1997; Whitman Citation1998; Dale & Johns Citation1999; Shi & Massague Citation2003). Thus, it has been used to evaluate the effect of micro-nutrients on the skeletal development of marine fish larvae (Villeneuve et al. Citation2005a, Citation2005b, Citation2006). Based on the assumption raised by Villeneuve et al. (Citation2006), the increase in BMP4 and RARγ expression reduces the number of osteoblasts available for bone formation and the loss of bone cells is counterbalanced by the cooperation between retinoic acid and BMP4. In the present study, the expression levels of BMP4 in fish fed non-enriched Artemia nauplii and Nannochloropsis enriched Artemia nauplii were significantly higher than fish fed Algamac 3080 enriched Artemia nauplii. Furthermore, jaw malformation in the treatment of non-enriched and Nannochloropsis was significantly higher than in the treatment of Algamac 3080. These results are consistent with the finding reported by Villeneuve et al. (Citation2006), in which jaw malformation increases when the expression of BMP4 is up-regulated.
Unlike other BMPs, BMP10 is expressed predominantly in the adult heart and to a lesser extent in the liver and lung (Neuhaus et al. Citation1999). During heart development, BMP10 is expressed in the trabeculae, a common ventricular chamber and atrium of the bulbus cordis (Neuhaus et al. Citation1999). In zebrafish, a high level of BMP10 expression was reported in the heart and liver, but a low expression in the brain and kidney (Bland Citation2001). In the present study, nutrient enhancement altered the expression of BMP10 in golden pompano larvae, and the expression of BMP10 was corresponding to jaw malformation.
In summary, nutrient enhancement can affect jaw malformation in fish larvae during the Artemia nauplii feeding phase. Feeding golden pompano larvae with enriched Artemia nauplii significantly reduced the jaw malformation rate, but also decreased survival at the same time. Reduction of jaw malformation may be due to the mortality of fish larvae during test time. However, this may need further verification. Nutritional manipulations can significantly affect the expression levels of BMP4 and BMP10, and the concentration of dietary DHA can significantly affect the expression of BMP10. The expressions of BMP4 and BMP10 varied between different dietary treatments, and the expressions of BMP4 and BMP10 correspond to jaw malformation in golden pompano larvae during the Artemia nauplii feeding phase. This study suggests that measures of BMP4 and BMP10 in golden pompano may serve as a suitable indicator for jaw malformation in the field and aquaculture facility, leading to rapid assessment of nutrient status affecting fish jaw malformation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andrades JA, Becerra J, Fernández-Llebrez P. 1996. Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.). Aquaculture. 141(1–2):1–11. doi: 10.1016/0044-8486(95)01226-5
- Bland RJ. 2001. Isolation, characterisation and evolution of zebrafish (Danio rerio) bmp9, bmp10, and gdf11. Auckland: University of Auckland; p. 334.
- Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagne S, Koumoundouros G. 2013. Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Rev Aquacult. 5:S121–S167. doi: 10.1111/raq.12016
- Bond JS, Beynon RJ. 1995. The astacin family of metalloendopeptidases. Protein Sci. 4:1247–1261. doi: 10.1002/pro.5560040701
- Cahu CL, Infante JLZ, Barbosa V. 2003. Effect of dietary phospholipid level and phospholipid: neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. British J Nutr. 90(1):21–28. doi: 10.1079/BJN2003880
- Canalis E, Economides AN, Gazzerro E. 2003. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocri Rev. 24:218–235. doi: 10.1210/er.2002-0023
- Castro J, Pino A, Hermida M, Bouza C, Chavarrias D, Merino P, Sanchez L, Martinez P. 2007. A microsatellite marker tool for parentage assessment in gilthead seabream (Sparus aurata). Aquaculture. 272:S210–S216. doi: 10.1016/j.aquaculture.2007.08.020
- Chen D, Zhao M, Mundy GR. 2004. Bone morphogenetic proteins. Growth Fac. 22(4):233–241. doi: 10.1080/08977190412331279890
- Cloutier R, Caron A, Grünbaum T, Le François NR. 2010. Effect of water velocity on the timing of skeletogenesis in the Arctic charr, Salvelinus alpinus (Salmoniformes: Teleostei): an empirical case of developmental plasticity. Int J Zool. 2010. Article ID 470456. doi: 10.1155/2010/470546
- Cobcroft JM, Battaglene SC. 2013. Skeletal malformations in Australian marine finfish hatcheries. Aquaculture. 396–399:51–58. doi: 10.1016/j.aquaculture.2013.02.027
- Dale L, Johns CM. 1999. BMP signalling in early Xenopus development. Bioessays. 21:751–760. doi: 10.1002/(SICI)1521-1878(199909)21:9<751::AID-BIES6>3.0.CO;2-I
- Dantagnan P, Borquez A, Hernandez A, Izquierdo M. 2010. Effect of EPA/DHA ratios on the growth and survival of Galaxias maculatus (Jenyns, 1842) larvae reared under different salinity regimes. Aquacult Res. 41:e239–e244. doi: 10.1111/j.1365-2109.2010.02512.x
- Darias MJ, Mazurais D, Koumoundouros G, Le Gall MM, Huelvan C, Desbruyeres E, Quazuguel P, Cahu CL, Zambonino-Infante JL. 2011. Imbalanced dietary ascorbic acid alter molecular pathways involved in skeletogenesis of developing European sea bass (Dicentrarchus labrax, Linnaeus, 1758). Comp Biochem Physio A. doi:10.1016/j.cbpa.2011.01.013
- Ferguson MM, Danzmann RG. 1998. Role of genetic markers in fisheries and aquaculture: useful tools or stamp collecting? Can J Fish Aquat Sci. 55:1553–1563. doi: 10.1139/f98-096
- Fernández I, Darias M, Andree KB, Mazurais D, Zambonino-Infante JL, Gisbert E. 2011. Coordinated gene expression during gilthead sea bream skeletogenesis and its disruption by nutritional hypervitaminosis A. BMP Develop Biol. 11:1–20. doi: 10.1186/1471-213X-11-1
- Fernández I, Gisbert E. 2011. The effect of vitamin A on flatfish development and skeletogenesis: a review. Aquaculture. 315:34–48. doi: 10.1016/j.aquaculture.2010.11.025
- Furuita H, Takeuchi T, Watanabe T, Fujimoto H, Sekiya S, Imaizumi K. 1996. Requirements of larval yellowtail for eicosapentaenoic acid, docosahexaenoic acid, and n-3 highly unsaturated fatty acid. Fish Sci. 62(3):372–379.
- Ge G, Greenspan DS. 2006a. BMP1 controls TGFβ1 activation via cleavage of latent TGFβ-binding protein. J Cell Biol. 175:111–120. doi: 10.1083/jcb.200606058
- Ge G, Greenspan DS. 2006b. Developmental roles of the BMP1/TLD metalloproteinases. Birth Def Res Part C: Embr Today. 78:47–68. doi: 10.1002/bdrc.20060
- Georgakopoulou E, Katharios P, Divanach P, Koumoundouros G. 2010. Effect of temperature on the development of skeletal deformities in gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture. 308:13–19. doi: 10.1016/j.aquaculture.2010.08.006
- Gjerde B, Pante MJR, Baeverfjord G. 2005. Genetic variation for a vertebral deformity in Atlantic salmon (Salmo salar). Aquaculture. 244:77–87. doi: 10.1016/j.aquaculture.2004.12.002
- Glozak MA, Rogers MB. 1996. Specific induction of apoptosis in P19 embryonal carcinoma cells by retinoic acid and BMP2 or BMP4. Develop Biol. 179:458–470. doi: 10.1006/dbio.1996.0275
- Grünbaum T, Cloutier R, Vincent B. 2012. Dynamic skeletogenesis in fishes: insight of exercise training on developmental plasticity. Develop Dynam. 241(10):1507–1524. doi: 10.1002/dvdy.23837
- Graff JM. 1997. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 89:171–174. doi: 10.1016/S0092-8674(00)80196-8
- Graham MA, Francis-West P, Brickell P, Lumsden A. 1994. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 372:684–686. doi: 10.1038/372684a0
- Grgurevic L, Macek B, Mercep M, Jelic M, Smoljanovic T, Erjavec I, Dumic-Cule I, Prgomet S, Durdevic D, Vnuk D, et al. 2011. Bone morphogenetic protein (BMP) 1-3 enhances bone repair. Biochem Biophys Res Commun. 408(1):25–31. doi: 10.1016/j.bbrc.2011.03.109
- Guo H, Ma Z, Jiang S, Zhang D, Zhang N, Li Y. 2014. Length-weight relationship of oval pompano, Trachinotus ovatus (Linnaeus 1758) (Pisces; Carangidae) cultured in open sea floating sea cages in South China Sea. Indian J Fish. 61:93–95.
- Haga Y, Du SJ, Satoh S, Kotani T, Fushimi H, Takeuchi T. 2011. Analysis of the mechanism of skeletal deformity in fish larvae using a vitamin A-induced bone deformity model. Aquaculture. 315:26–33. doi: 10.1016/j.aquaculture.2010.11.026
- Hamre K, Opstad I, Espe M, Solbakken J, Hemre G-I, Pittman K. 2002. Nutrient composition and metamorphosis success of Atlantic halibut (Hippoglossus hippoglossus, L.) larvae fed natural zooplankton or Artemia. Aquac Nutr. 8:139–148. doi: 10.1046/j.1365-2095.2002.00201.x
- Hamre K, Yufera M, Ronnestad I, Boglione C, Conceicao LEC, Izquierdo M. 2013. Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Rev Aquac. 5:S26–S58. doi: 10.1111/j.1753-5131.2012.01086.x
- Harel M, Koven W, Lein I, Bar Y, Behrens P, Stubblefield J, Zohar Y, Place A. 2002. Advanced DHA, EPA, and ARA enrichment materials for marine aquaculture using single cell heterotrophs. Aquaculture. 213:347–362. doi: 10.1016/S0044-8486(02)00047-9
- Hattori M, Sawada Y, Kurata M, Yamamoto S, Kato K, Kumai H. 2004. Oxygen deficiency during somitogenesis causes centrum defects in red sea bream, Pagrus major (Temminck et Schlegel). Aquacult Res. 35:850–858. doi: 10.1111/j.1365-2109.2004.01076.x
- Hogan BLM. 1996. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Devel. 10:1580–1594. doi: 10.1101/gad.10.13.1580
- Izquierdo MS, Scolamacchia M, Betancor M, Roo J, Caballero MJ, Terova G, Witten PE. 2013. Effects of dietary DHA and α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. British J Nutr. 109:1796–1805. doi: 10.1017/S0007114512003935
- Izquierdo MS, Socorro J, Roo J. 2010. Studies on the appearance of skeletal anomalies in red porgy: effect of culture intensiveness, feeding habits and nutritional quality of live preys. J App Ichth. 26:320–326. doi: 10.1111/j.1439-0426.2010.01429.x
- Jasuja R, Voss N, Ge G, Hoffman GG, Lyman-Gingerich J, Pelegri F, Greenspan DS. 2006. Bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis. Mech Develop. 123:548–558. doi: 10.1016/j.mod.2006.05.004
- Kishimoto Y, Lee K, Zon L, Hammerschmidt M, Schulte-Merker S. 1997. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 124:4457–4466.
- Kjørsvik E, Olsen C, Wold P-A, Hoehne-Reitan K, Cahu CL, Rainuzzo J, Olsen AI, Øie G, Olsen Y. 2009. Comparison of dietary phospholipids and neutral lipids on skeletal development and fatty acid composition in Atlantic cod (Gadus morhua). Aquaculture. 294(3–4):246–255. doi: 10.1016/j.aquaculture.2009.06.012
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely B, CKoble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. 1997. Fatty acid and eicosanoids regulated gene expression through direct interactions with peroxisome proliferator-activated recepors α and β. Proc Natl Acad Sci U S A. 94:4318–4323. doi: 10.1073/pnas.94.9.4318
- Kokkinos PP, Shaye R, Alam BS, Alam SQ. 1993. Dietary lipids, prostaglandin E2 levels, and tooth movement in alveolar bone of rats. Calcified Tis Int. 53:333–337. doi: 10.1007/BF01351839
- Koumoundourous G, Oran G, Divanach P, Stefanakis S, Kentouri M. 1997. The opercular complex deformity in intensive gilthead sea bream Sparus aurata L. larviculture. Moment of apparition and description. Aquaculture. 149:215–226. doi: 10.1016/S0044-8486(96)01443-3
- Koven W, van Anholt R, Lutzky S, Ben Atia IB, Nixon O, Ron B, Tandler A. 2003. The effect of dietary arachidonic acid on growth, survival, and cortisol levels in different-age gilthead seabream larvae (Sparus auratus) exposed to handling or daily salinity change. Aquaculture. 228:307–320. doi: 10.1016/S0044-8486(03)00317-X
- Koven WM, Tandler A, Kissil GW, Sklan D, Friezlander O, Harel M. 1990. The effect of dietary (n−3) polyunsaturated fatty acids on growth, survival and swim bladder development in Sparus aurata larvae. Aquaculture. 91(1–2):131–141. doi: 10.1016/0044-8486(90)90182-M
- Liang Q, Xie Y, Fang Z. 2012. Individual and joint toxicity of Zn2+ and Cd2+ during the early embryonic development of zebrafish (Danio rerio). J Fish Sci China. 19:283–292.
- Liu D, Veit HP, Denbow DM. 2004. Effects of long-term dietary lipids on mature bone mineral content, collagen, crosslinks, and prostaglandin E2 production in Japanese quail. Poult Sci. 83:1876–1883. doi: 10.1093/ps/83.11.1876
- Liu Z, Zhang S, Yang J. 2012. Toxic effects of chlorobenzene on embryonic development and larva of zebrafish. Env Sci Technol. 35:25–28.
- Ma Z, Guo H, Zhang D, Hu CQ, Jiang S. 2014a. Food ingestion, consumption, and selectivity of pompano, Trachinotus ovatus (Linnaeus 1758) under different rotifer densities. Aquacult Res. doi:10.1111/are.12413
- Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D. 2014b. Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem. 40:1157–1167.
- Ma Z, Qin JG. 2014. Replacement of fresh algae with commercial formulas to enrich rotifers in larval rearing of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquacult Res. 45:949–960. doi: 10.1111/are.12037
- Ma Z, Tan DAY, Qin JG. 2014c. Jaw deformities in the larvae of yellowtail kingfish (Seriola lalandi Valenciennes, 1833) from two groups of broodstock. Indian J Fish. 61(4):137–140.
- Ma Z, Zheng P, Guo H, Zhang N, Jiang S, Zhang D, Qin JG. 2014d. Jaw malfromation of hatchery reared golden pompano Trachinotus ovatus (Linnaeus 1758) larvae. Aquacult Res. doi:10.1111/are.12569
- Ma Z, Zheng P, Guo H, Zhang N, Wang L, Jiang S, Qin JG, Zhang D. 2014e. Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Nutr. doi:10.1111/anu.12183
- Mazurais D, Glynatsi N, Darias MJ, Christodoulopoulou S, Cahu CL, Zambonino-Infante J-L, Koumoundouros G. 2009. Optimal levels of dietary vitamin A for reduced deformity incidence during development of European sea bass larvae (Dicentrarchus labrax) depend on malformation type. Aquaculture. 294(3–4):262–270. doi: 10.1016/j.aquaculture.2009.06.008
- Mehler MF, Mabie PC, Zhang D, Kessler JA. 1997. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 20:309–317. doi: 10.1016/S0166-2236(96)01046-6
- Minina E, Wenzel HM, Karp S, Gaffield W, McMahon AP, Vortkamp A. 2001. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 128:4523–4534.
- Myers DC, Sepich DS, Solnica-Krezel L. 2002. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Develop Biol. 243:81–98. doi: 10.1006/dbio.2001.0523
- Neuhaus H, Rosen V, Thies RS. 1999. Heart specific expression of mouse BMP-10 a novel member of the TGF-β superfamily. Mech Dev. 80:181–184. doi: 10.1016/S0925-4773(98)00221-4
- Olsen RE, Myklebust R, Ringoe E, Mayhew TM. 2000. The influences of dietary linseed oil and saturated fatty acids on caecal enterocytes in Arctic char (Salvelinus alpinus L.): a quantitative ultrastructural study. Fish Phy Biochem. 22(3):207–216. doi: 10.1023/A:1007879127182
- Owen MG, Eynon B, Woodgate S, Davies SJ, Fox S. 2012. Increased water current induces micro-architectural changes to the vertebral bone of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture. 344–349:141–146. doi: 10.1016/j.aquaculture.2012.03.001
- Palomino J, Herrera G, Dettleff P, Martinez V. 2014. Growth differentiation factor 9 and bone morphogenetic protein 15 expression in previtellogenic oocytes and during early embryonic development of yellow-tail kingfish Seriola lalandi. Biol Res. 47(60):1–7.
- Planas M, Cunha I. 1999. Larviculture of marine fish: problems and perspectives. Aquaculture. 177:171-190.
- Rafael MS, Laize V, Cancela ML. 2006. Identification of Sparus aurata bone morphogenetic protein 2: molecular cloning, gene expression, and in silico analysis of protein conserved features in vertebrates. Bone. 39:1373–1381. doi: 10.1016/j.bone.2006.06.021
- Razdorov G, Vukicevic S. 2012. The use of mass spectrometry in characterization of bone morphogenetic protein from biological samples. In: J. K. Prasain, editor. Trandem mass spectrometry – applications and principles. Rijeka: InTech; p. 259–284.
- Rezek TC, Watanabe WO, Harel M, Seaton PJ. 2010. Effects of dietary docosahexaenoic acid (22:6n-3) and arachidonic acid (20:4n-6) on the growth, survival, stress resistance and fatty acid composition in black sea bass Centropristis striata (Linnaeus 1758) larvae. Aquacult Res. 41:1302–1314. doi: 10.1111/j.1365-2109.2009.02418.x
- Rickard DJ, Sullivan TA, Shenker BJ, Leboy PS, Kazhdan I. 1994. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Develop Biol. 161:218–228. doi: 10.1006/dbio.1994.1022
- Roo FJ, Hernandez-Cruz CM, Socorro JA, Fernandez-Palacios H, Montero D, Izquierdo MS. 2009. Effect of DHA content in rotifers on the occurrence of skeletal deformities in red porgy Pagrus pagrus (Linnaeus, 1758). Aquaculture. 287(1–2):84–93. doi: 10.1016/j.aquaculture.2008.10.010
- Salhi M, Hernández-Cruz CM, Bessonart M, Izquierdo MS, Fernández-Palacios H. 1999. Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut and liver histological structure of gilthead seabream (Sparus aurata) larvae. Aquaculture. 179(1–4):253–263. doi: 10.1016/S0044-8486(99)00194-5
- Salhi M, Izquierdo MS, Hernandez-Cruz CM, Socorro J, Fernandez-Palacios H. 1997. The improved incorporation of polyunsaturated fatty acids and changes in liver structure in larval gilhead seabream fed on microdiets. J. Fish Biol. 51:869-879.
- Sandel E, Nixon O, Lutzky S, Ginsbourg B, Tandler A, Uni Z, Koven W. 2010. The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture. 304(1–4):42–48. doi: 10.1016/j.aquaculture.2010.03.013
- Sargent J, Bell G, McEvoy L, Tocher D, Estevez A. 1999a. Recent developments in the essential fatty acid nutrition of fish. Aquaculture. 177(1–4):191–199. doi: 10.1016/S0044-8486(99)00083-6
- Sargent J, McEvoy L, Estevez A, Bell G, Bell M, Henderson J, Tocher D. 1999b. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture. 179(1–4):217–229. doi: 10.1016/S0044-8486(99)00191-X
- Sfakianakis DG, Koumoundouros G, Divanach P, Kentouri M. 2004. Osteological development of the vertebral column and of the fins in Pagellus erythrinus (L. 1758). Temperature effect on the developmental plasticity and morpho-anatomical abnormalities. Aquaculture. 232:407–424. doi: 10.1016/j.aquaculture.2003.08.014
- Shi Y, Massague J. 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 113:695–700. doi: 10.1016/S0092-8674(03)00432-X
- Sterchi EE, Stocker W, Bond JS. 2008. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 29:309–328. doi: 10.1016/j.mam.2008.08.002
- Takeuchi Y, Masuda R, Ishizaki Y, Watanabe T, Kanematsu M, Imaizumi K, Tsukamoto K. 1996. Determination of the requirement of larval striped jack for eicosapentaenoic acid and docosahexaenoic acid using enriched Artemia nauplii. Fish Sci. 62:760–765.
- Urist MR. 1965. Bone: formation by autoinduction. Science. 150:893–899. doi: 10.1126/science.150.3698.893
- Vainio S, Karavanova I, Jowett A, Thesleff I. 1993. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 75:45–58. doi: 10.1016/S0092-8674(05)80083-2
- Villeneuve L, Gisbert E, Le Delliou H, Cahu CL, Zambonino-Infante JL. 2005a. Dietary levels of all-trans retinol affect retinoid nuclear receptor expression and skeletal development in European sea bass larvae. Br J Nutr. 93(06):791–801. doi: 10.1079/BJN20051421
- Villeneuve L, Gisbert E, Zambonino-Infante JL, Quazuguel P, Cahu CL. 2005b. Effect of nature of dietary lipids on European sea bass morphogenesis: implication of retinoid receptors. Br J Nutr. 94(6):877–884. doi: 10.1079/BJN20051560
- Villeneuve LAN, Gisbert E, Moriceau J, Cahu CL, Infante JLZ. 2006. Intake of high levels of vitamin A and polyunsaturated fatty acids during different developmental periods modifies the expression of morphogenesis genes in European sea bass (Dicentrarchus labrax). Br J Nutr. 95:677–687. doi: 10.1079/BJN20051668
- Wan M, Cao X. 2005. BMP signaling in skeletal development. Biochem Biophys Res Commun. 328:651–657. doi: 10.1016/j.bbrc.2004.11.067
- Watkins BA, Shen CL, Memurtry JP, Xu H, Bain SD. 1997. Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J Nutr. 127:1084–1091.
- Whitman M. 1998. Smads and early developmental signaling by the TGF-β super-family. Genes Develop. 12:2445–2462. doi: 10.1101/gad.12.16.2445
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. 1988. Novel regulators of bone formation: molecular clones and activities. Science. 242:1528–1534. doi: 10.1126/science.3201241
- Yang Q, Zheng P, Ma Z, Li T, Jiang S, Qin JG. 2015. Molecular cloning and expression analysis of the retinoid X receptor (RXR) gene in golden pompano Trachinotus ovatus fed Artemia nauplii with different enrichements. Fish Physiol Biochem. doi:10.1007/s10695-015-0098-x
- Ytteborg E, Baeverfjord G, Torgersen J, Hjelde K, Takle H. 2010. Molecular pathology of vertebral deformities in hyperthermic Atlantic salmone (Salmo salar). BMC Phy. 10(12):1–16.
- Zheng P, Ma Z, Guo H, Zhang D, Fu M, Zhang N, Jiang S. 2014. Osteological ontogeny and malformations in larval and juvenile golden pompano Trachinotus ovatus (Linnaeu 1758). Aquacult Res. doi:10.1111/are.12600
- Zou H, Niswander L. 1996. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 272:738–741. doi: 10.1126/science.272.5262.738
Appendix 1. Partial sequences of BMP1


Appendix 2. Partial sequences of BMP2

Appendix 3. Partial sequences of BMP4

Appendix 4. Partial sequences of BMP5

Appendix 5. Partial sequences of BMP10

