ABSTRACT
This study aimed to evaluate the effect of reinserting controlled internal drug release (CIDR) devices after fixed-time artificial insemination (FTAI) on pregnancy rates in goats. Oestrus was synchronized with a short (5 days) CIDR protocol, and FTAI was conducted 54 hours after CIDR removal. According to the reinsertion of CIDR after FTAI, goats were assigned to three treatments: G0 control group (n = 29), no CIDR reinsertion; G7 group (n = 27), CIDR reinserted 7 days; G14 group (n = 29), CIDR reinserted 14 days. Blood samples were collected to determine progesterone levels. Oestrus appearance (92.9%), interval of CIDR removal to oestrus (IRE = 34.1 ± 1.1 hours), interval of oestrus onset to artificial insemination (IEAI = 20.2 ± 1.0 hours), mean duration of oestrus (38.4 ± 1.4 hours), and pregnancy rates (61.0%) were similar (P > .05) among groups. Progesterone concentrations were higher (P < .05) in G7 and G14 than G0 goats. G7 goats had lower (P < .05) oestrus rate, after CIDR reinsertion, than G0 and G14 goats. Overall pregnancy rate was similar (P > .05) for all groups. In conclusion, reinsertion of CIDR for 7 or 14 days after a short oestrus synchronization protocol and FTAI did not increase the overall pregnancy rate of goats.
Introduction
Goats are important contributors to the global food supply and play important roles in the economy of developing countries (Amiridis and Cseh Citation2012). Until 2014, according to FAO reports, the population of goats were of 1.011 million, having a 7% annual increase in the last 14 years. Also, goats produce about 19.2 million metric tons of milk, accounting for about 2% of the world’s total amount of milk produced by livestock species (FAOSTAT Citation2014). Goat meat is widely consumed in developing countries. According to FAO 2014, total meat inventory is about 315 metric tons, and goat meat represents only 2% of this total. The seasonal reproductive pattern of these ruminants imposes specific challenges to production systems. This has led to the development and implementation of assisted reproduction technologies and the genetic improvement of goats (Amiridis and Cseh Citation2012; Mpebe et al. Citation2017). Oestrus synchronization technologies frequently used are those progestagen-based protocols using either natural progesterone, fluoregestone, or medroxyprogesterone acetate, via intravaginal implants such as sponges or controlled internal drug release (CIDR) devices (Abecia et al. Citation2011; Wei et al. Citation2016). In the last decade, several studies have reported that a short progestogen protocol (6 days) is associated with higher levels of progesterone at the time of device removal, thus influencing the percentage of oestrus appearance (Ungerfeld and Rubianes Citation2002) as well as the pregnancy rates (Rubianes et al. Citation2001) outside the breeding season. However, Mellado (Citation2008) notes that extensive goat systems have other challenges, such as foetal loss (up to 15%), which can cause economic losses of up to 70% (Mellado et al. Citation2004; Diskin and Morris Citation2008). The development and survival of an embryo depend on an integrated sequence of biological events involving the ovary, embryo, oviduct, and uterus. A perturbation of this balanced system can lead to reduced embryo survival rates (Thatcher et al. Citation1994). According to Mann et al. (Citation2006), most embryonic losses occur during the first few days after fertilization and during the implantation process, inadequate luteal function being one of the main causes. The maintenance of pregnancy in ruminants depends on the continued secretion of progesterone by the corpus luteum, which inhibits luteolysis. Progesterone deficiency due to primary luteal insufficiency has been reported as a cause of embryonic death (Mann and Lamming Citation2001; Diskin and Morris Citation2008). Increasing concentrations of progesterone (P4) during meta-oestrus and early di-oestrus improves embryonic growth and the production of interferon-τ (IFN-τ) (Spencer Citation2013; Arosh et al. Citation2016), which in turn improves the relationship between embryo and uterus, and increases embryonic survival rates (Mann et al. Citation2006); in addition to the above, the beginning of calostrogenesis is regulated in part by this hormone in conjunction with oestradiol (Castro et al. Citation2011). The administration of exogenous progesterone at this time improves foetal growth (Diskin and Morris Citation2008). Progesterone supplementation is a common procedure during early gestation in bovines (Thatcher et al. Citation1994). Studies in cows show that treatment with CIDR before mid-cycle significantly increases pregnancy rates (13%); the effect is greater when the device is inserted between the sixth and eighth day after insemination (Stronge et al. Citation2005). Therefore, the aim of this study was to evaluate the effect of CIDR reinsertion after insemination on the oestrus response, onset of oestrus, plasma progesterone concentrations, and pregnancy rate of a commercial herd of goats.
Material and methods
Geographical location of the experimental site
This experiment was conducted at the beginning of the breeding season (June) in the ‘Los Castillos’ farm located in Cadereyta Jimenez, Nuevo Leon, Mexico (25° 29′ N, 99° 54′ W, 300 m.a.s.l). During the experiment, registered temperatures ranged from 18°C to 34°C.
Experimental animals, maintenance, and food supply
This study was approved by the Bioethics and Animal Welfare Committee of the Facultad de Medicina Veterinaria y Zootecnia of the Universidad Autónoma de Nuevo León (Act. No. 9). Ninety commercial goats were used with an average body weight (BW) of 39.1 ± 0.7 kg and a body condition (BC) score (Mendizabal et al. Citation2011) of 2.45 ± 0.49 SEM. The goats grazed daily for 8 hours on a pasture with the presence of xerophytic vegetation and shrubs (Acacia farnesiana, Acacia rigidula, Prosopis glandulosa, Cordia boissieri, Rhus microphylla, Chenopodium album). After returning from grazing, the goats were provided with commercial mineral salts containing 12% phosphorus and 6% calcium (Agronutrientes del Norte, Monterrey, Mexico). The mineral salt feeders were located close to the drinking troughs. Thirty days before the study, the goats were dewormed and given vitamins (Ivermectin + ADE®, 200 μg/kg, SC, Norvet, Mexico). At the beginning of the experiment, all animals were weighed and their BC score was determined (on a scale of 1–5) as recommended by Mendizabal et al. (Citation2011). In addition, a transrectal ultrasonography (Falco-ESAOTE, de 7.5 Mhz, Pie Medical, Boulder, CO, USA) was performed at the beginning of the experiment to determine the ovarian status of each goat.
Oestrus synchronization protocol
All goats were subjected to a short oestrus synchronization protocol (5 days) using an intravaginal CIDR device (0.3 g progesterone, Pfizer®, New Zealand). When the CIDR was removed, each goat received (IM) a 5-mg injection of Dinoprost Tromethamine (Lutalyse®, Pfizer, USA) and 200 IU of equine chorionic gonadotropin (Folligon®, Intervet, Holland). Oestrus detection was performed 24 hours after the CIDR removal. The presence of oestrus was checked hourly for 15 minutes, with a teaser buck, recording the starting and ending times of the oestrus of each goat. Transcervical artificial insemination (TAI) was performed at a fixed time, 54 hours after the CIDR removal (Menchaca et al. Citation2007) using semen stored at 4°C in 0.25 ml straws, each containing 100 million sperms.
Reinsertion of the CIDR device after artificial insemination: experimental group formation
Goats were blocked by BW and BC at the beginning of the experiment. After being removed, the CIDR devices were washed, allowed to dry, and kept in paper bags in a dry, dark place until reuse. Before reinsertion, which was performed 4 days after artificial insemination (AI), the devices were washed in a water-based iodine solution (5%) (Vanodine®, Pfizer, Mexico). The goats were assigned, according to live weight and BC, into three homogenous experimental groups: (1) Group G0 consisting of 29 goats with no CIDR reinsertion; (2) Group G7 consisting of 27 goats for which the CIDR device was reinserted for 7 days; (3) Group G14 consisting of 29 goats for which the CIDR device was reinserted for 14 days. Oestrus detection was performed 24 hours after the removal of the reinserted CIDR (the same way as after oestrus synchronization), and natural mating was allowed immediately after the positive detection of oestrus. Pregnancy diagnosis was performed 45 days after AI using the same methods described above.
Collection of blood samples and analysis of progesterone
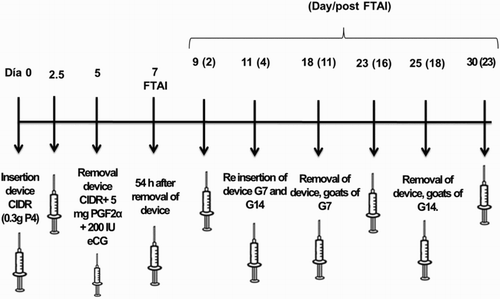
To quantify the concentration of serum progesterone, blood samples were drawn from the jugular vein with a vacutainer in 6 ml tubes without anticoagulant. The samples were centrifuged (Refrigerated centrifuge, Precision 200R, USA) at room temperature for 15 minutes at 1500 g. The serum obtained was stored in 1.5 ml conical vials at −20°C until analysis. shows the blood sampling schedule for goats in the control (G0) and experimental groups (G7 and G14). Progesterone concentration was determined using an ELISA kit (Bioelisa, MexLab, Mexico). The intra-assay coefficient of variation for the analysed samples was 4.48%, and the sensitivity of the progesterone assay was 0.58 ng/ml.
Statistical analysis
Data were analysed using SPSS-17 Software. The oestrus percentage and pregnancy rate were compared among treatments using the Chi-square test (χ2). For evaluation of the variables, interval between CIDR removal and oestrus (IRE), interval between oestrus and artificial insemination (IEAI), and length of oestrus and progesterone concentration, a linear model for repeated measurements was used. Means were compared using minimum significant difference methods, when differences were statistically significant.
Results
shows the results for each group of goats. In Stage 1 of oestrus synchronization (5-day protocol), no differences were found for each of the recorded variables (P > .05). However, after CIDR reinsertion (Stage 2), the goats in group G14 and G0 had a higher repeated oestrus appearance rate (31% and 24.1%, respectively) (P < .05) compared to the G7 (18.4%) group.
Table 1. Effect of short oestrus synchronization protocol on reproductive parameters of goats, using CIDR and effect of reinsertion (mean ± SEM).
presents the percentage of pregnancy in both stages of the experiment. In Stage 1 (5-day short protocol), there were no differences between the evaluated groups of goats (P > .05), obtaining on average 44.7% pregnancy. In Stage 2 (reinsertion of CIDR) of the experiment, a higher pregnancy rate was reported for G7 (100%) than for G0 (71.4%) and G14 (55.5%) groups. The overall average for all goats in the experiment was 61.0%.
Table 2. Effect of short oestrus synchronization protocol on pregnancy rate in goats, using CIDR and effect of reinsertion.
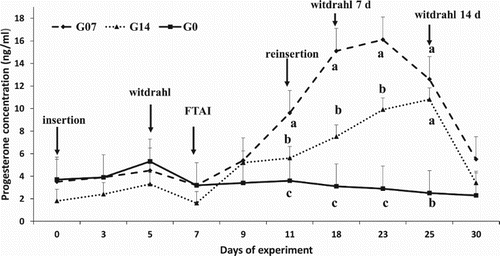
shows the results of progesterone concentration. Between day 0 and day 5 (CIDR device removal), there were no differences recorded among the groups of goats. However, progesterone levels increased over time due to the resynchronization of the G7 and G14 groups of goats (P < .05). Progesterone levels (P4) began to increase as of day 9, the increase being more pronounced when the CIDR devices were reinserted in the G7 and G14 groups of goats (day 11) compared to those of G0 (P < .05). The CIDR devices were reinserted 4 days after TAI when a tendency of increased progesterone levels in the G7 and G14 groups of goats (9.6 ± 1.9 ng/ml and 5.6 ± 1.7 ng/ml, respectively) was recorded compared to the G0 group of goats (3.6 ± 2.2 ng/ml) (P < .05). Thirty days after the start of the experiment, progesterone levels decreased in all groups (P > .05).
Figure 2. Plasma progesterone levels in three groups of goats during the experiment; before and after the reinsertion of the CIDR device. Events: day 0 insertion, day 5 – withdrawal of CIDR; day 7 – fixed-time insemination (FTI), day 11 – reinsertion of CIDR, day 18 – withdrawal of CIDR in the G7 group; day 25 – withdrawal in the G14 group.
Discussion
There were no differences recorded in measured traits among the groups in Stage 1, since the 5-day protocol was the same for all groups. The results of this study confirm that the CIDR device is an effective tool for the synchronization of the oestrus in goats at the beginning of the breeding season in semi-desert conditions (Fonseca et al. Citation2005; Riaz et al. Citation2012). The oestrus rate was 92.9%, similar to the figures reported by Menchaca et al. (Citation2002), who used a 6-day protocol and administered 300 IU of equine chorionic gonadotropin (eCG) when the device was removed. However, the results of this study are lower than those reported by Romano (Citation2004) in Nubian goats where new CIDR devices were used and the experiment was performed during high breeding season, where they recorded an oestrus rate of 100%. In most studies, it is mentioned that in adult animals the expected oestrus rate is greater than 90%, while nulliparous goats can reach up to 97.2% and lactating goats up to 85.7% (Fonseca et al. Citation2008; Abecia et al. Citation2011; Navanukraw et al. Citation2014; Alvarado-Espino et al. Citation2016).
Regarding the oestrus characteristics recorded in this study, oestrus appeared up to 2–3 hours later compared to those reported by Martemucci and D’Alessandro (Citation2011) and Menchaca et al. (Citation2002), but was similar to that reported by Romano (Citation2004). However, those authors used stabled and cycling goats and administered a higher dose of eCG (300 IU), which may explain the earliest appearance of oestrus in their studies (Nunes and Salgueiro Citation2011; Paramio and Izquierdo Citation2014). Also, the duration of oestrus in our experiment was up to 4 hours longer than in the experiment of Navanukraw et al. (Citation2014), which may be explained by the higher doses of eCG used in the mentioned study compared to this experiment, which shortened the duration of oestrus and facilitated earlier ovulation (Menchaca et al. Citation2007; Martemucci and D’Alessandro Citation2011). This is particularly the case in meat breeds such as the Boer during the breeding season (Al Yacoub et al. Citation2011). The interval between oestrus appearance and artificial insemination (IEAI) was 20.4 ± 3.1 hours on average in this study, which is consistent with the results reported by Cseh et al. (Citation2012), but clashes with the recommendation of insemination 12–18 hours after the positive detection of oestrus by Nuti (Citation2007).
The total average of pregnancy rate (61.0%) including Stage 1 and CIDR device reinsertion 4 days post-AI, and kept in the vagina for 0, 7, or 14 days in this study, was the same as reported by Menchaca and Rubianes (Citation2007), who used a short protocol of 5–6 days with vaginal sponges without device reinsertion post-AI. The goats in the present study were inseminated at a fixed time, 54 hours after removal of the device. It is likely that the 200 IU of eCG used in this study was not enough to stimulate the development of preantral follicles (Alvarado-Espino et al. Citation2016) and to synchronize ovulation (Scaramuzzi et al. Citation2006). Also, in sheep, it has been confirmed that higher dose of eCG increases the ovulation rate but also reduces the embryonic survival rate (Diskin and Morris Citation2008). In the present study, after reinsertion of the CIDR (Stage 2), G7 goats presented the lowest percentage of oestrus and the highest pregnancy rate, compared to G0 and G14 goats. Other authors reported different results than in this study, utilizing several alternative substances to increase circulating progesterone post-insemination, for example, Suguna et al. (Citation2009) used insulin in goats, Arndt et al. (Citation2009) used CIDR in dairy cows, and Fonseca et al. (Citation2006) used hCG in dairy goats.
Larson et al. (Citation2007) reported a pregnancy rate that was 3–13% higher when cows were supplemented with progesterone along with CIDR, which was inserted from day 3.5 to day 10 post-AI. In contrast, Ledezma-Torres et al. (Citation2015) obtained a pregnancy rate that was 6.6% higher in beef cows when inserting CIDR devices for 14 days post-AI. According to Spencer et al. (Citation2016), an increased concentration of IFN-τ is recorded when supplementation is done with progesterone 5–19 days post-insemination, with progesterone being more effective when administered earlier. The results of the present study are consistent with those reported by Macmillan and Peterson (Citation1993), who found that if treatment with CIDR begins before the middle of the sexual cycle, the pregnancy rate increases markedly due to a better embryonic development caused by the greater amount of progesterone, associated with an increased production of IFN-τ by the embryo (Mann et al. Citation2006), which facilitates maternal recognition of pregnancy. The low pregnancy rates found in the present study could also be due to the season during which the experiment was conducted (summertime, June), when most of the goats had a low ovarian activity of 10% (breeding onset) according to ultrasonography data and due to the limited availability of forage in the pasture (Viñoles et al. Citation2012). After the AI, the goats continued grazing in the pasture, walking 10 km per day on average. According to certain authors, the walking distance for goats should be reduced during early pregnancy, as long walking distances increase stress in goats and involve an additional adverse loss of energy (Mellado Citation2008). Early pregnancy is a critical period for maternal recognition, and the lack of nutrients that stimulate an increase in circulating progesterone can increase early embryonic losses (Viñoles et al. Citation2012). According to studies in cows exposed to heat stress, the concentration of progesterone decreases and the uterine secretion of prostaglandins increases (Weems et al. Citation2006; Arndt et al. Citation2009; Pohler et al. Citation2015). During this experiment, there was no rainfall, which affects nutrient availability and can affect embryonic loss, and therefore the percentage of pregnancy. The goats in this study were not supplemented with concentrate, which possibly resulted in a negative energy balance and BC loss, mainly at the hypothalamic-pituitary level (Wade and Jones Citation2004) characterized by decreased metabolism of steroid hormones (Diskin and Morris Citation2008). Since in our study most of the goats in all groups lost up to one unit in their BC, this could have caused an early embryo absorption, which can be raised up to 9 times after AI or natural mating (Humblot Citation2001; Mellado et al. Citation2004; Pohler et al. Citation2015).
During Stage 1 when CIDR was inserted pursuing the oestrus synchronization, the concentration of progesterone was higher for G0 (4.3 ± 1.1 ng/ml) followed by G7. Those values are higher than those reported by Uribe-Velasquez et al. (Citation2011) in a cyclic commercial herd of goats. When the CIDR devices were removed, progesterone concentration decreased (3.9 ± 1.7 ng/ml) coinciding with the results of Uribe-Velasquez et al. (Citation2011) who removed the CIDR on day 6 (3.7 ± 4.0 ng/ml), 2 days after AI (day 9) which is a proof of occurred ovulation (Dadarwal et al. Citation2013). Following the reinsertion of CIDR, and until the end of the experiment (day 30), progesterone concentration recorded in the G7 and G14 groups of goats was higher than the values measured in G0. These results coincide with those reported by Torres (Citation2013) in Suffolk–Dorset crossbred sheep, where new CIDR devices were inserted for 25 days after AI, followed by used CIDR devices until day 35. The administration of exogenous progesterone (CIDR) after AI increases serum progesterone levels during the luteal phase and improves embryonic growth and the production of IFN-τ (Spencer et al. Citation2016), enhancing the relationship between the embryo and the uterus and thus the survival rate of embryos (Spencer Citation2013). In the present study, although progesterone levels increased, the pregnancy rate of goats to which CIDR was reinserted for 7 and 14 days post-insemination was not significantly improved. There might be other factors affecting pregnancy rates, since studies have indicated that in goats, a high incidence of early abortion is associated with deficiencies in protein (Viñoles et al. Citation2012), phosphorus, magnesium, and copper supply (Mellado Citation2008). In the present study, progesterone levels decreased around day 25 after the reinsertion, which may affect the maintenance of pregnancy due to embryonic resorption, as mentioned earlier (Kenyon et al. Citation2013), These authors reported that Holstein cows with low progesterone levels (<5 ng/ml on day 14 post-AI) had higher embryonic loss rates between days 28 and 42 post-conception. In this study, the CIDR reinsertion after AI increased progesterone concentrations in blood but have not caused an increase in pregnancy rates in goats where CIDR was reinserted for 7 and 14 days post-AI. The increase of circulating progesterone is apparently not enough to increase the pregnancy rate itself in goats, since the increase in the metabolism of progesterone is more important in the reduction of embryonic losses in high-yielding cows and goats (Arndt et al. Citation2009; Samir et al. Citation2016). Also, other factors should be taken into consideration, such as the type of oestrus synchronization programme, breed, season of the year, and good post-breeding management which can reduce embryonic losses.
Conclusions
The short oestrus synchronization protocol using CIDR for 5 days is an effective way to synchronize more than 90% of anoestrous goats. This study tested the viability of reused CIDR devices with 0.3 g of progesterone to increase endogenous progesterone concentrations when the devices had previously been used for 5 days. Serum progesterone concentrations were different for groups of goats in which CIDR devices were reinserted for 0, 7, and 14 days post-insemination, but reusing CIDR devices failed to increase pregnancy rates in any of the groups.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abecia JA, Forcada F, González-Bulnes A. 2011. Pharmaceutical control of reproduction in sheep and goats. Vet Clin North Am Food Anim Pract. 27(1):67–79. doi: 10.1016/j.cvfa.2010.10.001
- Alvarado-Espino AS, Meza-Herrera CA, Carrillo E, González-Álvarez VH, Guillen-Muñoz JM, Ángel-García O, Mellado M. 2016. Reproductive outcomes of Alpine goats primed with progesterone and treated with human chorionic gonadotropin during the anestrus-to-estrus transition season. Anim Reprod Sci. 167:133–138. doi: 10.1016/j.anireprosci.2016.02.019
- Al Yacoub AN, Gauly M, Sohnrey B, Holtz W. 2011. Fixed-time deep uterine insemination in PGF2α-synchronized goats. Theriogenology. 76(9):1730–1735. doi: 10.1016/j.theriogenology.2011.07.005
- Amiridis GS, Cseh S. 2012. Assisted reproductive technologies in the reproductive management of small ruminants. Anim Reprod. 130(3):152–161. doi: 10.1016/j.anireprosci.2012.01.009
- Arndt WJ, Holle AJ, Bauer ML, Kirsch JD, Schimek DE, Odde KG, Vonnahme KA. 2009. Effect of post-insemination progesterone supplementation on pregnancy rate in dairy cows. Can J Vet Res. 73(4):271–274.
- Arosh JA, Banu SK, McCracken JA. 2016. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J Dairy Sci. 99(7):5926–5940. doi: 10.3168/jds.2015-10335
- Castro N, Capote J, Bruckmaier RM, Argüello A. 2011. Management effects on colostrogenesis in small ruminants: a review. J Appl Anim Res. 39(2):85–93. doi: 10.1080/09712119.2011.581625
- Cseh S, Faigl V, Amiridis GS. 2012. Semen processing and artificial insemination in health management of small ruminants. Anim Reprod Sci. 130(3):187–192. doi: 10.1016/j.anireprosci.2012.01.014
- Dadarwal D, Mapletoft RJ, Adams GP, Pfeifer LFM, Creelman C, Singh J. 2013. Effect of progesterone concentration and duration of proestrus on fertility in beef cattle after fixed-time artificial insemination. Theriogenology. 79(5):859–866. doi: 10.1016/j.theriogenology.2013.01.003
- Diskin MG, Morris DG. 2008. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim. 43(2):260–267. doi: 10.1111/j.1439-0531.2008.01171.x
- FAOSTAT. 2014. [accessed 2017 September 20]. http://www.fao.org/faostat/en/#data.
- Fonseca JF, Bruschi JH, Santos ICC, Viana JHM, Magalhães ACM. 2005. Induction of estrus in non-lactating dairy goats with different estrous synchrony protocols. Anim Reprod Sci. 85(1):117–124. doi: 10.1016/j.anireprosci.2004.03.005
- Fonseca JF, Maffili VV, Rodrigues MT, Santos ADF, Rovay H, Pinto Neto A, Brandao FZ, Torres CAA. 2006. Effects of hCG on progesterone concentrations and fertility in cyclic, lactating Alpine goats. Anim Reprod. 3(4):410–414.
- Fonseca JF, Torres CAA, Santos ADF, Maffili VV, Amorim LS, Moraes EA. 2008. Progesterone and behavioral features when estrous is induced in Alpine goats. Anim Reprod Sci. 103(3):366–373. doi: 10.1016/j.anireprosci.2007.05.013
- Humblot P. 2001. Use of pregnancy specific proteins and progesterone assays to monitor pregnancy and determine the timing, frequencies and sources of embryonic mortality in ruminants. Theriogenology. 56(9):1417–1433. doi: 10.1016/S0093-691X(01)00644-6
- Kenyon AG, Mendonça LGD, Lópes G, Lima JR, Santos JEP, Chebel RC. 2013. Minimal progesterone concentration required for embryo survival after embryo transfer in lactating Holstein cows. Anim Reprod Sci. 136(4):223–230. doi: 10.1016/j.anireprosci.2012.10.014
- Larson SF, Butler WR, Currie WB. 2007. Pregnancy rates in lactating dairy cattle following supplementation of progesterone after artificial insemination. Anim Reprod Sci. 102(1):172–179. doi: 10.1016/j.anireprosci.2007.02.023
- Ledezma-Torres RA, Garza DM, Moreno G, Manzanares N, Picón FJ, Ramírez R, Sánchez-Dávila F. 2015. Efecto del CIDR post inseminación sobre la tasa de preñez en vacas de carne. Ciencia UANL. 18(73):62–68.
- Macmillan KL, Peterson AJ. 1993. A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus. Anim Reprod Sci. 33(1-4):1–25. doi: 10.1016/0378-4320(93)90104-Y
- Mann GE, Fray MD, Lamming GE. 2006. Effects of time of progesterone supplementation on embryo development and interferon-τ production in the cow. Vet J. 171(3):500–503. doi: 10.1016/j.tvjl.2004.12.005
- Mann GE, Lamming GE. 2001. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction. 121(1):175–180. doi: 10.1530/rep.0.1210175
- Martemucci G, D’Alessandro AG. 2011. Synchronization of oestrus and ovulation by short time combined FGA, PGF2α, GnRH, eCG treatments for natural service or AI fixed-time. Anim Reprod Sci. 123(1):32–39. doi: 10.1016/j.anireprosci.2010.11.007
- Mellado M. 2008. Goat reproductive management under rangelands conditions. Trop Subtrop Agroecosys. 9:47–63.
- Mellado M, Valdez R, Lara LM, García JE. 2004. Risk factors involved in conception, abortion, and kidding rates of goats under extensive conditions. Small Ruminant Res. 55(1):191–198. doi: 10.1016/j.smallrumres.2003.10.016
- Menchaca A, Miller V, Salveraglio V, Rubianes E. 2007. Endocrine, luteal and follicular responses after the use of the short-term protocol to synchronize ovulation in goats. Anim Reprod Sci. 102(1):76–87. doi: 10.1016/j.anireprosci.2006.10.001
- Menchaca A, Pinczak A, Rubianes E. 2002. Follicular recruitment and ovulatory response to FSH treatment initiated on day 0 or day 3 postovulation in goats. Theriogenology. 58(9):1713–1721. doi: 10.1016/S0093-691X(02)01084-1
- Menchaca A, Rubianes E. 2007. Pregnancy rate obtained with short-term protocol for timed artificial insemination in goats. Reprod Domest Anim. 42(6):590–593. doi: 10.1111/j.1439-0531.2006.00827.x
- Mendizábal JA, Delfa R, Arana A, Purroy A. 2011. Body condition score and fat mobilization as management tools for goats on native pastures. Small Ruminant Res. 98(1):121–127. doi: 10.1016/j.smallrumres.2011.03.029
- Mpebe NA, González-Bulnes A, Lehloenya KC. 2017. Effect of breed and follicular status on response to superovulation in South African goats. J Appl Anim Res. 17:1–5. doi: 10.1080/09712119.2016.1277530
- Navanukraw C, Khanthusaeng V, Kraisoon A, Uriyapongson S. 2014. Estrous and ovulatory responses following cervical artificial insemination in Thai-native goats given a new or once-used controlled internal drug release with human chorionic gonadotropin. Trop Anim Health Pro. 46(8):1441–1446. doi: 10.1007/s11250-014-0662-x
- Nunes JF, Salgueiro CCM. 2011. Strategies to improve the reproductive efficiency of goats in Brazil. Small Ruminant Res. 98(1):176–184. doi: 10.1016/j.smallrumres.2011.03.036
- Nuti I. 2007. Techniques for artificial insemination of goats. In: Youngquist RS, Threlfall WR, editors. Current therapy in large animal theriogenology. 2nd ed. St. Louis (MO): Saunders-Elsevier; p. 529–534.
- Paramio MT, Izquierdo D. 2014. Current status of in vitro embryo production in sheep and goats. Reprod Domest Anim. 49(4):37–48. doi: 10.1111/rda.12334
- Pohler KG, Green JA, Geary TW, Peres RFG, Pereira MHC, Vasconcelos JLM, Smith MF. 2015. Predicting embryo presence and viability. In: Geisert RD, Bazer FW, editors. Regulation of implantation and establishment of pregnancy in mammals 2015. Cham: Springer International Publishing; p. 253–270
- Riaz H, Sattar A, Arshad MA, Ahmad N. 2012. Effect of synchronization protocols and GnRH treatment on the reproductive performance in goats. Small Ruminant Res. 104(1):151–155. doi: 10.1016/j.smallrumres.2011.10.008
- Romano JE. 2004. Synchronization of estrus using CIDR, FGA or MAP intravaginal pessaries during the breeding season in Nubian goats. Small Ruminant Res. 55(1):15–19. doi: 10.1016/j.smallrumres.2003.10.015
- Rubianes E, Ungerfeld R, Menchaca A. 2001. Advances in oestrous synchronisation techniques in sheep and goats. Proceedings of IV Simposio Internacional de Reproduccion Animal; p. 61–72.
- Samir H, Karen A, Ashmawy T, Abo-Ahmed M, El-Sayed M, Watanabe G. 2016. Monitoring of embryonic and fetal losses in different breeds of goats using real-time B-mode ultrasonography. Theriogenology. 85(2):207–215. doi: 10.1016/j.theriogenology.2015.09.039
- Scaramuzzi RJ, Campbell BK, Downing JA, Kendall NR, Khalid M, Muñoz-Gutiérrez M, Somchit A. 2006. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod Nutr Dev. 46(4):339–354. doi: 10.1051/rnd:2006016
- Spencer TE, Forde N, Lonergan P. 2016. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J Dairy Sci. 99(7):5941–5950. doi: 10.3168/jds.2015-10070
- Spencer TE. 2013. Early pregnancy: concepts, challenges, and potential solutions. Animal Frontiers. 3(4):48–55. doi: 10.2527/af.2013-0033
- Stronge AJH, Sreenan JM, Diskin MG, Mee JF, Kenny DA, Morris DG. 2005. Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology. 64(5):1212–1224. doi: 10.1016/j.theriogenology.2005.02.007
- Suguna K, Mehrotra S, Agarwal SK, Hoque M, Shanker U, Singh SK, Varshney VP. 2009. Effect of exogenous insulin administration on ovarian function, embryo/fetal development during pregnancy in goats. Anim Reprod Sci. 111(2):202–213. doi: 10.1016/j.anireprosci.2008.03.021
- Thatcher WW, Staples CR, Danet-Desnoyers G, Oldick B, Schmitt EP. 1994. Embryo health and mortality in sheep and cattle. J Anim Sci. 72(Suppl 3):16–30. doi: 10.2527/1994.72suppl_316x
- Torres Lemus FM. 2013. Infuelncia del aporte de progesterona exógena (CIDR) post-inseminación sobre la fertilidad de ovinos de las razas cruz Suffolk-Dorset [master thesis]. Texcoco, Mexico: Colegio de Posgraduados.
- Ungerfeld R, Rubianes E. 2002. Short term primings with different progestogen intravaginal devices (MAP, FGA and CIDR) for eCG-estrous induction in anestrus ewes. Small Ruminant Res. 46(1):63–66. doi: 10.1016/S0921-4488(02)00105-0
- Uribe-Velasquez LF, Gutierrez C, Carreno EE, Izquierdo JH, Lenz MI, Botero SA. 2011. Reutilizacion del dispositivo de progesterona (CIDR) asociado con protocolos de corta duración en cabras. Vet Zootec. 5(1):39–46.
- Viñoles C, Glover KMM, Paganoni BL, Milton JTB, Martin GB. 2012. Embryo losses in sheep during short-term nutritional supplementation. Reprod Fertility Dev. 24(8):1040–1047. doi: 10.1071/RD11281
- Wade GN, Jones JE. 2004. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physio. 287(6):1277–1296. doi: 10.1152/ajpregu.00475.2004
- Weems CW, Weems YS, Randel RD. 2006. Prostaglandins and reproduction in female farm animals. Vet J. 171(2):206–228. doi: 10.1016/j.tvjl.2004.11.014
- Wei S, Chen S, Wei B, Liu Z, Bai T, Lin J. 2016. Estrus synchronization schemes and application efficacies in anestrus Lanzhou fat-tailed ewes. J Appl Anim Res. 44(1):466–473. doi: 10.1080/09712119.2015.1091350