ABSTRACT
The underlying reasons for genetic differences in body fat mass remain unclear. The objective of this study was to investigate the contributions of hepatic de novo lipogenesis (DNL) to the genetic differences in fat mass in ducks. Ducks with distinct genetic backgrounds were selected for the genetically lean and fat animal models. The weights of fat tissue and organs as well as the gene expressions, enzyme activities and concentrations related to hepatic lipogenesis were measured and compared between the two genetically different duck breeds. Although a clear phenotypic difference in extra-liver fat mass between the two genetically different duck breeds was observed, the relative weights of the fat tissue to body weight in the two breeds were similar. There were no clear divergences regarding DNL-related gene expressions (Acc, Dgat2 and Fas), enzyme activities (ELVOL and FAS) and enzyme concentrations (ACC) between the two genetically different duck breeds, suggesting that hepatic DNL may contribute less to the individual fat mass difference in ducks; rather, body fat mass may depend more on the liver size. Our findings may provide a fundamental explanation for individual fat mass differences.
Introduction
Adipose tissue represents an endocrine metabolic organ that plays a critical role in the efficient storage and mobilization of lipids to fulfil bioenergetics demands (Galic et al. Citation2010). A specific amount of fat mass is necessary for sustaining normal physiological functions in the body. In farm animals that provide meats, fat in muscle tissues (i.e., intramuscular fatness) is considered an important economic trait because it influences meat quality (Wood et al. Citation2008). A thorough understanding of the exact mechanisms underlying the fat deposition process is important for developing strategies for producing high-quality meat. Hence, why some individuals have more fat than others is a fundamental question that needs to be addressed first.
It is clear that genetic backgrounds are responsible for individual differences in fat mass. In livestock and human medical research, genome-wide association studies have identified numerous single nucleotide polymorphisms (SNPs), many of which have been shown to be highly correlated with lipid metabolism (Hinney et al. Citation2007; Wu et al. Citation2013; Yang et al. Citation2013). The locations of some of these SNPs have been identified near or within the genes responsible for lipid metabolism. Gene expression and regulatory networks are involved in regulating fat mass through numerous physiological processes. Among them, de novo lipogenesis (DNL) is an endogenous pathway that involves lipid synthesis and plays an important role in directly affecting fat mass (Hellerstein Citation1999). In the body, some of the fatty acids are derived from the diet and some are derived from the DNL process. Carbohydrates could enhance the DNL pathway to contribute deposits and obesity (Diraison et al. Citation2003; Strable and Ntambi Citation2010). Therefore, the contribution of DNL is perhaps one of the main sources of individual fat mass differences.
The main DNL sites vary greatly among different species. In mammals, DNL mainly occurs in the adipose tissue (Shrago et al. Citation1971). The general view is that lipogenesis is equally active in the liver and adipose tissue based on observations in rodents (Leung and Bauman Citation1975). Hepatocytes prepared from lean rats starved for 48 h significantly lacked the capacity for lipogenesis, whereas hepatocytes isolated from starved obese rats exhibited detectable rates of lipogenesis. Enzymes that are normally associated with lipogenesis were elevated in the liver tissues from obese rats; the liver may be prominently involved in the development of excessive blood lipids and enlarged fat cells in the Zucker obese rat (McCune et al. Citation1981). In contrast, DNL in avian species predominantly, if not exclusively, occurs in the liver (Griffin et al. Citation1992; Bedu et al. Citation2002; Diraison et al. Citation2003), and the primary function of adipose tissue appears to be one of lipid storage rather than lipid synthesis (Griffin et al. Citation1992). Earlier studies have established that the in vitro incorporation of glucose into lipids was uniformly high in liver slices from ducks aged 2, 4 and 10 weeks but was very low in the adipose tissues of these animals (Evans Citation1972). Most of the endogenous body lipids are of hepatic origin, and the growth and subsequent fattening of adipose tissue depend more on the availability of plasma triglycerides (TG; O’Hea and Leveille Citation1969; Alvarenga et al. Citation2011). Our initial data in ducks also demonstrated that DNL mainly occurred in the liver instead of in adipose tissue (Ding et al. Citation2012).
Considering that DNL in birds mainly occurs in hepatic tissues and plays significant roles in avian fat mass, we hypothesized that hepatic DNL may be the key contributor to individual differences in fat mass. To test the hypothesis, ducks with distinct genetic backgrounds, Pekin duck (fast growth with high fat levels) and Heiwu (a new artificial selected duck breed, slow growth with low fat levels), were selected for the present study to serve as genetically obese and lean animal models, respectively, thus providing a valuable comparative model for investigating the role of hepatic DNL in the differences in fat mass. These findings may provide new insight into the mechanism of fat mass.
Materials and methods
Birds and sampling
Pekin ducks (Anas platyrhynchos, designated as PK) and Heiwu ducks (newly breed by artificial selection, designated as HW) were raised under natural temperature and light conditions at the experimental waterfowl breeding farm at Sichuan Agricultural University. The embryos were under the same hatching conditions of temperature and humidity, and the birds had free access to a starting diet containing 22.36% crude protein and 12.66 MJ/kg of metabolizable energy. From week 4, the ducks were fed a diet containing 18.32% protein and 12.35 MJ/kg of metabolizable energy. During the embryonic stages, 20 embryos of each species at each time point including embryonic days 15, 20 and 25 were isolated for sampling. During the post-hatching stages, six birds (three males and three females) were randomly selected at each sampling time point. The PK ducks were sacrificed for sampling every week, and the HW ducks were sacrificed every 2 weeks. Before sampling, the ducks were made to fast for 12 h, and then approximately 2 mL of blood was collected from the wing vein and mixed with EDTA (0.8 g/L) in a vacuum tube; then, the plasma was separated by centrifugation at 3000g for 10 min at 4°C. The blood serum was kept at −20°C until subsequent experiments. After the ducks were sacrificed, the organs and fat tissues including the liver, heart, leg fat tissues, abdominal fat tissues and subcutaneous fat tissues were isolated for weighing. Additionally, to produce an adequate comparison of abdominal fat and subcutaneous fat, 30 ducks (15 male and 15 female) of each species at weeks 8 and 16 were sacrificed, respectively. The tissues were immediately collected and quickly frozen at −80°C until subsequent analyses. All procedures in this study were conducted in compliance with the requirements of the Animal Ethics Committee of Sichuan Agricultural University.
Plasma lipid parameter determination
The lipid parameters of the blood plasma, including total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), in the livers of the genetically lean and fat ducks were compared. For each plasma parameter, six ducks (three male and three female) of each breed were analysed individually every 2 weeks from week 0 to week 16. Briefly, the plasma TG concentration was determined using a TG Assay Kit (GPO-PAP-LST, Sichuan Maker Biology Technology, China), and the TC was determined using a Cholesterol Kit (CHO Kit, Sichuan Maker Biology Technology, China). HDL-C was detected with an HDL ELISA kit (Shanghai Bangyi, China), and LDL-C was detected with an LDL ELISA kit (Mindray Biology, China). All assays were performed following the manufacturers’ instructions.
RNA isolation and real-time PCR
Total RNA was isolated from samples of liver, leg fat tissues, abdominal fat tissues and subcutaneous fat tissues from the two distinct duck breeds (PK & HW) at weeks 8 and 16, respectively. For each time point and breeds, the RNA was isolated from six individuals (three male and three female) to ensure six individual data points. The RNA was isolated using the Trizol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA) and then quantified based on spectrophotometric absorbance at 260 nm. First-strand cDNA was synthesized from total RNA using the PrimeScript RT reagent kit (TaKaRa Biotechnology, Dalian, China). The primers for the amplification of the duck Acc (acetyl-CoA carboxylase 2), dgat2 (diacylglycerol-O-acyltransferase 2), Fas (Fatty acid synthesis), cEBP(CCAAT/enhancer-binding protein) and PPARγ (peroxisome proliferator-activated receptor gamma) genes were designed using PrimerPremier 5.0 (Primer Biosoft International, USA). The duck β-actin and 18S rRNA sequences from GenBank were used as internal references. All GenBank accession numbers for each gene and the expected product size are provided in . The real-time PCR was performed using a reaction mixture containing 1 μL of the newly generated cDNA template, 12.5 μL of SYBR Premix Ex Taq, 10.5 μL of sterile water and 0.5 μL of the primers of the target genes. The reaction was performed using an IQ5 real-time PCR thermal cycle instrument (Bio-Rad, Munich, Germany). The thermal cycling parameters were as follows: 1 cycle at 95°C for 30 s, 40 cycles of 95°C for 10 s and 60°C for 40 s, with a subsequent 80-cycle melt curve to determine the primer specificity by starting at 55°C and increasing the temperature by 0.5°C every 10 s. All reactions were run in triplicate. The geometric means of the Ct value of the two internal control genes were calculated to analyse the relative expression of the target genes using the ‘normalized relative quantification’ method followed by 2−ΔΔCT (Livak and Schmittgen Citation2001).
Table 1. Primers for real-time PCR.
ELISA
For each duck, 0.5 g of liver tissues was homogenized. For each time point and breed, six individuals were prepared for analysis. The concentration of ACC, enzyme activities of FAS and ELOVL were determined using their corresponding ELISA kits, which were purchased from biotechnology companies (R&D, USA). The procedures were performed according to the manufacturer's instructions. Briefly, standard curves were first generated after measuring the optical density of the standard samples provided in the kits; then the optical densities of the tested samples were determined for a further analysis of enzyme amount based on the standard curves.
Data analysis
All the data were subjected to PROC GLM in SAS (Version 9.2, SAS Inc., Cary, NC) for testing, and the means were assessed for significance using Duncan's test. Correlations were assessed using Pearson's correlation coefficient using t-test. All statistical analyses were performed using SAS (version 9.3), with the significance level set at 0.05.
Results and analysis
Comparison of developmental characteristics of liver and adipose tissues between the two duck breeds
HW is a new artificially selected duck line with characteristics of slow growth and less fat deposits, whereas the PK duck is a type of domestic duck species with characteristics of fast growth and high fat content. These two distinct genetic backgrounds were used in this study to illustrate the contributions of hepatic DNL to body fat mass.
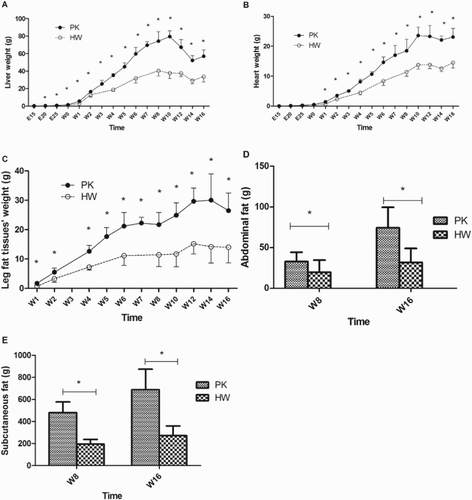
illustrates the phenotypic differences in weight in the liver, leg fat tissues, abdominal fat and subcutaneous fat between the two duck breeds. Overall, higher weights in the above-mentioned tissues were observed in the PK ducks, providing a reliable model for the study. The liver weights in the lean ducks were compared to those of the fat ducks at embryonic and post-hatching stages ((A)). An apparent difference in absolute weight of the liver began to emerge from as early as embryonic day 20. Correspondingly, the heart, which exhibits less fat deposition, was used as a contrast to the liver in the present study, and the results illustrated a similar trend in absolute weight of the heart compared with the liver, showing a clear difference beginning at week 1. The difference in the weights of the leg fat tissues between the HW and PK ducks emerged as early as 1 week post hatching. The weights of the abdominal fat tissues and the subcutaneous fat tissues were compared at weeks 8 and 16, and both showed a significantly higher fat content in the PK ducks than in the HW ducks.
Figure 1. Comparison of organ and adipose tissue weights of the HW and PK ducks. The liver weight, heart weight and leg fat tissue weights were recorded every five days in the embryonic phase and every two weeks post hatching, n = 6. The weights of the abdominal fat tissues and the subcutaneous fat tissues were recorded and compared at week 8 and week 16, respectively, n = 30. (A) Liver weight; (B) heart weight; (C) leg fat tissue weight; (D) abdominal fat tissue weight; (E) subcutaneous fat tissue weight. *Significance level of p < .05.
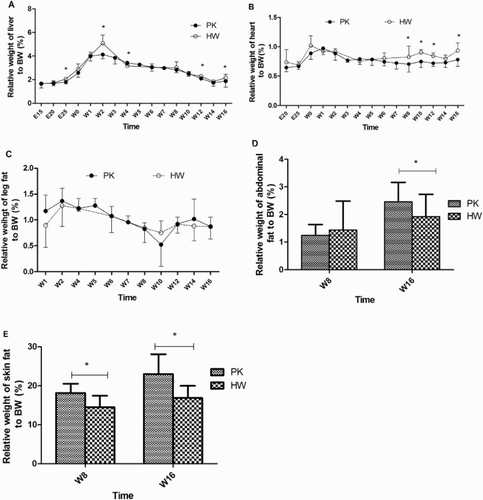
illustrates the relative weights of the fat tissues to overall body weight between the genetically different duck breeds. Firstly, in the heart, which is a non-fat deposition organ, no apparent difference for most of the examined stages was observed between the lean and fat ducks, except week 8 to week 16 where HW have a higher ratio (p < .05). Similarly, the relative weight of the livers showed no differences in the embryonic stages or in the post-hatching stages for most of the analysed stages. Some significant differences only appeared in E25, w2, w4, w12 and w16 (p < .05). Only significant differences were observed in abdominal fat tissues and subcutaneous fat tissues ().
Figure 2. Comparison of relative organ and fat tissue weights to body weight of the PK and HW ducks. The relative weight was calculated based on the percentage of organ and fat tissue weights in relation to the body weight. For all items at weeks 8 and 16, n = 30, and for other time points, n = 6. (A) Relative weight of the liver; (B) relative weight of the heart; (C) relative weight of the leg fat tissues; (D) relative weight of the abdominal fat tissues; (E) relative weight of the subcutaneous fat tissues. *Significance level of p < .05.
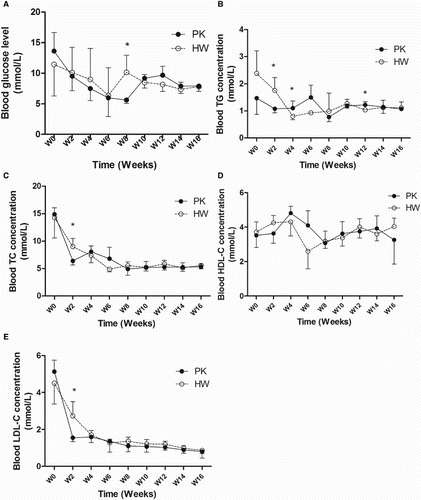
Variations in blood parameters between the two duck breeds
Blood glucose levels were monitored from the post-hatching stages until week 16. Overall, no apparent differences between the two duck species were observed during the entire study period. They exhibited similar trends, both showing higher levels in the early stages of post hatching compared to the later stages closer to the week 16 sampling point.
Blood TG and TC concentrations also showed similar trends in the two duck species at each time point analysed, exhibiting a decreasing trend during the early stages post hatching. The HDL concentration in the blood serum initially increased from the early stages post hatching until week 4, and then decreased from week 8. The LDL concentrations were decreased in both duck breeds at all examined time points.
DNL-related gene expression and enzyme amount in the two duck breeds
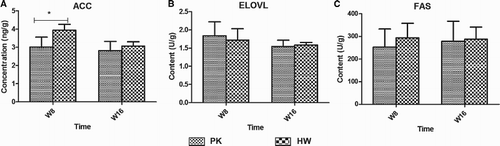
The DNL-related genes, including Acc, Dgat2 and Fas, were analysed in the liver tissues at weeks 8 and 16 (). At week 8, the relative mRNA expression levels of the three genes did not show any differences between the PK and HW ducks, whereas at week 16, the expression levels were increased in the HW ducks and apparent differences from the levels in the PK ducks began to emerge. However, still no significant differences were observed according to the large individual variance. The mRNA expression results were further confirmed based on the protein levels analysed using ELISA kits (). The ACC, ELVOL and FAS results still showed no significant differences between the genetically fat and thin ducks at weeks 8 and 16.
Figure 4. The expression levels of DNL- and adipocyte proliferation-related genes compared between genetically lean and fat ducks. (A–C) The relative mRNA expression of Acc, Dgat2 and Fas in the hepatic tissues at weeks 8 and 16; (D and E) the relative mRNA expression of cEBP and PPARγ in peripheral fat tissues, including leg fat (LF) tissues, abdominal fat tissues (AF) and subcutaneous fat (SF) tissues at weeks 8 and 16. For each gene and tissue, n = 6. *Significance level of p < .05.
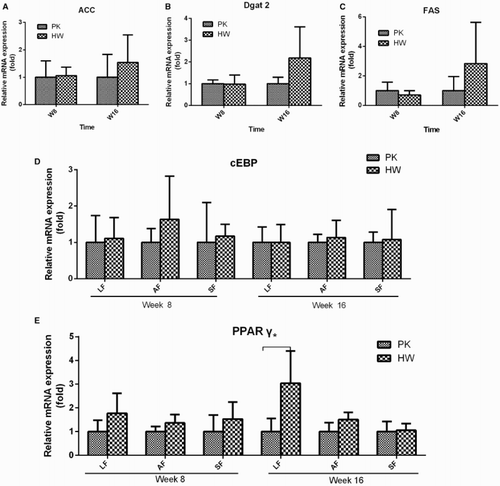
Figure 5. The enzyme activities and protein levels of DNL-related proteins in hepatic tissues compared between genetically lean and fat ducks. (A) Protein concentrations of ACC; (B) enzyme activity of ELVOL; (C) enzyme activity of FAS. n = 6. *Significance level of p < .05.
In the peripheral fat tissues, the mRNA expression levels of cEBP and PPARγ were measured and compared at weeks 8 and 16. The expression of cEBP showed a similar level in all three examined fat tissues in the PK and HW ducks. PPARγ was also expressed in all three peripheral adipose tissues studied, with a relatively higher level in the HW ducks at each time point; however, due to the large individual variance, a large difference in the leg fat tissues was observed only at week 16.
Correlation analysis
The contributions of DNL to fat deposition were evaluated based on the correlation coefficients between the fat phenotypic items and the molecular DNL indicators (). Heart weight was an important contributing factor that shaped the body weight during the entire study period (R = 0.37∼0.61, p < .05). The liver weight contributed greatly to the duck body weight only in the PK ducks at week 8 (R = 0.82, p < .01) and in the HW ducks at week 16 (R = 0.64, p < .01). The fat weight contributed greatly to the body weight except in the HW ducks at week 8 (R = 0.58∼0.87, p < .01). These data indicated that HW ducks are a late-maturing breed compared with the PK ducks. For the body fat mass, interestingly, the heart contributed more than the liver as reflected by the results that a significant correlation coefficient was observed between the heart weight and the fat weight at all stages studied; however, a significant correlation coefficient between the liver weight and the fat weight was observed only at week 8 in the PK ducks (R = 0.48, p < .01) and at week 16 in the HW ducks (R = 0.58, p < .01). The correlation between hepatic DNL molecular items and phenotypic fat deposit amounts was also analysed, and the results did not show any regular contributions of hepatic DNL to body fat mass, although some DNL molecular items appeared to play a negative role in fat mass.
Table 2. Correlation analysis of the items of phenotypes and DNL molecular indicators.
Discussion
The underlying reason for the individual differences in body fat mass remains unclear. The results of the present study supported different fat deposition capabilities in the PK and HW ducks. The phenotypic divergence in the weights of the liver and the fat tissues indicated that different genetic backgrounds existed within the two duck breeds, providing a suitable model for the subsequent analysis of the contribution of hepatic DNL to individual differences in duck fat mass.
In ducks, our initial data demonstrated that the main DNL site is always the liver in post-hatching ducks and that the adipose tissues are of little importance for DNL (Ding et al. Citation2012). It was therefore concluded that hepatic DNL as an endogenous pathway for lipid synthesis may play an important role in the individual fat mass differences. In the present study, we focused on the expression pattern of lipogenic genes (Acc, fas and Dgat2) and the levels of enzyme amount (ACC, ELVOL and FAS) in the hepatic tissues between the PK ducks and the HW ducks. These selected genes and enzymes have been shown to play roles in controlling the key lipogenesis processes and have been well established as good indicators of DNL (Daval et al. Citation2000; Yen et al. Citation2005; Zhao et al. Citation2007; Rosebrough et al. Citation2008; Herault et al. Citation2010; Strable and Ntambi Citation2010). Our results demonstrated that the expression of these genes and enzyme activities (or concentrations) showed no obvious changes in the two duck breeds, indicating that hepatic DNL may participate less in the fat mass difference. In chickens, the expression of lipid synthesis and secretion-related genes were analysed in the liver of lean and fat chickens, and only a few genes (stearoyl-CoA desaturase, sterol response element binding factor 1 and hepatocyte nuclear factor 4) were identified to be differentially expressed between the chicken breeds (Bourneuf et al. Citation2006). In ducks, Aijuan et al. identified the differential expression of proteins in hepatic tissues in lean and fat Pekin ducks (Zheng et al. Citation2014). Based on their data, no DNL-related proteins were identified, which was similar to our results that indicated less contribution of hepatic DNL in the genetic fat mass difference.
The fat mass difference can also be attributed to the accumulation of triacylglycerols in the adipose tissue and hypertrophy as well as hyperplasia of adipocytes, in addition to the contributions of hepatic DNL. Moreover, it was reported that the adipocyte proliferation mainly occurred in the early post-hatching stages in ducks (Kou et al. Citation2012). We further examined the mRNA expression levels of cEBP and PPARγ in the peripheral fat tissues, whose functions involve regulating adipocyte proliferation (Hu et al. Citation1995). However, no clear differences in the expression levels of these genes were observed in any of the fat tissues in the two duck breeds, indicating that the adipocyte proliferation status may contribute equally to fat tissues. It was concluded that adipocyte proliferation may not be the main contributing factor to individual fat mass differences.
In our present study, although there were some apparent phenotypic divergences in body fat mass amounts in the two duck breeds, they both grew at their normal rate and exhibited similar developmental status, which was reflected by the similar percentage of liver and fat tissues in relation to the body weight. The similar developmental status of the two duck breeds may be able to explain why the expressions and enzyme amount examined in our study showed no clear differences between the species. Our results were supported by a transcriptome study, which demonstrated that no DNL-related genes were identified to be differentially expressed in genetically thin and fat chickens (Carre et al. Citation2002). Given that the levels of gene expression and enzyme amount in per unit hepatic tissues were approximately the same in the two genotypic duck breeds, the liver size may account for the body fat mass. Interestingly in our results, the liver weight at its peak in the PK ducks was approximately 2.5-fold that in the HW ducks, which was similar to the fold change of the extra-hepatic adipose tissue between the two duck breeds; for example, the leg fat tissue weight at its peak in the PK ducks was approximately30 g, which was approximately three times that in the HW ducks. These findings were also supported by the correlation analysis, which showed that hepatic DNL items and phenotypic fat deposit amounts did not show any regular contributions of hepatic DNL to body fat. Therefore, it was suggested that a larger liver may represent higher levels of liver fatty acids generated in the liver.
It was suggested that hepatic lipogenesis is sensitive to the nutritional conditions, such as high-carbohydrate and high-fat diets. In humans, the DNL capabilities can be stimulated by high-carbohydrate and high-fat diets (Diraison et al. Citation2003; Strable and Ntambi Citation2010). In ducks, Hermier et al. investigated the effects of overfeeding on hepatic lipid channelling in two duck breeds, including the common duck (Anas platyrhynchos) and the Muscovy duck (Cairina moschata). The Muscovy duck exhibited a higher degree of hepatic steatosis and a lower increase in adiposity and in the concentration of plasma TG and VLDL response to overfeeding, indicating a genotypic influence on VLDL and fat mass (Hermier et al. Citation2003). Based on their data, several DNL-related genes were differentially expressed in the two duck species, which was similar to our findings. However, the common duck and the Muscovy duck are two duck species that belong to different genera and therefore have greater differences in their genetic backgrounds than do the PK and HW ducks in the current study. Herault et al. compared the feeding and species effect on differences in lipid metabolism and steatosis of Pekin and Muscovy overfed ducks, and they demonstrated a specific positive effect of feeding on the expression of the genes involved mainly in fatty acid and TG synthesis and glycolysis and a negative effect on genes involved in b-oxidation; a strong species effect was also observed in stearoyl-CoA desaturase 1 and, to a lesser extent, in diacylglycerol-O-acyltransferase 2 expression, leading to large differences in the expression levels in the Pekin and Muscovy overfed ducks(Herault et al. Citation2010). Their data did not definitively show a species effect on the expression of genes that encode the main enzymes involved in DNL. Taken together with our data, it is reasonable to believe that hepatic DNL may contribute less to the body fat mass difference in some duck species.
In summary, we investigated the possible effects of hepatic DNL on fat mass differences in genetically thin and fat ducks. Although a clear phenotypic divergence in extra-liver fat mass was observed in the two duck breeds, the relative weight of the fat tissues to the body weight was the same. Our results showed that there were no clear differences in the DNL-related gene expression and enzyme amount observed in the PK and HW ducks, suggesting that the hepatic DNL may contribute less to the fat mass difference in genetically different duck breeds than does liver size. Our findings may provide a fundamental explanation for the genetic differences in fat mass.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alvarenga RR, Zangeronimo MG, Pereira LJ, Rodrigues PB, Gomide EM. 2011. Lipoprotein metabolism in poultry. World’s Poult Sci J. 67(3):431–440. doi: 10.1017/S0043933911000481
- Bedu E, Chainier F, Sibille B, Meister R, Dallevet G, Garin D, Duchamp C. 2002. Increased lipogenesis in isolated hepatocytes from cold-acclimated ducklings. Am J Physiol Regul Integr Comp Physiol. 283(5):R1245–R1253. doi: 10.1152/ajpregu.00681.2001
- Bourneuf E, Herault F, Chicault C, Carre W, Assaf S, Monnier A, Mottier S, Lagarrigue S, Douaire M, Mosser J, Diot C. 2006. Microarray analysis of differential gene expression in the liver of lean and fat chickens. Gene. 372(1):162–170. doi: 10.1016/j.gene.2005.12.028
- Carre W, Bourneuf E, Douaire M, Diot C. 2002. Differential expression and genetic variation of hepatic messenger RNAs from genetically lean and fat chickens. Gene. 299(1–2):235–243. doi: 10.1016/S0378-1119(02)01077-6
- Daval S, Lagarrigue S, Douaire M. 2000. Messenger RNA levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet Sel Evol. 32(5):521–531. doi: 10.1186/1297-9686-32-5-521
- Ding F, Pan Z, Kou J, Li L, Xia L, Hu S, Liu H, Wang J. 2012. De novo lipogenesis in the liver and adipose tissues of ducks during early growth stages after hatching. Comp Biochem Physiol B Biochem Mol Biol. 163(1):154–160. doi: 10.1016/j.cbpb.2012.05.014
- Diraison F, Yankah V, Letexier D, Dusserre E, Jones P, Beylot M. 2003. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J Lipid Res. 44(4):846–853. doi: 10.1194/jlr.M200461-JLR200
- Evans AJ. 1972. In vitro lipogenesis in the liver and adipose tissues of the female Aylesbury duck at different ages. Br Poult Sci. 13(6):595–602. doi: 10.1080/00071667208415986
- Galic S, Oakhill JS, Steinberg GR. 2010. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 316(2):129–139. doi: 10.1016/j.mce.2009.08.018
- Griffin HD, Guo K, Windsor D, Butterwith SC. 1992. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J Nutr. 122(2):363–368.
- Hellerstein MK. 1999. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr. 53(S1):S53–S65. doi: 10.1038/sj.ejcn.1600744
- Herault F, Saez G, Robert E, Al Mohammad A, Davail S, Chartrin P, Baeza E, Diot C. 2010. Liver gene expression in relation to hepatic steatosis and lipid secretion in two duck species. Anim Genet. 41(1):12–20. doi: 10.1111/j.1365-2052.2009.01959.x
- Hermier D, Guy G, Guillaumin S, Davail S, Andre JM, Hoo-Paris R. 2003. Differential channelling of liver lipids in relation to susceptibility to hepatic steatosis in two species of ducks. Comp Biochem Physiol B Biochem Mol Biol. 135(4):663–675. doi: 10.1016/S1096-4959(03)00146-5
- Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, Grallert H, Illig T, Wichmann HE, Rief W, et al. 2007. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One. 2(12):e1361. doi: 10.1371/journal.pone.0001361
- Hu E, Tontonoz P, Spiegelman BM. 1995. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA. 92(21):9856–9860. doi: 10.1073/pnas.92.21.9856
- Kou J, Wang WX, Liu HH, Pan ZX, He T, Hu JW, Li L, Wang JW. 2012. Comparison and characteristics of the formation of different adipose tissues in ducks during early growth. Poult Sci. 91(10):2588–2597. doi: 10.3382/ps.2012-02273
- Leung TT, Bauman DE. 1975. In vivo studies of the site of fatty acid synthesis in the rabbit. Int J Biochem. 6(11):801–805. doi: 10.1016/0020-711X(75)90095-6
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25(4):402–408. doi: 10.1006/meth.2001.1262
- McCune SA, Durant PJ, Jenkins PA, Harris RA. 1981. Comparative studies on fatty acid synthesis, glycogen metabolism, and gluconeogenesis by hepatocytes isolated from lean and obese Zucker rats. Metabolism. 30(12):1170–1178. doi: 10.1016/0026-0495(81)90037-8
- O’Hea EK, Leveille GA. 1969. Lipid biosynthesis and transport in the domestic chick (Gallus domesticus). Comp Biochem Physiol. 30(1):149–159. doi: 10.1016/0010-406X(69)91309-7
- Rosebrough RW, Russell BA, Richards MP. 2008. Short term changes in the expression of lipogenic genes in broilers (Gallus gallus). Comp Biochem Physiol A Mol Integr Physiol. 149(4):389–395. doi: 10.1016/j.cbpa.2008.01.035
- Shrago E, Glennon JA, Gordon ES. 1971. Comparative aspects of lipogenesis in mammalian tissues. Metabolism. 20(1):54–62. doi: 10.1016/0026-0495(71)90059-X
- Strable MS, Ntambi JM. 2010. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 45(3):199–214. doi: 10.3109/10409231003667500
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78(4):343–358. doi: 10.1016/j.meatsci.2007.07.019
- Wu JH, Lemaitre RN, Manichaikul A, Guan W, Tanaka T, Foy M, Kabagambe EK, Djousse L, Siscovick D, Fretts AM, et al. 2013. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 6(2):171–183. doi: 10.1161/CIRCGENETICS.112.964619
- Yang B, Zhang W, Zhang Z, Fan Y, Xie X, Ai H, Ma J, Xiao S, Huang L, Ren J, Moore S. 2013. Genome-wide association analyses for fatty acid composition in porcine muscle and abdominal fat tissues. PLoS One. 8(6):e65554. doi: 10.1371/journal.pone.0065554
- Yen CF, Jiang YN, Shen TF, Wong IM, Chen CC, Chen KC, Chang WC, Tsao YK, Ding ST. 2005. Cloning and expression of the genes associated with lipid metabolism in Tsaiya ducks. Poult Sci. 84(1):67–74. doi: 10.1093/ps/84.1.67
- Zhao S, Ma H, Zou S, Chen W, Zhao R. 2007. Hepatic lipogenesis in broiler chickens with different fat deposition during embryonic development. J Vet Med A. 54(1):1–6. doi: 10.1111/j.1439-0442.2007.00898.x
- Zheng A, Chang W, Hou S, Zhang S, Cai H, Chen G, Lou R, Liu G. 2014. Unraveling molecular mechanistic differences in liver metabolism between lean and fat lines of Pekin duck (Anas platyrhynchos domestica): a proteomic study. J Proteomics. 98(4):271–288. doi: 10.1016/j.jprot.2013.12.021