ABSTRACT
Immunomodulatory properties of fucoidan have been extensively studied in fish. However the effect of dietary fucoidan on the metabolic enzymes is not studied in detail with respect to aquatic organisms. An investigation of the effect of dietary fucoidan on the metabolic and haematological status of Labeo rohita fingerlings is presented. One hundred and eighty fingerlings were distributed into four experimental groups in triplicates. Each group was fed isonitrogenous and isoenergetic diets containing 0% fucoidan (T1), 1% fucoidan (T2), 2% fucoidan (T3) and 3% (T4) seaweed powder to satiation for a period of sixty days. Dietary fucoidan was found to significantly (P < 0.05) reduce malate dehydrogenase and aspartate aminotransferase activity in the liver tissue. Alanine aminotransferase activity in the muscle tissue of fucoidan fed groups was significantly higher than the control. The liver and gill superoxide dismutase activity was significantly reduced in the fucoidan fed groups compared to the control. The catalase activity in the liver and gill was significantly lowered in the T3 group. The blood profile of the different experimental groups also revealed beneficial effects of dietary fucoidan yielding a superior haematological status in the groups fed with fucoidan.
KEYWORDS:
Introduction
Intensification of culture practices is a major contributor for the growth of global farmed fish production. However, fish reared in intensive culture systems are subjected to a variety of stressful conditions due to economic realities of large-scale production (Burton Citation1997) and sanitary shortcomings of the culture environment. A number of stressors are at play simultaneously in a culture environment inducing responses, which involve all three regulatory systems, neural, endocrine, and immune (Tort Citation2011). Stress in aquatic animals causes immunosuppression (Magnadóttir Citation2006) and reduced feed intake (Mackett et al. Citation1992), which may lead to undesirable effects like disease outbreak (Snieszko Citation1974) and poor growth (Pankhrust and Van Der Craak Citation1997). The use of immunostimulants is a sustainable approach to avoid disease outbreaks in aquaculture systems. Immunostimulants work by stimulating the innate immune system of the fish (Ringø et al. Citation2012) and dietary polysaccharides from a variety of sources have the immunomodulatory ability (Wang et al. Citation2016). Fucoidan is one such polysaccharide that has well known bioactive properties (Church et al. Citation1989; Colliec et al. Citation1991; Nishino et al. Citation1991; Sinniger et al. Citation1993). This water-soluble polysaccharide, which is mostly obtained from macroalgae, has been extensively studied for its immunomodulatory capacities in fish (Kitikiew et al. Citation2013; El-Boshy et al. Citation2014; Prabu et al. Citation2016). However, the effect of dietary fucoidan on the key metabolic enzymes and haematological status of fish is unknown. Metabolic enzymes and blood profile are important indicators of the health and metabolism of fish. Enzymes like glutamate oxalacetate transaminases (GOT), glutamate pyruvate transaminase (GPT) and lactate dehydrogenase (LDH) not only function as links between the protein and carbohydrate metabolism, but also serve as indicators of stress (Abhijith et al. Citation2016). When exposed to stressors, fish modulate and adjust their metabolism (Malarvizhi et al. Citation2012) and the changes induced in the enzymes such as GOT, GPT, LDH, acid phosphatase (ACP), alkaline phosphates (ALP) reflect the health status of fish. Fishes also possess powerful antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT), which are widely distributed among different tissues (Dröge Citation2002). SOD and catalase, along with the non-enzymatic antioxidants neutralize the free radicals (Lushchak Citation2011). The enzymes SOD and CAT can be used as biomarkers of oxidative stress in aquatic organisms (Borkovi et al. Citation2008). Haematological analysis has proven to be a valuable tool for gaining insights into the health status of farmed animals as these indices provide reliable information on chronic stress (Bahmani et al. Citation2001). Haematological parameters of fish are known to be highly responsive to feed additives (Yilmaz Erguin and Celik Citation2012; Roohi et al. Citation2017). In this context, we conducted the present study to determine the effect of dietary fucoidan on the antioxidant and metabolic enzymes and haematological parameters of Labeo rohita.
Materials and methods
Extraction of fucoidan
The brown seaweed, Sargassum wightii, required for extraction of fucoidan was collected from Mandapam, Ramanthapuram district in Tamil Nadu. Fresh brown seaweed samples were collected from the sea with the help of fishermen. Then from the heaps, the Sargassum wightii specimens were sorted out, dried and pulverized to fine powder. Fucoidan was extracted by the method of Prabu et al. (Citation2016). The fucoidan content in the FRSE was estimated by the method of Dubois et al. (Citation1956).
Evaluation of bioactive properties of fucoidan
The stable free radical Diphenylpiclrylhydrazyle (DPPH) was used to determine the antixodant activity of crude fucoidan extract (Molyneux Citation2004). Briefly, two mililitres of 0.06 M methanolic DPPH was added to crude fucoidan extract, mixed well and kept in dark for 30 min. Then the OD value was measured at 517 nm against the reagent blank. The control containing no crude fucoidan extract was also run along with the samples. The ability of each concentration of fucoidan to scavenge DPPH radical was represented as percentage inhibition (%).
Total phenolic content
Total phenolic content in the crude fucoidan extract was estimated by the method of Singleton and Rosy (Citation1965). Gallic acid was used as the standard and the total phenolics content was expressed in mg phenols/100 g sample.
Total antioxidant activity
Antioxidant activity of the fucoidan extract was evaluated by using Cayman Antioxidant assay kit (Item No. 709001). The standard antioxidant used was trolox. Briefly, gradient concentrations of the trolox were prepared in the standard wells to which 10 ul of metmyoglobin and 150 ul chromogen were added. Similarly different concentrations of crude FRSE sample were prepared in the ELISA plate. Finally, 441 uM of hydrogen peroxide was added to all the wells and the absorbance at 750 nm was checked after 5 min in the ELISA reader.
Minimum inhibitory concentration
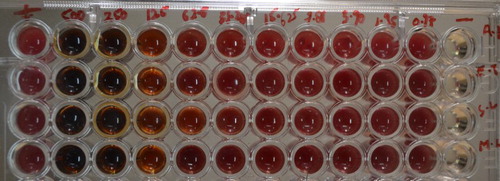
Minimum inhibitory concentration of crude fucoidan against different bacterial strains was determined by Micro Broth Dilution method according to the National Committee for Clincal Laboratory Standards (NCCLS) (NCCLS Citation1999) guidelines using a 96 well microtitre plate. Four different bacteria, Aeromonas hydrophila, Staphylococcus ludgensis, Edwardsiella tarda and Micrococcus luteus were tested for minimum inhibitory concentrations of crude fucoidan extract. Briefly, hundred microliters of sterilized Mueller Hinton Broth (MHB) was added in each well of plate. Then, 100 ul of crude fucoidan extract was added and subsequently serial dilution was performed. 10 ul inoculums of different bacteria grown in Muller Hinton Broth were added to all wells and incubated for 24 h at 37°C. A positive control containing inoculums but no fucoidan and a negative control containing fucoidan but no inoculums were included in each plate. The MIC was determined by adding 10 ul of 1% TTC (2,3,5-tripenyltetrazolium chloride) was added to each well. Plates were then incubated at 30°C for 1 h and the colour change to deep pinkish red was taken as positive.
Experimental fish and diets
One hundred and eighty fingerlings of L. rohita with an average weight of 8.852 ± 0.2 g were used for the experiment. The fishes were procured from R.A. Farm, Mumbai, India. Fish were acclimatized for 15 days in laboratory condition in 1000 L tanks at 30°C, under continuous aeration before starting the experiment. After sufficient acclimatization the fish were randomly transferred to plastic tanks of 100 L capacity with 15 fingerlings per tank in triplicates following a completely randomized design (CRD). Four experimental diets (crude protein and lipid levels at 35% and 8%, respectively) were fed to different experimental groups (). The first group was maintained as control in which fish were given no dietary fucoidan. In second and third groups, fish were given 1% and 2% dietary fucoidan, respectively. For the preparation of feed for T2, 1.78 L of FRSE containing 10 g fucoidan was concentrated in a rotary evaporator to a volume of 200–250 ml. This concentrated extract was then mixed with experimental diet so that the diet may have a final fucoidan content of 1%. Similarly, the feed for T3 was prepared by concentrating 3.57 L of FRSE in the rotary evaporator into a volume of 200–250 ml. This was then mixed with experimental diet so that the diet may have a final fucoidan content of 2%. After the feed preparation, the feeds were dried in a hot air oven at 40°C in order to remove the moisture that came from the FRSE paste. In the fourth group (T4), the fish were provided finely ground seaweed powder at the level of 3% of the diet. Continuous aeration was provided to all the tubs throughout the experimental period. The fish were fed to satiation daily at 10.00 h in the morning and 18.00 h in the evening under normal light regime.
Table 1. Composition of the experimental diets (g/100 g).
Hepatosomatic index (HSI) and viscerosomatic index (VSI)
The liver and intestine weights and weight of fishes of different treatment groups were recorded and the hepatosomatic index and viscerosomatic index was calculated as
Sampling and enzyme assays
The muscle, liver, gill and intestine of the fishes were removed carefully and were weighed. Tissues were homogenized using tissue homogenizer in chilled sucrose solution (0.25 M) added to obtain 5% tissue homogenate concentration. The tube was continuously kept in ice to avoid heating. The homogenate was centrifuged at 5000 rpm for 10 min at 4°C. The supernatant was stored at −20°C till further use. Total protein of each tissue homogenate for enzyme assays was estimated by Bradford method (Bradford Citation1976).
Lactate dehydrogenase (E.C.1.1.1.27) activity was assayed in different tissues by the method of Wroblewski and Ladue (Citation1955). The total 3 ml of the reaction mixture comprised of 2.7 ml of 0.1 M phosphate buffer (pH 7.5), 0.1 ml of NADH solution (2 mg NADH dissolved in 1 ml of phosphate buffer solution), 0.1 ml of tissue homogenate and 0.1 ml of sodium pyruvate. The enzymatic activity was expressed as units/mg protein at 25°C where 1 unit was equal to Δ0.01OD/min.
Malate dehydrogenase (E.C.1.1.1.37) activity was assayed in different tissues by the method of Ochoa (Citation1955). Total 3 ml of the reaction mixture comprised of 2.7 ml of 0.1 M phosphate buffer (pH 7.5), 0.1 ml of NADH solution (2 mg NADH dissolved in 1 ml of phosphate buffer solution), 0.1 ml of tissue homogenate and 0.1 ml of freshly prepared oxaloacetate solution (2 mg oxaloacetate dissolved in 2 ml chilled distilled water). The OD was recorded at 340 nm and enzymatic activity was expressed as units/mg protein at 25°C where 1 unit was equal to Δ0.01 OD/min.
Aspartate aminotransferase (E.C.2.6.1.1) and Alanine aminotransferase activity (E.C.2.6.1.2) was assayed in different tissue homogenates as described by Wootton (Citation1964). For AST the reaction mixture comprised of 0.2 M D,L-aspartic acid and 2 mM α-ketoglutarate in 0.05 M phosphate buffer (pH 7.4). The reaction was started by adding 0.1 ml of tissue homogenate. The assay mixture was incubated at 25°C for 60 min. The reaction was terminated by adding 0.5 ml of 1 mM 2,4-dinitrophenylhydrazine (DNPH). The OD was recorded at 540 nm against the blank. Enzyme activity was expressed as nanomoles of sodium pyruvate formed/ mg protein/ minute at 25°C. For alanine amonitransferase, D,L-alanine was used instead of D,L-aspartic acid.
Alkaline phosphatase (ALP) (E.C. 3.1.3.1) activity was determined by the method of Garen and Levinthal (Citation1960). The assay mixture consisted of bicarbonate buffer (0.2 M, pH 9.5), 0.1 M MgCl2, tissue homogenate and freshly prepared 0.1 M para-Nitrophenylphosphate (p-NPP) as substrate. The reaction mixture was incubated in water bath at 25°C for 15 min and then stopped with 0.1 N NaOH. Optical density was recorded at 410 nm. ALP activity was expressed as nanomoles p-nitrophenol released/min/mg protein at 25°C. Acid phosphatase (ACP) (E.C. 3.1.3.2) activity was estimated using the same method as ALP, except that acetate buffer (0.2 M, pH 5) was used in place of bicarbonate buffer.
Superoxide dismutase (EC 1.15.1.1) was assayed in the liver and gill homogenates by the method of Marklund and Marklund (Citation1974). The reaction was initiated by the addition of 0.5 ml pyrogallol reagent, and the change in optical density was measured at 480 nm for 3 min. Fifty percent inhibition of pyrogallol by the enzyme was taken as one enzyme unit. The enzyme activity was expressed as units per milligramm of protein in tissues. Catalase (EC 1.11.1.6) activity was assayed by the method of Sinha (Citation1972). Absorbance was read at 570 nm and catalase activity was expressed as micromoles of H2O2 consumed per minute per milligramme of protein.
Haematological parameters
For collection of blood, each fish was anaesthetized with clove oil (50 μl of clove oil per litre of water) before taking blood from fish. Blood was taken from caudal vein using a medical syringe (1 ml), which was previously rinsed with 2.7% EDTA solution. Blood collected was then transferred immediately to test tube containing thin layer of EDTA powder (as an anticoagulant) and shaken well in order to prevent haemolysis of blood. The hemoglobin level of blood was analysed following the Cyanmethemoglobin method using Drabkins Fluid (Qualigens). Blood (20 μl) was mixed with 5 ml of Drabkin’s working solution. The absorbance was measured using a spectrophotometer at wavelength of 540 nm. The final concentration was calculated by comparing with the standard cyanmethemoglobin (Qualigens Diagnostics). The haemoglobin concentration was then calculated by using the following formula:where, OD (T) = Absorbance of test; OD (S) = Absorbance of standard
Total erythrocyte count (TEC) and total leucocyte count (TLC)
Blood (20 μl) was mixed with 3980 μl of RBC diluting fluid and WBC diluting fluid separately in clean test tubes. The mixtures were shaken well to suspend the cells uniformly in the solution. Small drop of this mixture was charged to Neubauer’s counting chamber. To determine RBC count, the cells lying inside the five small squares were counted under high power (40X) light microscope, whereas the WBCs were determined by counting the cells in four big squares under high power (40X) magnification light microscope The following formula was used to calculate the number of RBC per mm3 of the blood sample:where N is the total number of cells counted.
Packed cell volume (PCV) was determined by drawing non-dotted blood by capillary action into microhaematocrit tubes. One end of the tubes was sealed with synthetic sealant. The sealed tube was centrifuged in a microhaemotocrit centrifuge for 5 min at 10,500 rpm. The PCV measured using microhaemotocrit reader and expressed as percentage.
Statistical analysis
To validate the reproducibility of results, each assay was done in triplicate. The results were treated by one-way analysis of variance (ANOVA) using SPSS v. 16. All analyses were performed considering a level of 95% of confidence (P < 0.05).
Results
Quantification of fucoidan
The dialysed fucoidan rich seaweed extract was quantified by measuring the L-fucose content, which was multiplied by a factor of 1.75 to get the total fucoidan content. The L-fucose content of the concentrated and dialyzed extract was 32 mg/g dried seaweed powder. Hence, the fucoidan yield was 56 mg/g or 5.6 g/100 g dried seaweed powder.
DPPH radical scavenging method
The crude extract exhibited varying degrees of scavenging capacity depending on the concentration (). All the concentrations of fucoidan vary significantly (P < 0.05), except that there was no significant difference in the scavenging capacity of 5.5 mg/ml and 7 mg/ml concentrations. The maximum concentration (10 mg/ml) of the extract showed the highest scavenging effect (52.05%), whereas the lowest concentration (0.5 mg/ml) of the extract showed the least scavenging capacity (8.50%).
Table 2. The total phenolic content and DPPH inhibition of crude FRSE extracted from Sargassum wightii.
Total phenolics assay
The total phenolic contents of all the crude fucoidan extract with different concentrations were significantly different (P < 0.05) exhibiting similar trend as that of DPPH. Total phenolic compounds increased with the increasing concentration of fucoidan extract.
Metmyoglobin inhibition antioxidant assay
The metmyoglobin based antioxidant assay results showed a consistently increasing trend with the concentration of the extract with the highest up to 1.6 mg/ml concentration of fucoidan extract (). All the values were significantly different from each other (P < 0.05).
Table 3. Antioxidant activity (mM Trolox equivalents) of crude FRSE at different concentrations.
Minumum inhibitory concentration
MIC values of fucoidan rich seaweed extracts against the different bacterial strains was 250 ul/ml (). The fucoidan content of the extract was 5.6 mg/ml, which indicated MIC of 1.4 mg/ml.
Hepatosomatic index and viscerosomatic index
The HSI and VSI values of the different experimental groups were not found to be significantly different ()
Table 4. HSI and VSI of different experimental groups at the end of the 60-days feeding experiment.
Enzyme assays
The liver malate dehydrogenase activity was found to be significantly (P < 0.05) lower in the groups fed with fucoidan compared to the control and seaweed fed groups (). However, the muscle MDH activity did not show any significant difference among the treatment groups. Also the LDH activities in muscle and liver were not significantly affected by dietary fucoidan or seaweed. AST activity in liver tissues of the group fed 2% fucoidan was significantly lower than the control group (). Also, the ALT activity in the muscle tissue of T3 groups was significanty higher than the control group. There was no significantly difference in the liver ALT activity in the different experimental groups, but the muscle AST was significantly higher in the fucoidan fed groups compared to control group. The liver and gill SOD activity was significantly reduced in the fucoidan fed group compared to the control group (). Also, the catalase activity in the liver and gill was significantly lowered in the T3 group. There was no significant difference in the intestinal ALP and ACP enzymes of the different experimental groups ()
Table 5. LDH and MDH activity of liver and muscle tissues of different experimental groups.
Table 6. ALT and AST activity of liver and muscle of the different experimental groups.
Table 7. SOD and Catalase activities in liver and gill tissues of different experimental groups.
Table 8. ALP and ACP activity in the intestine of the different experimental groups.
Haematological parameters
The blood haemoglobin value of the T3 treatment was found to be significantly higher than the other groups (). In the 2% fucoidan fed group, the TEC was also significantly higher than the other experimental groups. Dietary fucoidan was also found to significantly affect the blood TLC with the highest value found in T3 group. Significantly higher haematocrit values were found in the T3 group compared to other treatment groups. The lowest haematocrit value was found in the control group (T1) and the seaweed fed group (T4). Blood profile of the T2 and T4 group had no significant difference from the control group (T1).
Table 9. Haematological parameters of different experimental groups.
Discussion
The extraction yield of fucoidan depends upon the method employed. The earliest report of fucoidan extraction from seaweeds (Kylin Citation1913) used dilute acetic acid followed by purification. However, over the course of time the method was modified and improved. Precipitation of sulphate groups by ethanol was also effective in order to isolate fucoidan from brown seaweeds (Bird and Haas Citation1931). Dilute hydrochloric acid was also used by many workers (Nelson and Cretcher Citation1931; Black et al. Citation1952). The bioactivity of the polysaccharide is related to its molecular weight, the monosaccharide composition, the sulphate content and the position of the sulphate ester group (Ale et al. Citation2011), the isolation procedure should avoid the loss of the sulphate and the structural alteration of the target compound. Therefore mild aqueous conditions are commonly preferred in order to cause least damage to the structure and hence bioactivity of fucoidan during extraction. The method followed in the present study uses hot water extract and the yield of 5.6% is in agreement with the previous results, which reported the yield of fucoidan 3.9% to 7.15% (Wang and Zhae Citation1985; Velayutham and Jayachandran Citation1991; Eluvakkal et al. Citation2010; Prabu et al. Citation2013). Also the method is amenable to large-scale extraction as least inputs are involved compared to previously developed methods (Athukorala et al. Citation2006; Rodriguez-Jasso et al. Citation2011).
A wide range of methods is available which can be employed to determine the antioxidant capacity of a compound (Pisoschi and Negulescu Citation2011). However, determining the antioxidant activity can be highly variable and may not be explained by one single method. The variability observed may be due to the different methodologies used. This is important because some methods may be based on single electron transfer mechanism and others on hydrogen atom transfer (Ioannou et al. Citation2015), leading to contradicting results. Therefore, to avoid this, we evaluated antioxidant activity by different methods, ABTS assay (Re et al. Citation1999) and the DPPH assay (Blois Citation1958) both of which are based on the principle of single electron transfer. Our results are supportive of the antioxidant properties of fucoidan confirmed previously (Choi et al. Citation2007; Balboa et al. Citation2013). The extract used in the present study was in crude form. High phenolic content is reported to be associated with the crude hot water extraction method (Prabu et al. Citation2016). Although, fucoidan is not a phenolic compound, but it is possible that some phenolics may have been co-extracted from the seaweed, correlating with and adding to the antioxidant power of the crude extract (Piluzza and Bullita Citation2011). For this reason, the total phenolics assay of the crude fucoidan was carried out. We observed an increase in total phenolic activity with the concentration of the extract that is coinciding with the previous studies (Zubia et al. Citation2008; Namvar et al. Citation2013). Fucoidan has been found to have antibacterial properties against different strains of bacteria like Staphylococcus spp. (Lee et al. Citation2013), Vibrio and Salmonella (Marudhupandi and Kumar Citation2013), E. coli (Vijayabaskar et al. Citation2012), Aeromonas hydrophila and Micrococcus luteus (Prabu et al. Citation2013). We found fucoidan to have antibacterial effect with the minimum inhibitory concentration of 1.4 mg/ml. Lee et al. (Citation2013) who reported MIC of fucoidan within the range of 0.125 to 1 mg/ml against different bacteria. Also, Prabu et al. (Citation2013) reported MIC of fucoidan as 6.8 to 27.5 mg/ml against different strains of bacteria.
The hepatosomatic index and viscerosomatic index of fish are indicators of well-being and fitness of fish (Jensen Citation1979; Delahunty and Vlaming Citation1980). These indices indicate the metabolic stores of the body and exhibit fluctuation in response to nutritional status of fish (Larsen et al. Citation2001) and exposure to anthropogenic pollutants (Al-Ghais Citation2013). However, the present study revealed no significant variations in the HSI and VSI. This supports the non toxic effect of dietary fucoidan implies that fucoidan does not adversely affect the nutritional status of Labeo rohita.
Oxidative stress results when pro-oxidant forces overcome anti-oxidant defences and reactive oxygen species are not removed adequately (Winston and Di Giulio Citation1991). Living organisms are protected from the ROS by several defence mechanisms, including antioxidant enzymes such as SOD and catalase. SOD and catalase enzyme activity are good indicators of oxidative stress in fish (Martinez-Álvarez et al. Citation2005; Monteiro et al. Citation2006). Fucoidan is known to modulate SOD and catalase activity and provide protection in response to strong pro-oxidants (Veena et al. Citation2007). Similar mechanisms have been observed in fishes (Prabu et al. Citation2016). The present study revealed the SOD and catalase activity was diminished significantly with the increasing level of dietary fucoidan. The results confirm that fucoidan acts as a powerful antioxidant in vivo and can scavenge free radicals, thereby protecting lipids, membranes and other compounds being oxidized or destroyed (Popeskovic et al. Citation1980). Correlation between the antioxidant power of fucoidan with several factors like its molecular weight (Hou et al. Citation2012) and sulphate content (Wang et al. Citation2008) has been observed. Indeed, it will be interesting to study whether or not the correlation observed in vitro also hold true for in vivo experiments.
AST and ALT are the enzymes that redistribute amino nitrogen among the amino acids, forming new amino acid with the amino group from the pre-existing ones. These enzymes act as link between carbohydrate and protein metabolism in fish and also are indicators of liver health status. Alanine aminotransferase (ALT) and aspartate transaminase (AST) are two most important amino acid metabolizing enzymes, which can shed light on the intensity of fish amino acids metabolism. The liver is the main organ for amino acid catabolism, which results in the production of 50–99% ammonia (Ballantyne Citation2001). We found significant reduction of liver AST and ALT activities due to dietary fucoidan. This means the metabolism is gearing up for sparing the protein to be used as an energy source (Jurss Citation1981). However, an increase in the muscle ALT in the fish fed fucoidan was observed contrary to the trend observed in liver. This is because the metabolism of the muscle tissue is different from that of the liver in terms of the function of transaminases, which play an important role in protein synthesis and degradation (Lin and Luo Citation2011). Therefore, the increased activity of the transaminase in the muscle suggests that fucoidan may direct the metabolic balance of the fish towards growth.
Lactate dehydrogenase helps in maintaining the glycolysis cycle by supplying NAD+. Under stressed conditions, anaerobic pathway is activated and high levels of lactate are being produced by inter-conversion of pyruvate in the cell and thus an increase of LDH activity is expected (Kumar et al. Citation2010). Similarly, malate dehydrogenase reversibly converts malate to oxaloacetate with release of NADH+. There was no effect of dietary fucoidan on the lactate dehydrogenase enzyme, but the liver malate dehydrogenase activity was reduced in the fucoidan fed experimental groups. Malate dehydrogenase is a Kreb’s cycle enzyme and a reduction in MDH activity in fucoidan fed groups is an indicator of reduced metabolic activity in these groups (Yengkokpam et al. Citation2010). The reduced activity may be in response to reduced liver AST activity, which must have caused a reduction in the substrate oxaloacetate (Verma et al. Citation2007). This reveals that dietary fucoidan has some role on the cellular bioenergetics, which must be explored more deeply.
Intestinal acid phosphatase and alkaline phosphatase catalyse the hydrolysis of various phosphate-containing compounds and acts as transphosphorylases at acid and alkaline pH, respectively. Intestinal alkaline phosphatase has a pivotal role in intestinal homeostasis and its activity could be increased through the diet (Lalles Citation2010). The enzyme acid phosphatase, present mainly in lysosomes, has been related to intracellular digestive activity and maturation of the intestinal epithelium and constitutes an auxiliary element of the digestion process (Baintner Citation1994). We found no significant effect of dietary fucoidan on the intestinal acid phosphatase and alkaline phosphatase enzymes in fish indicating no effect on phosphorus metabolism in fish.
Immunostimulants have the potential to improve the function of head kidney meanwhile augmenting the erythropoiesis and haematosynthesis. This in turn can influence the haematological status of fish as observed in different studies (Prabu et al. Citation2016; Ribeiro et al. Citation2016). The present study also revealed positive effect of dietary fucoidan on the RBC count and haemoglobin content. Since, the TEC value increased, haematocrit value was also increased gradually with the increasing concentration of fucoidan in the diet. Our results are in agreement with Sahu et al. (Citation2007) and Mohamad and Abasali (Citation2010). The leucocyte count is considered as a good indicator of the health and immune status of the fish. We observed an increase in leucocyte count in the experimental group fed with fucoidan. Similar result have been observed by Choudhury et al. (Citation2005) and Misra et al. (Citation2006) which used dietary yeast RNA and n-3 PUFA as immunostimulants, respectively.
Seaweed powder is obtained from drying and pulverizing of the seaweed Sargassum wightii. The seaweed powder is the base material for the extraction of crude fucoidan. In the T4 group, seaweed powder was mixed with the feed at the rate 3%. Syad et al. (Citation2013) found that Sargassum wightii has a fibre content of 17% dry weight and crude protein content of 1.4 mg/g dry weight. This means that the chance of any nutritional supplementation of the fish by feeding dry seaweed powder at the rate of 3% is very meagre. Seaweed was included in one of the treatment (T4) to evaluate any effect of dietary seaweed powder on the fish. The metabolic enzyme activity and blood profile of the T4 group had no significant difference from the control group. We observed that only fucoidan extracted from the seaweed powder has beneficial effect on the metabolism and blood profile of fish. This implies that although, there must be some fucoidan in T4 feed coming through seaweed powder, it is either not enough to incite any metabolic response or is in some inactive form.
Conclusion
The present study represents an attempt to investigate the effect of dietary fucoidan on the metabolic enzyme response of fish. The study revealed that dietary fucoidan significantly affects the activities of the important metabolic enzymes of the fish. In addition, fucoidan also reduced the antioxidant enzymes SOD and catalase activities in the different tissues of Labeo rohita. The experiment further revealed that fucoidan can improve the haematological status of the fish by increasing the haemoglobin content and the count of WBCs and RBCs. Therefore, it may be concluded that dietary fucoidan can provide benefits other than immunostimulation to the cultured fish.
Acknowledgement
The authors are grateful to Director, ICAR-Central Institute of Fisheries Education, Mumbai, India for providing support and facilities to carry out this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Adnan Gora http://orcid.org/0000-0001-6207-9410
Additional information
Funding
References
- Abhijith BD, Ramesh M, Poopal RK. 2016. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J Basic Appl Zool. 77:31–40. doi: 10.1016/j.jobaz.2015.11.002
- Ale MT, Mikkelsen JD, Meyer AS. 2011. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. 9(10):2106–2130. doi: 10.3390/md9102106
- Al-Ghais SM. 2013. Acetylcholinesterase, glutathione and hepatosomatic index as potential biomarkers of sewage pollution and depuration in fish. Mar Poll Bull. 74(1):183–186. doi: 10.1016/j.marpolbul.2013.07.005
- Athukorala Y, Jung W-K, Vasanthan T, Jeon Y-J. 2006. An anticoagulative polysaccharide from an enzymatic hydrolysate of ecklonia cava. Carbohydr Polym. 66(2):184–191. doi: 10.1016/j.carbpol.2006.03.002
- Bahmani M, Kazemi R, Donskaya P. 2001. A comparative study of some hematological features in young reared sturgeons (Acipenser persicus and Huso huso). Fish Physiol Biochem. 24(2):135–140. doi: 10.1023/A:1011911019155
- Baintner K. 1994. Demonstration of acidity intestinal vacuoles of the suckling rat and pig. J Histochem Cytochem. 42(2):231–238. doi: 10.1177/42.2.7507141
- Balboa EM, Conde E, Moure A, Falqué E, Domínguez H. 2013. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 138(2):1764–1785. doi: 10.1016/j.foodchem.2012.11.026
- Ballantyne JS. 2001. Nitrogen excretion. Vol. 20. San Diego, CA: Academic Press. (Fish Physiology; vol. 20).
- Bird GM, Haas P. 1931. On the nature of the cell wall constituents of Laminaria spp. mannuronic acid. Biochem J. 25(2):403–411. doi: 10.1042/bj0250403
- Black WAP, Dewar ET, Woodward FN. 1952. Manufacture of algal chemicals. IV—laboratory-scale isolation of fucoidin from brown marine algae. J Sci Food Agric. 3(3):122–129. doi: 10.1002/jsfa.2740030305
- Blois MS. 1958. Antioxidant determinations by the Use of a stable free radical. Nature. 181(4617):1199–1200. doi: 10.1038/1811199a0
- Borkovi SS, Pavlovic SZ, Kovacevic TB, Stajn AS, Petrovic VM, Saicic ZS. 2008. Antioxidant defence enzyme activities in hepatopancreas, gills and muscle of spiny cheek crayfish (orconectes limosus) from the river danube. Comp Biochem Physiol C Toxicol Pharmacol. 147(1):122–128. doi: 10.1016/j.cbpc.2007.08.006
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3
- Burton BA. 1997. Stress in finfish: past, present and future—a historical perspectives. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB, editors. Fish stress and health in aquaculture. Cambridge: Cambridge University Press; p. 1–33.
- Choi DS, Athukorala Y, Jeon YJ, Senevirathne M, Cho KR, Kim SH. 2007. Antioxidant activity of sulfated polysaccharides isolated from sargassum fulvellum. Prev Nutr Food Sci. 12(2):65–73. doi: 10.3746/jfn.2007.12.2.065
- Choudhury D, Pal AK, Sahu NP, Kumar S, Das SS, Mukherjee SC. 2005. Dietary yeast RNA supplementation reduces mortality by aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shellfish Immunol. 19(3):281–291. doi: 10.1016/j.fsi.2005.01.004
- Church FC, Meade JB, Treanor RE, Whinna HC. 1989. Antithrombin activity of fucoidan – the interaction of fucoidan with heparin cofactor-ii, antithrombin- iii, and thrombin. J Biol Chem. 264(6):3618–3623.
- Colliec S, Fischer AM, Tapon-Bretaudiere J, Boisson C, Durand P, Jozefonvicz J. 1991. Anticoagulant properties of a fucoidan fraction. Thromb Res. 64(2):143–154. doi: 10.1016/0049-3848(91)90114-C
- Delahunty G, Vlaming VD. 1980. Seasonal relationships of ovary weight, liver weight and fat stores with body weight in the goldfish, Carassius auratus (L.). J Fish Biol. 16(1):5–13. doi: 10.1111/j.1095-8649.1980.tb03683.x
- Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol Rev. 82(1):47–95. doi: 10.1152/physrev.00018.2001
- Dubois M, Gilles KA, Hamilton JK, Rebers DA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analyt Chem. 28:350–356. doi: 10.1021/ac60111a017
- El-Boshy M, El-Ashram A, Risha E, Abdelhamid F, Zahran E, Gab-Alla A. 2014. Dietary fucoidan enhance the non-specific immune response and disease resistance in African catfish, Clarias gariepinus, immunosuppressed by cadmium chloride. Vet Immunol Immunopathol. 162(3):168–173. doi: 10.1016/j.vetimm.2014.10.001
- Eluvakkal T, Sivakumar SR, Arunkumar K. 2010. Fucoidan in some Indian brown seaweeds found along the Coast Gulf of Mannar. Int J Botany. 6(2):176–181. doi: 10.3923/ijb.2010.176.181
- Garen A, Levinthal C. 1960. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 38:470–483. doi: 10.1016/0006-3002(60)91282-8
- Hou Y, Wang J, Jin W, Zhang H, Zhang Q. 2012. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr Polym. 87(1):153–159. doi: 10.1016/j.carbpol.2011.07.031
- Ioannou I, Chaaban H, Slimane M, Ghoul M. 2015. Origin of the variability of the antioxidant activity determination of food material. In: Ekinci D, editor. Biotechnology. Rijeka (Croatia): In Tech. http://dx.doi.org/10.5772/60453.
- Jensen AJ. 1979. Energy content analysis from weight and liver index measurements of immature pollock (pollachius virens). J Fish Board Can. 36(10):1207–1213. doi: 10.1139/f79-174
- Jurss K. 1981. Influence of temperature and ratio of lipid to protein in diets on aminotransferase activity in the liver and white muscle of rainbow trout (Salmo gairdneri Richardson). Comp Biochem Physiol. 68B(4):527–533.
- Kitikiew S, Chen JC, Putra DF, Lin YC, Yeh ST, Liou CH. 2013. Fucoidan effectively provokes the innate immunity of white shrimp Litopenaeus vannamei and its resistance against experimental vibrio alginolyticus infection. Fish Shellfish Immunol. 34(1):280–290. doi: 10.1016/j.fsi.2012.11.016
- Kumar V, Sahu NP, Pal AK, Kumar S, Sinha AK, Ranjan K, Baruah K. 2010. Modulation of key enzymes of glycolysis, gluconeogenesis, amino acid catabolism, and TCA cycle of the tropical freshwater fish Labeo rohita fed gelatinized and non-gelatinized starch diet. Fish Physiol Biochem. 36(3):491–499. doi: 10.1007/s10695-009-9319-5
- Kylin H. 1913. Biochemistry of sea algae. HZ Physiol Chem. 83:171–197. doi: 10.1515/bchm2.1913.83.3.171
- Lalles JP. 2010. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 68(6):323–332. doi: 10.1111/j.1753-4887.2010.00292.x
- Larsen DA, Beckman BR, Dickhoff WW. 2001. The effect of low temperature and fasting during the winter on metabolic stores and endocrine physiology (insulin, insulin-like growth factor-I, and thyroxine) of coho salmon, Oncorhynchus kisutch. Gen Comp Endocrinol. 123(3):308–323. doi: 10.1006/gcen.2001.7677
- Lee KY, Jeong MR, Choi SM, Na SS, Cha JD. 2013. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch Oral Biol. 58(5):482–492. doi: 10.1016/j.archoralbio.2012.11.002
- Lin S, Luo L. 2011. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus× O. aureus. Anim Feed Sci Technol. 168(1):80–87. doi: 10.1016/j.anifeedsci.2011.03.012
- Lushchak VI. 2011. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 101(1):13–30. doi: 10.1016/j.aquatox.2010.10.006
- Mackett DB, Tam WH, Fryer JN. 1992. Histological changes in insulin-immunoreactive pancreatic β-cells, and suppression of insulin secretion and somatotrope activity in brook trout (Salvelinus fontinalis) maintained on reduced food intake or exposed to acidic environment. Fish Physiol Biochem. 10(3):229–243. doi: 10.1007/BF00004517
- Magnadóttir B. 2006. Innate immunity of fish (overview). Fish Shellfish Immunol. 20(2):137–151. doi: 10.1016/j.fsi.2004.09.006
- Malarvizhi A, Kavitha C, Saravanan M, Ramesh M. 2012. Carbamazepine (CBZ) induced enzymatic stress in gill, liver and muscle of a common carp, Cyprinus carpio. J King Saud Univ. 24(2):179–186. doi: 10.1016/j.jksus.2011.01.001
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. FEBS J. 47(3):469–474.
- Martínez-Álvarez RM, Morales AE, Sanz A. 2005. Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish. 15(1–2):75–88. doi: 10.1007/s11160-005-7846-4
- Marudhupandi T, Kumar TTA. 2013. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int Curr Pharm J. 2(10):156–158. doi: 10.3329/icpj.v2i10.16408
- Misra S, Sahu NP, Pal AK, Xavier B, Kumar S, Mukherjee SC. 2006. Pre- and post-challenge immuno-haematological changes in Labeo rohita juveniles fed gelatinised or non-gelatinised carbohydrate with n-3 PUFA. Fish Shellfish Immunol. 21(4):346–356. doi: 10.1016/j.fsi.2005.12.010
- Mohamad S, Abasali H. 2010. Effect of plant extracts supplemented diets on immunity and resistance to aeromonas hydrophila in common carp (Cyprinus carpio). Res J Animal Sci. 4:26–34. doi: 10.3923/rjnasci.2010.26.34
- Molyneux P. 2004. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 26(2):211–219.
- Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL. 2006. Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C Toxicol Pharmacol. 143(2):141–149. doi: 10.1016/j.cbpc.2006.01.004
- Namvar F, Mohamad R, Baharara J, Zafar-Balanejad S, Fargahi F, Rahman HS. 2013. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). BioMed Res Int. 2013:1–9. Article ID 604787. doi: 10.1155/2013/604787
- [NCCLS] National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A. Wayne (PA): NCCLS.
- Nelson WL, Cretcher LH. 1931. The carbohydrate acid sulfate of Macrocystis pyrifera. J Biol Chem. 94(1):147–154.
- Nishino T, Aizu Y, Nagumo T. 1991. The influence of sulfate content and molecular- weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thromb Res. 64(6):723–31. doi: 10.1016/0049-3848(91)90072-5
- Ochoa S. 1955. Malic dehydrogenase and ‘malic’ enzyme. In: Coloric SP, Kaplan N, editors. Methods of enzymology I. New York: Academic Press; p. 735–745.
- Pankhurst NW, Van Der Kraak G. 1997. Effects of stress on reproduction and growth of fish. In: Iwama GK, editor. Fish stress and health in aquaculture. Fish stress and health in aquaculture. Cambridge (UK): Cambridge University Press; p. 73–93.
- Piluzza G, Bullitta S. 2011. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm Biol. 49(3):240–7. doi: 10.3109/13880209.2010.501083
- Pisoschi AM, Negulescu GP. 2011. Methods for total antioxidant activity determination: a review. Biochem Anal Biochem. 1:106. doi:10.4172/2161-1009.1000106.
- Popeskovic D, Kepcija D, Dimitrijevic M, Stojanovic N. 1980. The anti oxidative properties of propolis and some of its components. Acta Veterinaria (Belgrade). 30(3/4):133–136.
- Prabu DL, Sahu NP, Pal AK, Dasgupta S, Narendra A. 2016. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquacult Res. 47(1):199–218. doi: 10.1111/are.12482
- Prabu DL, Sahu NP, Pal AK, Narendra A. 2013. Isolation and evaluation of antioxidant and antibacterial activities of fucoidan rich extract (fre) from Indian brown seaweed, Sargassum wightii. Cont J Pharm Sci. 7(1):9–16.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3
- Ribeiro SC, Castelo AS, Silva BMPD, Cunha ADS, Proietti Junior AA, Oba-Yoshioka ET. 2016. Hematological responses of tambaqui Colossoma macropomum (Serrassalmidae) fed with diets supplemented with essential oil from Mentha piperita (Lamiaceae) and challenged with Aeromonas hydrophila. Acta Amazon. 46(1):99–106. doi: 10.1590/1809-4392201501284
- Ringø E, Olsen RE, Vecino JLG, Wadsworth S, Song SK. 2012. Use of immunostimulants and nucleotides in aquaculture: a review. J Marine Sci Res Development. 2:104. doi:10.4172/2155-9910.1000104.
- Rodriguez-Jasso RM, Mussatto SI, Pastrana L, Aguilar CN, Teixeira JA. 2011. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr Polym. 86(3):1137–1144. doi: 10.1016/j.carbpol.2011.06.006
- Roohi Z, Imanpoor MR, Jafari V, Taghizadeh V. 2017. The use of fenugreek seed meal in fish diets: growth performance, haematological and biochemical parameters, survival and stress resistance of common carp (Cyprinus carpio L.). Aquacult Res. 48(3):1209–1215. doi: 10.1111/are.12962
- Sahu S, Das BK, Pradhan J, Mohapatra BC, Mishra BK, Sarangi NN. 2007. Effect of Mangifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immunol. 23(1):109–118. doi: 10.1016/j.fsi.2006.09.009
- Singleton VL, Rosy JA. 1965. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am J Enol Viticult. 16(3):144–158.
- Sinha AK. 1972. Colorimetric assay of catalase. Analyt Biochem. 47(2):389–394. doi: 10.1016/0003-2697(72)90132-7
- Sinniger V, Tapon-Bretaudiere J, Millien C, Muller D, Jozefonvicz J, Fischer AM. 1993. Affinity chromatography of sulphated polysaccharides separately fractionated on antithrombin III and heparin cofactor II immobilized on concanavalin A—sepharose. J Chromatogr B Biomed Sci Appl. 615(2):215–23. doi: 10.1016/0378-4347(93)80335-2
- Snieszko SF. 1974. The effects of environmental stress on outbreaks of infectious diseases of fishes. J Fish Biol. 6(2):197–208. doi: 10.1111/j.1095-8649.1974.tb04537.x
- Syad AN, Shunmugiah KP, Kasi PD. 2013. Seaweeds as nutritional supplements: analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomed Prev Nutr. 3(2):139–144. doi: 10.1016/j.bionut.2012.12.002
- Tort L. 2011. Stress and immune modulation in fish. Develop Comp Immunol. 35(12):1366–1375. doi: 10.1016/j.dci.2011.07.002
- Veena CK, Josephine A, Preetha SP, Varalakshmi P. 2007. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 100(4):1552–1559. doi: 10.1016/j.foodchem.2005.12.040
- Velayutham P, Jayachandran P. 1991. Isolation and purification of fucoidan, laminarian and alginate from Sargassum ilicifolium. Seaweed Res Utilin. 13:49–50.
- Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, Chandrachoodan PP, Ravi PM, Apte SK. 2007. Persistent sub-lethal chlorine exposure elicits the temperature induced stress responses in Cyprinus carpio early fingerlings. Pestic Biochem Physiol. 87(3):229–237. doi: 10.1016/j.pestbp.2006.08.001
- Vijayabaskar P, Vaseela N, Thirumaran G. 2012. Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin J Nat Med. 10(6):421–428.
- Wang E, Chen X, Wang K, Wang J, Chen D, Geng Y, Lai W, Wei X. 2016. Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol. 59:196–202. doi: 10.1016/j.fsi.2016.10.039
- Wang Z, Zhae X. 1985. Extraction and isolation of alginic acid, laminaria and fucoidan from Sargassum horneri. J Fish China. 9(1):279–311.
- Wang J, Zhang Q, Zhang Z, Li Z. 2008. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromolec. 42(2):127–132. doi: 10.1016/j.ijbiomac.2007.10.003
- Winston GW, Di Giulio RT. 1991. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol. 19(2):137–161. doi: 10.1016/0166-445X(91)90033-6
- Wootton IDP. 1964. Enzymes in blood. In: Churchill J, Churchill A, editors. Microanalysis in medical biochemistry. 4th ed. London (UK): Churchill; p. 101–107.
- Wroblewski L, Ladue JS. 1955. Lactic dehydrogenase activity in blood.. Proc Soc Exp Biol Med. 90(1):210–213. doi: 10.3181/00379727-90-21985
- Yengkokpam S, Debnath D, Pal AK, Sahu NP, Jain KK, Norouzitallab P, Baruah K. 2013. Short-term periodic feed deprivation in Labeo rohita fingerlings: effect on the activities of digestive, metabolic and anti-oxidative enzymes. Aquaculture. 412–413:186–192. doi: 10.1016/j.aquaculture.2013.07.025
- Yilmaz S, Ergun S, Celik ES. 2012. Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): change in body composition and some blood parameters. J BioSci Biotechnol. 1(3):217–222.
- Zubia M, Payri C, Deslandes E. 2008. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (phaeophyta: fucales), from tahiti (French Polynesia). J Appl Phycol. 20(6):1033–1043. doi: 10.1007/s10811-007-9303-3