ABSTRACT
Lumpy skin disease (LSD) is an important disease of cattle. This study was conducted to determine alterations in hematological, biochemical and oxidative stress markers in cattle that have been naturally infected with LSD virus (LSDV). Blood samples and skin nodular lesions were collected from clinically infected, recovered and healthy animals. Quantitative real-time PCR was used to screen for Capripoxvirus DNA in samples from clinically infected animals. Hematological, biochemical and oxidative stress markers were measured. LSDV nuclic acids were detected in the collected samples using PCR. Hematological results revealed erythrocytosis, thrombocytopenia and leukopenia in infected cattle. Biochemical analyses showed that total protein and globulin levels were significantly elevated, while albumin and glucose were significantly reduced in these cattle. Aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), and creatinine levels were markedly elevated. Moreover, serum levels of reduced glutathione (GSH) were markedly lowered, whereas lipid peroxidation (MDA) and inflammatory cytokines (IL-4 and TNF-α) were elevated. Recovered cattle exhibited significant amelioration of the alterations resulting from LSDV infection. The results of this study suggest that LSDV infection induces changes in hematological and biochemical parameters and stimulates oxidative stress; these findings may be helpful for choosing a good strategy for rapidly detecting and diagnosing LSDV infection.
1. Introduction
Lumpy skin disease (LSD), which is an acute viral disease of cattle that mainly originated in Africa and is endemic in most African countries, causes high morbidity and low mortality in cattle (Davies Citation1991). LSD is currently emerging in the Middle East and poses a threat to Europe and the rest of the world (Tuppurainen and Oura Citation2012). This disease is caused by LSD virus (LSDV), which belongs to the genus Capripoxvirus and the subfamily Chordopoxvirinae of the family Poxviridae (Buller et al. Citation2005). LSDV is transmitted among cattle by biting insects (Lubinga et al. Citation2014). LSDV infections cause significant economic losses in various respects, such as by inducing severely reduced milk production, weight loss, abortion, infertility and/or hide damage. These infections produce marked hematological, biochemical and immunological alterations (Neamat-Allah Citation2015). LSD is characterized by fever, nodules (2–5 cm in diameter) on the skin and mucous membranes, lesions in the respiratory and gastrointestinal tracts, and enlarged superficial lymph nodes (Salib and Osman Citation2011). Affected cattle may exhibit widespread pox lesions, and such lesions can even be found in internal organs (Babiuk et al. Citation2008). The acute form of LSD is characterized by severe degeneration of the epidermis, furunculosis, and folliculitis with vasculitis that affects dermal capillaries and arterioles (El-Neweshy et al. Citation2013).
This study sought to investigate hematological, biochemical, oxidative stress markers and inflammatory cytokine alterations due to LSD in naturally infected and recovered cattle and assess the presence of LSDV in skin lesions via real-time PCR.
2. Materials and methods
2.1. Animal population and clinical presentation
Healthy cattle (the control farm where LSD was not reported, native breed animals aged between 1 and 3 years) were not recently vaccinated against any disease and were free of external, internal and blood parasites, they were used as controls. Naturally infected and recovered cattle (native breed animals aged between 1 and 3 years). A large sized farms (n > 50 cattle) were voluntarily used for this study, all research animals from farms located in Dakahlia Governorate, Egypt. All procedures were performed and approved by a research ethics committee of Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Clinical examination of the diseased cattle was performed and recorded on the infected farm. The farm owner reported that diseased animals had a typical fever for three days and marked decrease in the milk production at the first stage (acute form). Nasal discharge, lacrimation, anorexia, emaciation, enlarged lymph nodes and lesions in the skin and oral mucous membranes were observed in sever infected animals. Skin nodules (3–5 cm in diameter) occurred mostly on the legs and areas of the neck and back. Recovered cattle of the same infected farm exhibited less severe clinical signs and alleviated skin lesions.
2.2. Samples collection and preparation
Blood samples (with and without EDTA) were collected aseptically from the jugular vein of healthy, acute infected and recovered animals by traumatic venipuncture using Vacutainer tubes. Whole blood samples with EDTA were used for hematological examination, and samples without EDTA were centrifuged at 3000 rpm for 15 min for serum separation and stored at −20°C for biochemical analysis. Skin nodules were removed from clinically diseased cattle (n = 10), preserved in a glycerin and stored at −20°C for PCR analysis.
2.3. Virus isolation and identification
Skin nodules were prepared in a 10% suspension as described by Burleson et al. (Citation1997) for isolation and identification of the virus via real-time PCR. In accordance with the manufacturer’s instructions, Applied Biotechnology kits were used to extract LSD virus DNA from the collected nodules via the method described by Gubbels et al. (Citation1999).
2.4. Hematological parameters
An automatic cell counter (Hospitex Hemascreen 18, Italy) was used to determine red blood cells (RBCs), hemoglobin (Hb), packed cell volume (PCV), platelets and white blood cells (WBCs) in blood collected at 10% EDTA. As described by Feldman et al. (Citation2000), blood smears were prepared, fixed with absolute methanol (95%) and stained with Giemsa stain to determine differential leukocyte counts (DLCs).
2.5. Biochemical parameters
Total protein and albumin levels were measured in serum samples as described by Doumas et al. (Citation1981) and Drupt (Citation1974), respectively. Serum globulin concentration was calculated by subtracting the measured albumin level from the total protein level (Doumas and Biggs Citation1972). Serum activities of aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were measured as described by Reitman and Frankel (Citation1975) and Kind and King (Citation1954), respectively. Lactate dehydrogenase (LDH) was measured using the method employed by Buhl and Jackson (Citation1978). Serum creatine phosphokinase (CKP) was determined as described by Szasz et al. (Citation1979). Total and direct bilirubin were determined as described by Colombo (Citation1974), and indirect bilirubin was calculated by subtracting the direct bilirubin level from the total bilirubin level. Creatinine was assessed using the method employed by Larsen (Citation1972). Serum glucose was measured as the method used by Trinder (Citation1969).
2.6. Cytokine and oxidative stress markers assays
Serum inflammatory cytokines were evaluated by utilizing commercial ELISA kits to measure interleukin-4 (IL-4) and tumor necrosis factor-alpha (TNF-α) in accordance with the method described in standard kits from MyBioSource and ALPCO Diagnostic, respectively. Glutathione (GSH) and lipid peroxidation (MDA) in the serum were determined via the approaches used by Owens and Belcher (Citation1965) and Ohkhawa et al. (Citation1979), respectively.
2.7. Statistical analysis
All data are expressed as mean ± standard error, were statistically analyzed by the computer program SPSS/PC (2001) using one way ANOVA test.
3. Results
3.1. Real-time PCR results
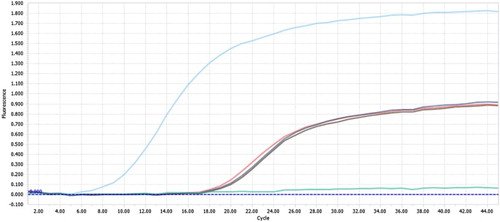
Skin samples were examined via real-time PCR to determine the presence of LSDV. Positive results with CT values of 18.46, 17.97 and 18.97 were obtained, indicating the existence of high viral concentrations ().
3.2. Hematological findings
Hematological parameters for naturally infected and recovered cattle are shown in . We observed that compared with healthy controls, infected cattle exhibited significant increases (P ≤ 0.05) in certain blood parameters (RBCs, Hb, and PCV), significant decreases (P ≤ 0.05) in MCV, platelets, WBCs, neutrophils, eosinophils, lymphocytes and monocytes, and no significant changes in MCHC. Recovered cattle showed significant improvement in hematological parameters compared with naturally infected cattle.
Table 1. Hematological parameters in the control healthy, infected and recovered cattle with LSDV, mean ± SD.
3.3. Biochemical findings
The biochemical parameters recorded for healthy controls, cattle naturally infected with LSDV and recovered cattle are shown in . In the naturally infected and recovered groups, serum levels of total protein, globulin, total bilirubin, direct bilirubin, AST, ALP, LDH, and CPK were significantly increased (P < 0.05), whereas albumin and glucose levels were significantly decreased (P < 0.05), although albumin level in recovered cattle was not significantly different from those observed in the control group. Serum levels of indirect bilirubin and creatinine were not significantly altered in infected and recovered cattle compared with healthy control.
Table 2. Biochemical parameters in the control healthy, infected and recovered cattle with LSDV, mean ± SD.
3.4. Serum antioxidant/oxidative stress and inflammatory cytokine biomarkers
The effects of LSDV on serum levels of GSH, MDA, IL-4 and TNF-α are shown in . Compared with the control group, the naturally infected group exhibited significantly reduced serum GSH levels (P ≤ 0.05) but significantly increased (P ≤ 0.05) MDA, IL-4 and TNF-α levels. In the recovered group, we observed significantly increased serum GSH (P ≤ 0.05) and significantly decreased MDA, IL-4 and TNF-α levels (P ≤ 0.05) compared with the naturally infected group.
Table 3. Serum antioxidant/oxidative stress and inflammatory cytokine markers in the control healthy, infected and recovered cattle with LSDV, mean ± SD.
4. Discussion
Lumpy skin disease is considered a transboundary animal disease due to its significant impacts on trade and food security as well as its capacity to spread to other countries (Rossiter and Al Hammadi Citation2009). However, LSD has been eradicated at a high cost by rapidly diagnosing cattle, slaughtering all diseased or in-contact cattle and small ruminants, and vaccinating the remaining cattle (Davies Citation1991). LSDV was diagnosed in this study based on RT–PCR findings and clinical signs. PCR was the best technique for rapidly detecting and identifying the causative agent of the examined viral outbreak. PCR exhibited high sensitivity for detecting LSD virus DNA in skin nodular samples, a finding consistent with the results obtained by Tuppurainen et al. (Citation2005) and Sharawi and Abd El-Rahim (Citation2011); this sensitivity may be attributed to the virus tropism to skin tissues and its persistence in high concentrations in the skin. In this study, the infected cattle had increased erythrocyte counts, which may have been related to dehydration or absolute erythrocytosis. Infected cattle typically exhibit dehydration exacerbated by fever, anorexia, and lethargy, which are common manifestations of LSDV infections. In addition, chronic diseases in large animals can be associated with absolute erythrocytosis (Morris Citation2002). LSDV-infected cattle produced leucopenia with eosinopenia, lymphopenia and monocytopenia, which may result from viral infections (Coles Citation1986); a release of high quantities of corticosteroid hormones also induces lymphopenia (Ismail and Yousseff Citation2006). Our results agree with those of Neamat-Allah (Citation2015). Thrombocytopenia occurred in all infected cattle and was mainly attributed to the shortening of the platelet life span. This phenomenon is typically caused by excessive platelet consumption due to systemic vasculitides, which were widespread in our study due to the tropism of LSDV to endothelial cells (House et al. Citation1990; Radostitis et al. Citation2000).
With respect to biochemical results, total protein and globulin levels were elevated in LSDV-infected cattle; however, dehydration may elevate the total protein level, also albumin levels were reduced, likely due to increased protein catabolism or decreased protein synthesis as well as hepatic damage (Hassan et al. Citation2011). Globulin elevation is correlated with the body’s immune response against infection (Agag et al. Citation1992). Hyperbilirubinemia (total and direct) was observed in infected animals; this condition was attributed to the presence of LSDV in the blood, which may have damaged hepatocytes surrounding the bile duct, and may also have been induced by intrahepatic cholestasis and biliary disease (Stockham and Scott Citation2008). LSDV infection significantly increased serum AST activity, a phenomenon that reflects hepatocyte damage, even if such damage is subclinical (Kauppinen Citation1984; Meyer and Harvey Citation1998). In addition, AST is present in cardiac and skeletal muscle cells; therefore, AST elevation in our study may have been related to injury or inflammation of cardiac muscle due to the presence of LSDV in the heart (Stockham and Scott Citation2008; Zilva et al. Citation1988). Serum ALP was elevated in infected cattle, this phenomenon may have been caused by the effects of inflammation on cells lining and surrounding the biliary ducts; related to the presence of hepatic cholestasis (Abutarbush Citation2015; Stockham and Scott Citation2008), which was confirmed by elevated direct bilirubin; or attributable to renal or intestinal infections (Coles Citation1986). Therefore, elevated AST and ALP in LSDV-infected cattle could be related to viremia-induced hepatic injury (Sevik et al. Citation2016). Serum LDH and CPK were elevated in LSD-infected cattle; based on results indicating that LDH and CPK levels may be elevated due to heart injury, these manifestations may reflect myocardial damage (Marmor et al. Citation1988). In addition, LDH may increase due to muscle breakdown resulting from muscular rigidity or activity during fever. Infected cattle exhibited reduced glucose levels, which may indicate decreased food intake and increased glucose catabolism in the body during viral infection. Moreover, creatinine concentration was significantly increased in LSDV-infected cattle, which may be attributed to the damaging effects of LSDV on the kidneys (Coles Citation1986) and decreased blood flow to the kidneys during the viremic stage of LSD to decrease the virus’s toxic effects.
There is a lack of available studies that describe blood antioxidant and lipid peroxidation status in skin diseases of cattle. The present study revealed significantly reduced serum GSH and elevated MDA in LSDV-infected cattle; these manifestations were attributed to increased oxidative stress due to free radical production and lipid peroxidation with the exhaustion of antioxidants in the blood, resulting in tissue injury (Elsayed et al. Citation2016; Rantnam et al. Citation2006). Moreover, decreases in GSH and TAC have been reported in LSD-infected animals with excessive MDA production (Nashwa et al. Citation2017).
Oxidative stress increases the production of oxidants, such as MDA, that can influence the release of proinflammatory mediators, such as cytokines; these mediators play important roles in the induction of inflammation in certain skin diseases (Meffert et al. Citation1976). Our study revealed that infected cattle exhibited elevated IL-4 and TNF-α concentrations, which may be attributed to the stimulation of macrophages and lymphocytes to release different cytokines that initiate inflammation in different tissues during the viremic stage of the disease. In this study, relative to infected cattle, recovered cattle exhibited significantly improved hematological, biochemical and oxidative stress/antioxidant markers.
5. Conclusions
The present study clearly established that LSDV-infected cattle exhibited alterations in hematological, biochemical and oxidative stress markers and that these alterations were diminished in recovered animals; thus, rapid detection and diagnosis of LSD and good treatment are recommended to prevent high losses.
Acknowledgments
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abutarbush SM. 2015. Hematological and serum biochemical findings in clinical cases of cattle naturally infected with lumpy skin disease. J Infect Dev Countr. 9(3):283–288. doi: 10.3855/jidc.5038
- Agag B, Mousa S, Hassan H, Saber M, El-Deghidy N, Abdel-Aziz A. 1992. Clinical, serological and biochemical studies on lumpy skin disease. J Appl Anim Res. 1(1):13–23. doi: 10.1080/09712119.1992.9705904
- Babiuk S, Bowden TR, Parkyn G, Dalman B, Manning L, Neufeld J, Embury-Hyatt C, Copps J, Boyle DB. 2008. Quantification of lumpy skin disease virus following experimental infection in cattle. Transbound Emerg Dis. 55:299–307. doi: 10.1111/j.1865-1682.2008.01024.x
- Buhl SN, Jackson KY. 1978. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactateto-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37 degrees C. Clin Chem. 24:828–831.
- Buller RM, Arif BM, Black DN, Dumbell KR, Esposito JJ, Lefkowitz EJ, McFadden G, Moss B, Mercer AA, Moyer RW, et al. 2005. Poxviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editor. Virus taxonomy: eight report of the international committee on the taxonomy of viruses. Oxford: Elsevier Academic Press; p. 117–133.
- Burleson F, Chambers T, Wiedbrauk D. 1997. Virology: a laboratory manual. San Diego: Academic Press, Harcourt Brace Jovanovich.
- Coles E. 1986. Veterinary clinical pathology. Philadelphia and London: W B Saunders Company.
- Colombo G. 1974. Determination of bilirubin with single reagent using diazotized dichloroaniline. Clin Chem Acta. 51:217. doi: 10.1016/0009-8981(74)90033-3
- Davies FG. 1991. Lumpy skin disease, an african capripox virus disease of cattle. Br Vet J. 147:489–503. doi: 10.1016/0007-1935(91)90019-J
- Doumas BT, Bayso DD, Carter RJ, Peters T, Schaffer R. 1981. Determination of serum total protein. Clin Chem. 27:1642.
- Doumas BT, Biggs HG. 1972. Determination of serum globulin in : standard methods of cilnical chemistry. Vol. 7. Edited by Cooper. New York: Academic Press.
- Drupt F. 1974. Colorimetric determination of albumin. Pharm Biol. 9:777.
- El-Neweshy MS, El-Shemey TM, Youssef SA. 2013. Pathologic and immunohistochemical findings of natural lumpy skin disease in Egyptian cattle. Pak Vet J. 33(1):60–64.
- Elsayed HK, Mohamed HG, Abdel Hafiz NN, Rushdi M. 2016. Evaluation of blood total antioxidant capacity and lipid peroxidation in cows infected with lumpy skin disease.13th Sc. cong. Hurghada: Society For Cattle Diseases.
- Feldman BF, Zinki JG, Jain VC. 2000. Schalm's veterinary hematology, 5th ed. Philadelphia: Lippincott Williams and Wilkins.
- Gubbels JM, De Vos AP, Van Der Weide M, Viseras J, Schouls LM, De Vries E, Jongejan F. 1999. Simultaneous detection of bovine theileria and babesia species by reverseline blot hybridization. J Clin Microbiol. 37:1782–1789.
- Hassan H, Elkhrdosy A, Ali M. 2011. Immunobiochmical profile in cattle infected with lumpy skin disease. J Basi Chem. 1:21–25.
- House JA, Wilson TM, El Nakashly S, Karim IA, Ismail I, El Danaf N, Moussa AM, Ayoub NN. 1990. The isolation of lumpy skin disease virus and bovine herpesvirus- from cattle in Egypt. J Vet Diagn Invest. 2:111–115. doi: 10.1177/104063879000200205
- Ismail SM, Yousseff FM. 2006. Clinical, hematological, biochemical and immunological studies on lumpy skin disease in ismailia governorate. SCVMJ. 1:393–400.
- Kauppinen K. 1984. ALAT, AP, ASAT, GGT, OCT, activities and urea and total bilirubin concentrations in plasma of normal and ketotic dairy cows. Zentralblatt für Veterinärmedizin Reihe A. 31:567–576. doi: 10.1111/j.1439-0442.1984.tb01316.x
- Kind PRN, King EG. 1954. Colorimetric determination of alkaline phosphatase activity. J Clin Pathol. 7:322. doi: 10.1136/jcp.7.4.322
- Larsen K. 1972. Creatinine assay in the presence of protein with LKB 8600 reaction rate analyser. Clin Chim Acta. 38:475–476. doi: 10.1016/0009-8981(72)90146-5
- Lubinga JC, Tuppurainen ESM, Coetzer JAW, Stoltsz WH, Venter EH. 2014. Evidence of lumpy skin disease virus over-wintering by transstadial persistence in amblyomma hebraeum and transovarial persistence in rhipicephalus decoloratus ticks. Exp Appl Acarol. 62(1):77–90. doi: 10.1007/s10493-013-9721-7
- Marmor AT, Klein R, Plich M, Groshar D, Schneeweiss A. 1988. Elevated CK-MB isoenzyme after exercise stress test and atrial pacing in patients with ischemic heart disease. Chest. 94(6):1216–1220. doi: 10.1378/chest.94.6.1216
- Meffert H, Diezel W, Sonnichsen N. 1976. Stable lipid peroxidation products in human skin: detection, ultraviolet light-induced increase, pathogenic importance. Experientia. 32:1397–1398. doi: 10.1007/BF01937397
- Meyer DJ, Harvey JW. 1998. Evaluation of hepatobiliary system and skeletal muscle and lipid disorders. In: Veterinary laboratory medicine, interpretation and diagnosis. 2nd ed. Philadelphia (PA): W.B. Saunders Company; p. 157–187.
- Morris DD. 2002. Alterations in the erythron. In: Smith BP, editor. Large animal internal medicine. 2nd ed. New York: Mosby; p. 415–419.
- Nashwa MH, Ahmed AS, Mohamed ZY. 2017. Molecular, clinico-pathological and sero-diagnosis of LSDV in cattle at sharkia and fayoum governorates. J Virol Sci. 1:1–11.
- Neamat-Allah ANF. 2015. Immunological, hematological, biochemical, and histopathological studies on cows naturally infected with lumpy skin disease. Vet World. 8(9):1131–1136. doi: 10.14202/vetworld.2015.1131-1136
- Ohkhawa H, Ohsini N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. doi: 10.1016/0003-2697(79)90738-3
- Owens C, Belcher R. 1965. Calometeric method for the determined glutathione. Biochem J. 94(3):705–711. doi: 10.1042/bj0940705
- Radostitis OM, Gay CC, Blood DC, Hinchcliff KW. 2000. Diseases caused by viruses and chlamydia II. In: Radostitis OM, Gay CC, Blood DC, editors. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 9th ed. Philadelphia: WB Saunders Co; p. 1135–1260.
- Rantnam D, Ankola D, Bhardy V, Sahara D. 2006. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 113:189–207. doi: 10.1016/j.jconrel.2006.04.015
- Reitman S, Frankel S. 1975. Colorimetric method for determination of serum transaminase activity. Am J Clin Pathol. 28:56–68. doi: 10.1093/ajcp/28.1.56
- Rossiter PB, Al Hammadi N. 2009. Living with transboundary animal diseases (TADs). Trop Anim Health Pro. 41:999–1004. doi: 10.1007/s11250-008-9266-7
- Salib FA, Osman AH. 2011. Incidence of lumpy skin disease among Egyptian cattle in giza governorate, Egypt. Vet World. 4(4):162–167.
- Sevik M, Avci O, Dogan M, Ince OB. 2016. Serum biochemistry of lumpy skin disease virus-infected cattle. BioMed Res Int. 2016 (2):1–6. ID 6257984. doi: 10.1155/2016/6257984
- Sharawi S, Abd El-Rahim I. 2011. The utility of polymerase chain reaction for diagnosis of lumpy skin disease in cattle and water buffaloes in Egypt. Rev Sci Tech. 30(3):821–830. doi: 10.20506/rst.30.3.2075
- Stockham SL, Scott MA. 2008. Fundamentals of veterinary clinical pathology, 2nd ed. Ames (Iowa): Blackwell.
- Szasz G, Waldenstrom J, Gruber W. 1979. Creatine kinase in serum: 6. inhibition by endogenous polyvalent cations, and effect of chelators on the activity and stability of some assay components. Clin Chem. 25:446–452.
- Trinder P. 1969. Enzymatic methods for glucose determination. Ann Clin Biochem. 6:24–28. doi: 10.1177/000456326900600108
- Tuppurainen ES, Oura CA. 2012. Review: lumpy skin disease: an emerging threat to Europe the Middle East and Asia. Transbound Emerg Dis. 59:5940–5948. doi: 10.1111/j.1865-1682.2011.01242.x
- Tuppurainen E, Venter E, Coetzer J. 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort J Vet Res. 72:153–164. doi: 10.4102/ojvr.v72i2.213
- Zilva JF, Pannall PR, Mayne D. 1988. Clinical chemistry in diagnosis and management, 5th ed. London (UK): Hodder Arnold.