ABSTRACT
The aim of this study was to investigate the effect of single or double Gonadotropin releasing hormone (GnRH) injections at the time of mating and following on day 9 on pregnancy rates and litter size in ewes. Oestrus was synchronized in 130 adult ewes using intravaginal sponges containing 20 mg of fluorogestone acetate for 14 days. All ewes received 10 IU/kg of pregnant mare serum gonadotropin (PMSG) at the sponge withdrawal. Then animals were divided into three groups. Ewes in group 1 (G1; Control, n = 68) received 1 ml placebo injection. In group 2 (G2; n = 30), ewes in oestrus were treated with 1 ml GnRH (4 μg/ml) at the time of mating. In group 3 (G3; n = 32), ewes were injected 1 ml GnRH not only at the time of mating but also on day 9 post-mating. There was significantly higher in duration of oestrus G1 compared with the G2 and G3. Pregnancy rate was found to be higher in G3 (96.0%) than in G1 (72.7%) and G2 (83.3%). The litter size was significantly higher for G3 (1.26 ± 0.14) in comparison with the G1 (1.18 ± 0.07) and G2 (1.22 ± 0.10). In conclusion, Post-mating GnRH injection enhanced the pregnancy rate and litter size, because of its beneficial effects on embriyo viability by increasing luteal formations.
1. Introduction
In small ruminants, synthetic progesterone analogs, such as controled internal drug release, fluorogestone acetate (FGA) and medroxyprogesterone acetate (MAP), have been used to provide oestrus synchronization, and thus, to improve fertility parameters (Wildeus Citation2000). Progesterone-based oestrus synchronization methods have been applied either long term (14 days) (Ahmadi and Mirzaei Citation2016) or short–medium term (7–12 days) (Karaca et al. Citation2009). Equine chorionic gonadotropin (eCG) injections are generally used prior to the end of the progesterone treatment in order to induce ovarian activity (Powell et al. Citation1996).
Gonadotropin releasing hormone (GnRH) or human chorionic gonadotropin (hCG) injections are applied concurrently with mating or just after in order to prevent embryonic and foetal loss or to increase reproductive performance in ewes (Beck et al. Citation1994; Nephew et al. Citation1994; Cam and Kuran Citation2004; Khan et al. Citation2007; Ataman et al., Citation2013; Ahmadi and Mirzaei Citation2016; Rostami et al. Citation2017). Embryonic loss generally results from inadequate early luteal function and insufficient progesterone levels during pre-implantation period (Spencer et al. Citation2004).
Because insufficient progesterone concentrations during early embrionic stage may cause embrionic lost, because of unenough sentencing and producing of interferone tau (IFN-τ) providing maternal recognition (Spencer et al. Citation2004; Antoniazzi et al. Citation2013). As a natural result of these mechanism, IFN-τ cannot show its anti-leucotic effect, and this mechanism causes a decline in pregnancy rates (Nephew et al. Citation1994).
Progesterone from ovaries has an important role in embryonic implantation, maintainence of gestation following fertilization in ewes (Spencer et al. Citation2004; Dorniak and Spencer Citation2013). During early luteal phase, inadequate or low progesterone levels generally cause to strike against embryonic development (Nephew et al. Citation1991). For this reason, progesterone from corpus luteum is needed for embryonic implantation and development in uterus (Thatcher et al. Citation2001) or exogenous progesterone applications may be needed to continue these vital processes for embryo (Davies and Beck Citation1992; Nephew et al. Citation1994; Rostami et al. Citation2017).
In many studies having carried out until now (Beck et al. Citation1994; Nephew et al. Citation1994; Cam and Kuran Citation2004; Khan et al. Citation2006; Anjum et al. Citation2009; Lashari and Tasawar Citation2010; Ataman et al. Citation2013; Coleson et al. Citation2015; Ahmadi and Mirzaei Citation2016), it has been reported that GnRH treatment applied until 12th day of gestation supports luteal function and it has some beneficial effects on embryonic development. These studies reveal that GnRH injections in various days following mating may be important because of improving progesterone levels, providing embryonic developments, preventing embryonic lost and supporting good implantation. Therefore, GnRH injection enhanced reproductive performance of ewes when injected at the time of mating (Lashari and Tasawar Citation2010). On the other hand, GnRH injection on day of mating support the luteinization of developing follicles, to increase in LH and improved luteal function (Beck et al. Citation1996). In this way, GnRH stimulates the development of embryo and its implantaion (Khan et al. Citation2007) likewise hCG injections at the time of mating increases embryo viability and fertility (Khan et al. Citation2003). Consequently, the present study aimed to investigate the effect of GnRH injections on the day of mating and 9 days after mating on pregnancy rate, litter size and oestrus synchronization in the Awassi ewes receiving long-term progesterone during breeding season.
2. Material and methods
2.1. Animals and location
The present study was carried out in a village located at Tek Tek mountains, 70 km away from Sanliurfa city, in Southeast Anatolia region during breeding season (August–September). This area is located at 37° 10′ N – 39°03′E and 518 m higher from sea level. In this research, a total of 130 Awassi-bred ewes clinically healthy, 3–5 years old, 45–50 kg body weight and 2–3 body condition score were used.
All animals in the study were allowed field grazing natural pasture near the village on day. When animals were kept indoors at night, they fed a diet of chopped straw and barley, Fresh ad libitum.
2.2. Study groups
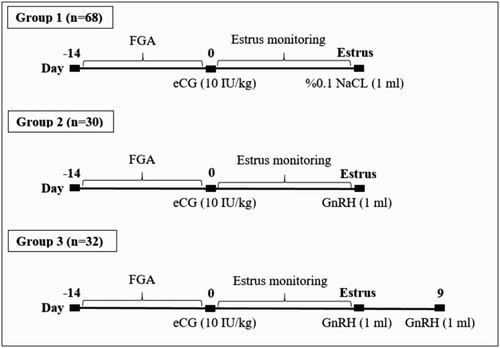
Sponges containing progesterone (20 mg FGA, Chronogest®, Intervet, Turkey) were intra-vaginally applied to all animals for 14 days in order to provide oestrus synchronization in flock. At the time of removal of FGA sponges, 10 IU/kg PMSG (Chorono-Gest®/PMSG, 6000 IU/flacon, Intervet International B.V., Boxmeer, Netherlands) was intramuscularly injected to all animals. After this application, ewes were randomly assigned to three groups. Ewes in group 1 (n = 68) received 1 ml placebo injection (physiologic saline solution, 0.9% NaCl, i.m.) and these animals were considered as a control group. In group 2 (n = 30), ewes showing oestrus were intramuscularly treated with 1 ml GnRH (4 µg/ml, buserelin asetat, Receptal®; Intervet, Istanbul, Turkey) at the time of breeding. Ewes of group 3 (n = 32), two doses of GnRH (4 µg/ml) were intramuscularly injected at the time of mating and on 9 days later. The hormonal treatments of the study are shown in .
2.3. Oestrus determination
Thirteen Awassi rams, with proven fertility and 2–4 years old, were used to determine oestrus response of ewes after the removal of sponges. Rams were sent to ewes flock after the removal of sponges. Teaser rams were used in order to determine initial and ending of oestrus sings and duration of oestrus. Teaser rams were stayed three times a day for 1 h duration during 5 days after removal of sponges. In these periods, behaviours of ewes, especially gathering around the ram, sniffing, urination and standing oestrus were watched and ewes showing this behaviours were considered in oestrus. Ewes in oestrus were mated with a proven Awassi rams (ewe: ram ratio of 10:1) in this period. Naturally mated animals were determined, recorded and followed until the end of the oestrus behaviours. The end of oestrus were considered as following behaviours, i.e. running from teaser rams, disallowing to mount, no standing on rams and dispersing of flock (Ucar et al. Citation2005).
2.4. Determination of reproductive parameters
The reproductive paremeters, calculated following treatment, were oestrus response rate (number of ewes showing oestrus behaviours/number of ewes receiving intravaginal sponge and PMSG ×100), elapsed time for oestrus behaviour (time from removal of sponge to first oestrus behaviour), oestrus duration (time from first oestrus sings to end of oestrus behaviours for standing oestrus), pregnancy rate (number of pregnant ewes/number of mated/mounted ewes ×100), gestation length (time from mated to birth), lambing rate (number of lambing ewes/number of mated ewes ×100) and litter size (number of total lambs/number of lambing ewes in the group ×100) (Zarkawi et al. Citation1999; Karaca et al. Citation2009; Zonturlu et al. Citation2011).
Pregnancies were determined with transrectal real time B Mod ultrasonography with linear prob (5.0 MHz, VED 3000, Hasvet, Turkey) on day 35 following mating. Number of lambs and twining were determined after parturitions. Individual birth weights of lambs were determined and recorded by flock owners.
2.5. Statistical analysis
In group 1 and 2, two aborts were determined while one abort was occured in group 3 with unknown reasons. These aborted lambs in these groups were not included to statistical analyses. Data obtained during present study were statistically analysed with SPSS 18. Parameters each group were tested for normality using the Kolmogorov–Smirnov test. Differences between/among groups were revealed with ANOVA test. Oestrus response and reproductive performance were analysed using the Chi-squared test. Mean numbers of lambs (litter size) in each of the treated groups were compared to that of the control group using the Independent Samples t-test. Data for duration and onset of oestrus, gestation length and birth weight were subjected to analysis of variance, and differences between means were tested with ANOVA test. Data are presented as the percentage or mean (±SE). The differences were considered significant at P < .05.
3. Results
Results regarding oestrus synchroniztion and mean (±SE) oestrus response, time to onset of oestrus and duration of oestrous of period were summarized in . There were no significant differences in oestrus response, time to onset of oestrus betveen all groups. However, differences in duration of oestrus were found to be significantly different when GnRH injection groups were compared with the group 1 (P < .05).
Table 1. Oestrus response (%), interval between end of treatment and the onset of oestrus (h), and duration of oestrus (h).
Results for reproductive performance are present in . The highest pregnancy rate observed was 96.0% in group 3, compared to group 1 and 2. There was significant differences in the pregnancy rate of treatment GnRH post-mating ewes in comparison with the group 1 and 2 (P < .05). No significnat differences were observed in the lambing rate and multiple birth rate among any of the groups. However, litter size was found to be significnatly different between in the group 1 and group 3 (P < .05). The gestation length was similar between groups and averaged 148.9 ± 1.9 day. No significant differences were observed among any of groups in terms of gestation period. Average birth weight of lambs was highest in group 2 compared to group 1 and 3, which had similarly average birth weight of lambs, while there was no significant differnce in birth weigth all of groups. In group 1 and 2, two aborts were determined while one abort was occured in group 3 with unknown reasons.
Table 2. Reproductive performance of ewes: PMSG and GnRH injection days after progesterone administration.
4. Discussion
The aim of the study was to improve reproductive performance of ewes using GnRH at the time of mating and post-mating. Because, 12–13 days following mating, when regression of CL and luteolysis are started in this period during natural sexual cycles, is a critical term for gestation in sheep (Bazer Citation2013). Therefore, it may be usefull that gonadotropic hormone injections are applied in order to support luteal structure and to increase progesterone levels in this critical term (Khan et al. Citation2006).
Rostami et al. (Citation2017) pointed out that GnRH injections applied between 7 and 19 days following mating may be important, although 12th day of gestation is critical for preventing luteolysis, because insufficient and abnormal luteal function in this period detrimentally affect embryonic development and cause embryonic loss (Spencer et al. Citation2004). A study made by Beck et al. (Citation1996) reported that GnRH injection on day 12 post-mating improves reproductive outcome; these applications are essential for successful implantation and progression of gestation, supports follicular and luteal function with increased LH pulses, survival and quality of developing embryos can be improved during pre-implantation period (Khan et al. Citation2007). Moreover, injection of gonadotropins on day 4 post-mating increase number of corpus lutem (Coleson et al. Citation2015). These hormones can be used on different days of the cycle after mating, such as on days 2, 4, 5, 11, 12 and 16. Injections on day 12 post-mating are suggested to be the critical period for maternal recognition of pregnancy in the ewe (Beck et al. Citation1996; Bazer et al. Citation1998; Cavalcanti et al. Citation2012; Ataman et al. Citation2013) and cattle (Kaçar et al. Citation2007).
From the results of the present study, it was determined that GnRH injections did not have any effect on oestrus response rate and time to onset of oestrus betveen all groups. However, duration of oestrus were significantly different between GnRH injected groups (group 2 and group 3) and group 1 (P < .05). Gonadotrophins have been used to induce ovulation and shorten the time to onset of oestrus in combination with progestagen (Jabbour and Evans Citation1991). On the contrary, Lashari and Tasawar (Citation2010) also reported that GnRH injections at the time of mating are effects on synchronization. Another study by Cavalcanti et al. (Citation2012) expressed that the injection of 25 μg GnRH 24 h after sponge removal had no significant effect on the time of oestrus. Luther et al. (Citation2007) also tested GnRH treatment did not affect the efficiency of oestrus synchronization in ewes during the breeding season (GnRH, 85.1% versus without GnRH, 90.3%). Kaya et al. (Citation2013) reported that hCG injections on day 7 following progesteron applications increased pregnancy in Tushin sheep during non-breeding season. Gonadotropins treatment at the time of insemination may be beneficial in increasing fertility of ewes from farms with low reproductive performance (Gomez et al. Citation2007).
The results of the present study showed that GnRH injection caused the increase of pregnancy rate and litter size. Highest pregnancy rate (96.0%) was observed in group 3, compared to that in group 1 (72.72%) and 2 (83.33%). There was significant differences in the pregnancy rate of treatment GnRH post-mating ewes in comparison with the group 1 and 2 (P < .05). This situation may be explained with the luteotrophic or embriyotrophic as positive on embryonic of development GnRH injections post-mating. There are a number of explanations regarding effect of GnRH on oocyte maturation, conceptus growth (Kleemann et al. Citation1994) and embryonic development (Khan et al. Citation2007). It may have helped improve embryo and suppressing the luteolysis and allowing more time for establishment of pregnancy (Nephew et al. Citation1994). Moreover, it is well known that GnRH injections beneficially affect placentation and increase placentom numbers during gestation period (Lashari and Tasawar Citation2010). Ahmadi and Mirzaei (Citation2016) reported that the injections hCG and GnRH two days after long-term progesterone enhanced twin lambing rate during breeding season and litter size. This study provides evidence that double GnRH causes enhanced litter size, double injection of GnRH increased litter size. However, multiple birth rate, twin and triplet were not affected by GnRH injections. No significant differences were observed in twinning and lambing rate or multiple birth rates among any of the treatment groups. Although studies by Ahmadi and Mirzaei (Citation2016) and Lashari and Tasawar (Citation2010) revealed that GnRH injections significantly increases twining, removal of vaginal sponge, Ataman et al. (Citation2013) determined that GnRH injections at the time of mating did not increase twinning rates in ewes. Mirzaei et al. (Citation2014) also recorded that there were no significant differences between hCG and GnRH groups in comparison with the control group in twin rate. This may be due to breed, nutrition, breeding conditions and poor reproductive performance in awassi ewes.
Cam and Kuran (Citation2004) reported that hCG and GnRH appeared to act differently on embryo survival because only hCG administration increased foetal growth, not GnRH. In the present study, average birth weight of lambs was highest in group 2 compared to group 1 and 3, which had similar average birth weight of lambs, while there was no significant difference in birth weight all of groups (P > .05). It was determined that differences between GnRH treatment and control groups were statistically insignificant for gestation periods. These results and data were consistent with other studies, including GnRH injections at the time of or following mating (Cam et al. Citation2002; Lashari and Tasawar Citation2010).
5. Conclusion
The results of this study indicated that GnRH administration at the time of mating and on day 9 later increased pregnancy rate and litter size. However, multiple birth rate, twin and triplet were not affected by GnRH injections in awassi ewes. Post-mating GnRH injections may be embryotrophic and protect embryo viability.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmadi E, Mirzaei A. 2016. High twin lambing rate of synchronized ewes using progestagen combined with the gonadotropins injection in breeding season. Revue Méd Vét. 167(1–2):28–32.
- Anjum IA, Usmani RH, Tunio MT, Abro SH. 2009. Improvement of conception rate in crossbred cattle by using GnRH analogue therapy. Pakistan Vet J. 29(2):93–94.
- Antoniazzi AQ, Webb BT, Romero JJ, Ashley RL, Smirnova NP, Henkes LE, Bott RC, Oliveira JF, Niswender GD, Blazer FW, et al. 2013. Endocrine delivery of interferon tau protects the corpus luteum from prostaglandin F2 alpha-induced luteolysis in ewes. Biol Reprod. 88:1–12. doi: 10.1095/biolreprod.112.105684
- Ataman MB, Aköz M, Sarıbay M K, Erdem H, Bucak MN. 2013. Prevention of embryonic death using different hormonal treatments in ewes. Turk J Vet Anim Sci. 37:6–8.
- Bazer FW. 2013. Pregnancy recognition signaling mechanisms in ruminants and pigs. J Anim Sci Biotechnol. 4:23–32. doi: 10.1186/2049-1891-4-23
- Bazer FW, Ott TL, Spencer TE. 1998. Maternal recognition of pregnancy: comparative aspects: a review. Placenta. 12:375–386. doi: 10.1016/S0143-4004(98)80055-6
- Beck NFG, Jones M, Davies B, Mann GE, Peters AR. 1996. The effect of the GnRH analogue (Buserelin) on day 12 post mating on ovarian structure and plasma oestradiol and progesterone concentrations in ewes. Anim Sci. 63:407–412. doi: 10.1017/S1357729800015290
- Beck NFG, Peters AR, Williams SP. 1994. The effect of GnRH agonist (buserelin) treatment on day 12 post mating on there productive performance of ewes. Anim Sci. 58:243–247. doi: 10.1017/S1357729800042557
- Cam MA, Kuran M. 2004. Effects of a single injection of hCG or GnRH agonist on day 12 post mating on fetal growth and reproductive performance of sheep. Anim Reprod Sci. 80:81–90. doi: 10.1016/S0378-4320(03)00158-1
- Cam MA, Kuran M, Yildiz S, Selcuk E. 2002. Fetal growth and reproductive performance in ewes administered GnRH agonist on day 12 post-mating. Anim Reprod Sci. 72:73–82. doi: 10.1016/S0378-4320(02)00071-4
- Cavalcanti ADS, Brandão FZ, Nogueira GLA, Fonseca JFD. 2012. Effects of GnRH administration on ovulation and fertility in ewes subjected to estrous synchronization. R Bras Zootec. 41(6):1412–1418. doi: 10.1590/S1516-35982012000600014
- Coleson MPT, Sanchez NS, Ashley AK, Ross TT, Ashley RL. 2015. Human chorionic gonadotropin increases serum progesterone, number of corpora lutea and angiogenic factors in pregnant sheep. Reproduction. 150:43–52. doi: 10.1530/REP-14-0632
- Davies MCG, Beck NFG. 1992. Plasma hormone profiles and fertility in ewe lambs given progestagen supplementation after mating. Theriogenology. 38:513–526. doi: 10.1016/0093-691X(92)90071-X
- Dorniak P, Spencer TE. 2013. Biological roles of progesterone, prostaglandins, and interferon tau in endometrial function and conceptuse longation in ruminants. Anim Reprod. 10(3):239–251.
- Gomez BA, Santiagom J, Montoro V, Garde J, Pons P, Gonzalez BA, Lopezs A. 2007. Reproductive performance and progesterone secretion in estrusinduced Manchega ewes treated with hCG at the time of AI. Small Rumin Res. 71:117–122. doi: 10.1016/j.smallrumres.2006.04.015
- Jabbour HN, Evans G. 1991. Ovarian and endocrine responses of Merino ewes to treatment with PMSG and/or FSH-p. Anim Reprod Sci. 93–106. doi: 10.1016/0378-4320(91)90068-B
- Kaçar C, Yıldız S, Pancarcı ŞM, Kaya M, Güngör Ö, Arı UÇ. 2007. Effect of administration of lesireline five days after artificial insemination on pregnancy rates and luteal function in cows. Kafkas Üniv Vet Fak Derg. 13(2):139–142.
- Karaca F, Ataman MB, Coyan K. 2009. Synchronization of estrus with short and long-term progestagen treatment and the use of GnRH prior to short-term progestagen treatment in ewes. Small Rumin Res. 81:185–188. doi: 10.1016/j.smallrumres.2008.12.002
- Kaya S, Kaçar C, Kaya D, Aslan S. 2013. The effectiveness of supplemental administration of progesterone with GnRH, hCG and PGF2α on the fertility of Tuj sheep during the non-breeding season. Small Rumin Res. 113(2–3):365–370. doi: 10.1016/j.smallrumres.2013.03.018
- Khan TH, Beck NFG, Khalid M. 2007. The effects of GnRH analogue (buserelin) or hCG (Chorulon) on Day 12 of pregnancy on ovarian function, plasma hormone concentrations, conceptus growth and placentation in ewes and ewe lambs. Anim Reprod Sci. 102:247–257. doi: 10.1016/j.anireprosci.2006.11.007
- Khan TH, Beck NFG, Mann GE, Khalid M. 2006. Effect of post-mating GnRH analogue (buserelin) treatment on PGF2α release in ewes and ewe lambs. Anim Reprod Sci. 95:107–115. doi: 10.1016/j.anireprosci.2005.09.010
- Khan TH, Hastie PM, Beck NFG, Khalid M. 2003. hCG treatment on day of mating improves embryo viability and fertility in ewe lambs. Anim Reprod Sci. 76:81–89. doi: 10.1016/S0378-4320(02)00194-X
- Kleemann DO, Walker SK, Seamark RF. 1994. Enhanced fetal growth in sheep administered progesterone during the first three days of pregnancy. J Reprod Fertil. 102:411–417. doi: 10.1530/jrf.0.1020411
- Lashari MH, Tasawar Z. 2010. The effect of GnRH given on day of mating on ovarianfunction and reproductive performance in Lohi sheep. Pakistan Vet J. 30(1):29–33.
- Luther JS, Grazul-Bilska AT, Kirsch JD, Weigl RM, Kraft KC, Navanukraw C, Pant D, Reynolds LP, Redmer DA. 2007. The effect of GnRH, eCG and progestin type on estrous synchronization following laparoscopic AI in ewes. Small Rumin Res. 72:227–231. doi: 10.1016/j.smallrumres.2006.10.015
- Mirzaei A, Rezaei M, Asadi J. 2014. Reproductive performance after hCG or GnRH administration of long term progestagen treatment of fat tailed ewes during seasonal anestrus. J Fac Vet Med Istanbul Univ. 40:176–182.
- Nephew KP, Cardenas H, McClure KE, Ott TL, Bazer FW, Pope WF. 1994. Effects of administration of human chorionic gonadotropin or progesterone before maternal recognition of pregnancy on blastocyst development and pregnancy in sheep. J Anim Sci. 72:453–458. doi: 10.2527/1994.722453x
- Nephew KP, Mcclure KE, Ott TL, Dubois DH, Bazer FW, Pope WF. 1991. Relationship between variation in conceptus development and differences in estrous cycle duration in ewes. Biol Reprod. 44:536–539. doi: 10.1095/biolreprod44.3.536
- Powell M, Kaps M, Lamberson WR, Keisler DH. 1996. Use of melengestrol acetate-based treatments to induce and synchronize estrus in seasonally anestrous ewes. J Anim Sci. 74:2292–2302. doi: 10.2527/1996.74102292x
- Rostami B, Hajizadeh R, Shahir MH, Aliyari D. 2017. The effect of post-mating hCG or progesterone administration on reproductive performance of Afshari × Booroola-Merino crossbred ewes. Trop Anim Health Prod. 49:245–250. doi: 10.1007/s11250-016-1183-6
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC. 2004. Implantation mechanisms: insights from the sheep. J Reprod Fertil. 128:657–668. doi: 10.1530/rep.1.00398
- Thatcher WW, Moreira F, Santos JEP, Mattos RC, Lopes FL, Pancarci SM, Risco CA. 2001. Effects of hormonal treatments on reproductive performance and embryo production. Theriogenology. 55:75–89. doi: 10.1016/S0093-691X(00)00447-7
- Ucar O, Kaya M, Yıldız S, Onder F, Cenesiz M, Uzun M. 2005. Effect of progestagen/PMSG treatment for oestrus synchronization of Tuj ewes to be bred after the natural breeding season. Acta Vet Brno. 74:385–393. doi: 10.2754/avb200574030385
- Wildeus S. 2000. Current concepts in synchronization of estrus: sheep and goats. J Anim Sci. 77:1–14. doi: 10.2527/jas2000.00218812007700ES0040x
- Zarkawi M, Merestani MR AI-, Wardeh MF. 1999. Induction synchronized oestrous and early pregnancy diagnosis in Syrian Awassi ewes, outside the breeding season. Small Rumin Res. 33:99–102. doi: 10.1016/S0921-4488(99)00007-3
- Zonturlu AK, Özyurtlu N, Kaçar C. 2011. Effect of different doses PMSG on estrus synchronization and fertility in Awassi ewes synchronized with progesterone during the transition period. Kafkas Univ Vet Fak Derg. 17(1):125–129.