ABSTRACT
This study was carried out to evaluate the effect of electroejaculation (EEJ) on the serum concentrations of the acute phase proteins (APPs) serum amyloid A (SAA) and haptoglobin (Hp), and on the bone metabolism biomarkers osteocalcin (OC), bone-specific alkaline phosphatase (b-ALP) and pyridinoline cross-links (PYD). Twenty sexually mature apparently healthy male camels were assigned to EEJ. Parallel, 8 naturally mated male camels were enrolled as a control group. Three blood samples were collected from each camel: just before (T0), directly after (T1) and 24 h after (T2) EEJ or natural mating camels. The serum concentrations of the APPs and bone biomarkers were determined. The serum concentrations of SAA increased significantly at T1 compared to T0. In none of the 2 groups was the serum concentration of Hp differed significantly. The serum concentrations of OC increased significantly at T1 compared to T0. In none of the 2 groups was the serum concentration of b-ALP differed significantly among T0, T1 and T2. It is concluded that acute phase reaction, manifested by significant increases of SAA, had occurred in male camels as a result of EEJ. In addition, EEJ increased serum concentration of the bone formation biomarker OC.
1. Introduction
Electroejaculation (EEJ) is used frequently to collect semen from infertile dromedary camels (Skidmore et al. Citation2013; Ali et al. Citation2014; Tharwat, Ali, et al. Citation2014). However, the procedure has raised concerns and implications in the area of animal welfare; it is painful and stressful (Palmer Citation2005; Damian and Ungerfeld Citation2011; Fumagalli et al. Citation2012; Whitlock et al. Citation2012). In addition, it is recently reported that EEJ causes cardiac injury; however the damage is temporary and reversible (Tharwat, Ali, et al. Citation2014).
The acute phase proteins (APPs) are a class of blood proteins that can be used to assess the systemic response of the innate immune system to infection, inflammation or trauma (Murata et al. Citation2004; Petersen et al. Citation2004; Ceron et al. Citation2005). In response to injury, local inflammatory cells (neutrophil granulocytes and macrophages) secrete a number of cytokines into the bloodstream. The liver responds by producing a large number of APPs (Petersen et al. Citation2004). Serum APPs concentrations decrease (negative APPs) or increase (positive APPs) in animals subjected to external or internal challenges (Murata et al. Citation2004; Eckersall and Bell Citation2010).This response is called the acute-phase reaction or acute-phase response (APR). The APR is a rapid, nonspecific, systemic response occurring secondary to many types of tissue injury and might be a physiological protective mechanism during inflammatory events (Yazwinski et al. Citation2013). The origin of APR can be attributed to infection, inflammation, surgical trauma, or other causes (Petersen et al. Citation2004; Ceron et al. Citation2005), and the purpose of the response is to restore homeostasis and to remove its disturbance (Ebersole and Cappelli Citation2000; Ceron et al. Citation2005).
In ruminants, the APPs have been proposed as sensitive and rapid indicators of inflammatory disturbances (Eckersall Citation2000; Eckersall and Bell Citation2010; Schneider et al. Citation2013). The major APPs in ruminants are haptoglobin (Hp) and serum amyloid A (SAA) (Murata et al. Citation2004). In cattle, Hp and SAA are effective in the diagnosis and prognosis of mastitis, enteritis, peritonitis, pneumonia, endocarditis, and endometritis (Gronlund et al. Citation2003; Murata et al. Citation2004; Petersen et al. Citation2004). It has also been suggested that APPs may be useful in the assessment of animal welfare (Eckersall Citation2000; Murata et al. Citation2004; Murata Citation2007; Baghshani et al. Citation2010). In ruminants, the major APPs are Hp and SAA (Murata et al. Citation2004). In domestic animals, a critical mass of knowledge on the use of APPs as biomarkers of inflammatory conditions has accumulated over recent years, so there is now sufficient understanding of the pathophysiology of the response to support the use of these compounds as diagnostic tools in clinical settings (Eckersall and Bell Citation2010; Tharwat, Al-Sobayil, et al. Citation2014; Tharwat and Al-Sobayil Citation2015).
The common biomarkers of bone formation include osteocalcin (OC), bone-specific alkaline phosphatase (b-ALP) and amino and carboxy propeptides of collagen type I. The most common biomarkers of bone resorption include pyridinoline cross-links (PYD), deoxypyridinoline enzyme tartrate resistant acid phosphatase and amino and carboxy telopeptides of collagen type I. Biochemical markers of bone turnover are widely used in human clinical practice, mainly for non-invasive monitoring of bone metabolism and response to therapy of certain musculoskeletal and bone disorders (Swaminathan Citation2001; Watts et al. Citation2001; Kanakis et al. Citation2004; Sabour et al. Citation2014). In veterinary medicine, bone biomarkers are mostly used in preclinical and clinical studies as a rapid and sensitive method for assessment of bone response to medical treatment and surgical interventions and for the detection of musculoskeletal injuries (Allen Citation2003; DeLaurier et al. Citation2004; Frisbie et al. Citation2008; Frisbie et al. Citation2010; Tharwat, Al-Sobayil, et al. Citation2014; Tharwat and Al-Sobayil Citation2015).
The influences of EEJ on the serum concentrations APPs (Hp, SAA) and on bone metabolism biomarkers (OC, b-ALP, PYD) have not been reported in veterinary literature. Therefore, the present study was designed. The results could prove increasingly useful for the future health and welfare of dromedary camels. In addition, understanding the effects of EEJ on stimulation of the inflammatory response and on bone remodelling is important for the development of strategies to control stress and pain during EEJ.
2. Materials and methods
2.1. Camels
The experimental protocol was approved by the Ethics Committee for Animal Research of the Scientific Research Deanship of Qassim University, Saudi Arabia. The experimental design was reported recently in details (Tharwat, Ali, et al. Citation2014). Briefly, twenty sexually mature, healthy male dromedary camels were presented to the Veterinary Teaching Hospital, Qassim University for assessment of fertility soundness. Eight naturally mating camels were used as controls. All the camels were considered healthy on the basis of physical and laboratory examinations. The animals were treated according to the regulations of the Laboratory Animal Control Guidelines of Qassim University, which basically conform to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publications No. 86 to 23, revised 1996).
2.2. Electroejaculation
Each camel was positioned firstly before EEJ in a lateral recumbency as described previously (Tingari et al. Citation1986). The animal was then restrained physically with ropes and slightly sedated with xylazine (0.2 mg/kg body weight, IV, Bomazine 10%, BOMAC Laboratories Ltd, New Zealand). An IV jugular catheter was then inserted. Before EEJ, the feces were evacuated from the rectum. The electroejaculator (ElectroJac 6©, Neogen, Lexington, KY) was adjusted on the automatic mode, where the circuit delivers a series of 40 cycles, with each cycle delivering a slightly higher intensity. Each cycle lasts 2 s, followed by a 2 s pause. The stimulators were operated on a power source of 220 v. and 40 cycles A.C. The voltage to the probe was regulated by two control knobs. The power step control regulated the maximum voltage to the probe in seven increments (power steps) ranging from 10 to 40 v. Within each power step, a bull was stimulated by turning the stimulator knob smoothly from zero to the maximum voltage, then back to zero, over a period of 2–3 s. The voltage was then increased to the next higher power step and the process repeated. Once ejaculation had begun, no further increase in voltage was used unless it was judged necessary to obtain a complete ejaculate (Ali et al. Citation2014; Tharwat, Ali, et al. Citation2014).
2.3. Blood sampling
Three blood samples were collected from each camel; the first just before EEJ (T0), the second directly after EEJ (T1) and the third at 24 h after EEJ (T2). Parallel, in the control group, blood samples were collected just before mating (T0), directly after mating (T1) and at 24 h after mating (T2). From both experimental and control groups, 10 mL of jugular blood was collected at each sampling time. Sera were harvested and stored at −20°C pending analyses.
2.4. Acute-phase proteins assays
The serum concentrations of Hp and SAA were determined as recently reported in the literature for camels (Tharwat and Al-Sobayil Citation2015; Tharwat et al. Citation2015). Haptoglobin was measured according to the prevention of the peroxidase activity of hemoglobin, which is directly proportional to the amount of Hp (Tridelta Development Ltd., Ireland).The analytical sensitivity of the assay was 0.0005 mg/mL, and the intra- and inter-assay CVs were 5–6% and 4–6%, respectively. The SAA was determined by a solid phase sandwich ELISA (Tridelta Development Ltd., Ireland). The analytical sensitivity of the assay was 0.15 µg/mL and the intra- and inter-assay CVs were 4.5% and 6%, respectively.
2.5. Bone metabolism biomarkers assays
The bone biomarkers OC, b-ALP and PYD serum concentrations were determined in the serum using commercial immunoassay kits (Quidel Corp., CA, USA). The serum concentrations of the bone biomarkers in the camels were determined as recently reported (Al-Sobayil Citation2010; Tharwat and Al-Sobayil Citation2015). The limit of quantification of OC ranged from 2 to 32 ng/mL, and precision CVs within runs and between runs were 5–10%. The dynamic range of b-ALP was from 2 to 140 U/L, and the precision CVs within and between runs were 4–6% and 5–8%, respectively. The dynamic range of PYD was from 15 to 750 nM/L, and the precision CVs within and between runs were 6–10% and 3–11%, respectively.
2.6. Cortisol assay
Cortisol was determined in serum samples using electrochemiluminescent immunoassay kit (Roche Diagnostics, Indianapolis, IN, USA), with measuring ranges of 0.018–63.4 µg/dL. The intra- and inter-assay coefficient of variance was 1.22% and 1.54%.
2.7. Statistical method
Data are presented as means ± SD and were analyzed statistically using the SPSS statistical package, version 18, Citation2009. A repeated measures analysis of variance was used as the statistical model to evaluate the differences among T0, T1 and T2 in both the control and experimental groups. The Duncan test was used to calculate multiple comparisons. Results were considered significant at P < 0.05.
3. Results
Compared to a mean heart rate value of 35 ± 5 bpm at T0, the heart rate had increased significantly (P = 0.0001) to 67 ± 11 bpm at T1. The respiratory rate had also increased significantly (P = 0.0001) to a mean value of 23 ± 6 bpm at T1 compared to 9 ± 3 bpm at T0. At T2, the heart and respiratory rates were 38 ± 8 bpm and 11 ± 5 bpm, respectively; neither differed significantly compared to T0. Compared to a baseline value of 2.94 ± 0.11 µg/dL (T0), the serum concentration of cortisol increased significantly at T1 to 4.66 ± 0.60 µg/dL (P = 0.0002) then decreased to 2.84 ± 0.27 µg/dL at T2 that did not differ significantly compared to T0 (P = 0.4).
shows the serum concentration of SAA in EEJ compared to naturally ejaculated control camels. In EEJ camels, the serum concentrations of SAA increased significantly (P = 0.02) immediately after EEJ (T1) compared to baseline (T0) values. The values remained significantly elevated (P = 0.001) 24 h after EEJ (T2). In the control group, the mean serum concentrations of SAA did not differ among T0, T1 and T2 values.
Figure 1. Effect of stimulation by electroejaculation (EEJ) on concentrations of serum amyloid A in male dromedary camels (mean ± SD, n = 20) compared to control group (n = 10). T0: just before EEJ; T1: directly after EEJ; T2: 24 h after EEJ. a,b,cValues differ significantly.
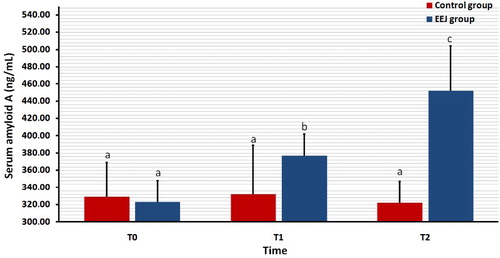
illustrates the serum concentration of Hp both EEJ and naturally ejaculated controls. In none of the 2 groups was the serum concentration of Hp differed significantly (P > 0.05) among T0, T1 and T2 values.
Figure 2. Effect of stimulation by electroejaculation (EEJ) on serum concentrations of haptoglobin in male dromedary camels (mean ± SD, n = 20) compared to control group (n = 10). T0: just before EEJ; T1: directly after EEJ; T2: 24 h after EEJ. aValues did not differ significantly.
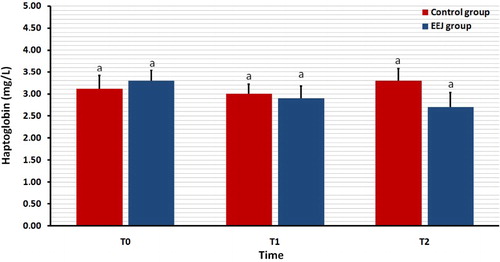
summarizes the serum concentration of OC in EEJ compared to naturally mated control camels. In EEJ camels, the serum concentrations of OC increased significantly (P = 0.04) immediately after EEJ (T1) compared to baseline (T0) values. The values declined at T2 that did not differ significantly from T0 (P = 0.3). In the controls, the mean serum concentrations of OC did not differ among T0, T1 and T2 values.
Figure 3. Effect of stimulation by electroejaculation (EEJ) on concentrations of serum osteocalcin in male dromedary camels (mean ± SD, n = 20) compared to control group (n = 10). T0: just before EEJ; T1: directly after EEJ; T2: 24 h after EEJ. a,bValues differ significantly.
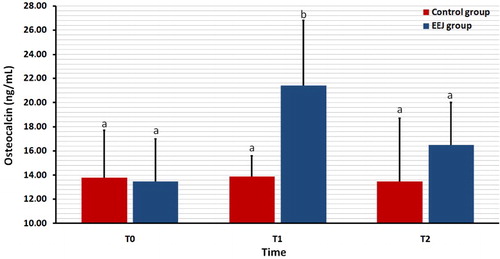
illustrates the serum concentration of b-ALP in both EEJ and naturally ejaculated controls. In none of the 2 groups was the serum concentration of b-ALP differed significantly (P > 0.05) among T0, T1 and T2 values.
Figure 4. Effect of stimulation by electroejaculation (EEJ) on serum concentrations of bone-specific alkaline phosphatase (b-ALP) in male dromedary camels (mean ± SD, n = 20) compared to control group (n = 10). T0: just before EEJ; T1: directly after EEJ; T2: 24 h after EEJ. aValues did not differ significantly.
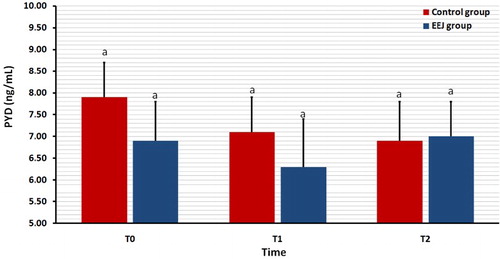
shows the serum concentration of the bone biomarker PYD in EEJ and naturally ejaculated camels. In none of the 2 groups was the serum concentration of PYD differed significantly (P > 0.05) among T0, T1 and T2 values.
Figure 5. Effect of stimulation by electroejaculation (EEJ) on serum concentration of pyridinoline cross-links (PYD) in male dromedary camels (mean ± SD, n = 20) compared to control group (n = 10). T0: just before EEJ; T1: directly after EEJ; T2: 24 h after EEJ. aValues did not differ significantly.
4. Discussion
To the authors’ knowledge, this is the first study in male camels emphasizing the influence of EEJ on serum concentrations of APPs (SAA and Hp), and bone formation (OC, b-ALP) and bone resorption (PYD) biomarkers.
The APR is a rapid, nonspecific, systemic response occurring secondary to many types of tissue injury and might be a physiological protective mechanism (Yazwinski et al. Citation2013). APR is induced by the pro-inflammatory cytokines IL-1, TNF-α and especially IL-6 (Petersen et al. Citation2004; Ceron et al. Citation2005; Tizard Citation2009). These cytokines activate receptors on various target cells and promote hormonal and metabolic changes leading to local and systemic effects, including APP synthesis in the liver (Petersen et al. Citation2004; Tizard Citation2009). The APPs can be used in diagnosis, prognosis and in monitoring response to therapy, as well as in general health screening and animal welfare (Eckersall Citation2000; Murata et al. Citation2004; Murata Citation2007; Eckersall and Bell Citation2010). The changes in APPs due to various inflammatory and non-inflammatory conditions have been studied intensively in many animal species (Murata et al. Citation2004; Murata Citation2007). The APPs have received attention as biomarkers for APR due to its low physiological levels, a fast incline, marked rise in concentration during APR that eases detection and a fast decline after cessation of a stimulus (Ceron et al. Citation2005).
Generally, APPs are secreted during the inflammatory response (Eckersall and Bell Citation2010). However, in this study, the significant increases of the APP SAA as a result of EEJ cannot be associated with pathological conditions, as the WBCs did not change significantly among T0, T1 and T2 values, thus confirming the absence of pathological conditions (Tharwat, Ali, et al. Citation2014). Therefore, these elevations, together with the cortisol increases, could be due to a physical ‘stress’ resulting in the numerous changes (Huzzey et al. Citation2011; Bauer et al. Citation2012; Tharwat, Al-Sobayil, et al. Citation2014). EEJ, often considered as a physical stress, represents a variety of physical and psychological stimuli that alter homeostasis and metabolism. The effects of EEJ include behavioural patterns in the animals such as vocalizing, struggling or displaying strong muscular contractions (Mosure et al. Citation1998). In humans, EEJ without anaesthesia is known to be painful (Ohl Citation1993), and complications such as nausea and vomiting, elevated blood pressure and headaches have been reported (VerVoort et al. Citation1988). Similarly in animals, EEJ is reported to be painful and stressful (Palmer Citation2005; Damian and Ungerfeld Citation2011; Fumagalli et al. Citation2012; Whitlock et al. Citation2012; Skidmore et al. Citation2013).
The mechanism behind the stress-induced SAA release after EEJ is not known, but a hypothesis based on a neuroendocrine-immune network concept has recently been reported (Murata Citation2007). This indicates that the non-inflammatory and psychophysical stressors activate the combined action of the sympatho-adrenal axis and the hypothalamic–pituitary–adrenal axis. This would affect both the immunity-related cells and the release of glucocorticoids, and would ultimately lead to the production and release of APPs (Murata Citation2007). In addition, glucocorticoids have been shown to induce or facilitate hepatic synthesis of APPs in vitro (Alsemgeest et al. Citation1995).
Haptoglobin is an α2-globulin synthesized in the liver (Eckersall and Bell Citation2010) and is a major APP in numerous species of livestock and domesticated animals as well as in camels (Baghshani et al. Citation2010; Nazifi et al. Citation2012). Elevated Hp concentrations occur not only with inflammation, but also with some conditions not generally associated with inflammation or tissue damage (Murata et al. Citation2004; Baghshani et al. Citation2010; Tharwat, Al-Sobayil, et al. Citation2014). In this study, the serum concentration of Hp did not change significantly post-EEJ.
The non-collagenous protein OC, a product of the osteoblasts, is regarded as a sensitive indicator of bone formation (Pullig et al. Citation2000). In addition to its increases that accompany skeletal growth, weight-bearing exercise induces changes in serum concentrations of OC (Eliakim et al. Citation1997). In this study, the serum concentrations of OC were significantly increased as a result of EEJ. The b-ALP, a glycoprotein found on the surface of osteoblasts, has also been shown to be a sensitive and reliable indicator of bone metabolism (Kress Citation1998). In contrast to OC, our results showed non-significant changes in b-ALP. Although OC and b-ALP are considered bone formation biomarkers, their correlation in the serum of camels was reported to be weak (Al-Sobayil Citation2010). The lack of a strong correlation between the two biomarkers has been attributed to the fact that each of them reflects different stages of osteoblast function (Delmas et al. Citation1990).
Increased concentrations of PYD in the blood or urine are most commonly considered as indicators of bone resorption (Thompson et al. Citation1992). The PYD cross-links, indicators of type I collagen resorption, are found in the mature collagen of bone. In this study, we observed non-significant changes in the serum concentration of PYD post-EEJ. These findings may indicate that increased physical activity may have the potential to decrease collagen resorption in male camels. This result may be influenced, in part, by a systemic suppression of collagen resorption through the systemic actions of calciotropic hormones, with emphasis on testosterones. It has been shown that increased levels of testosterone significantly reduce bone loss (Steffens et al. Citation2012; Wiren et al. Citation2012), decrease collagen and glycosaminoglycan loss in the articular tissues (Ganesan et al. Citation2008) and increase the repair strength of the ligaments and tendons (Tipton et al. Citation1975).
5. Conclusions
Acute phase reaction had occurred in male camels as a result of EEJ. This was manifested by significant increases of serum SAA. EEJ increased serum concentration of the bone formation biomarker OC. Although semen in male camels is frequently collected by EEJ, it should be taken into consideration to maintain the welfare status of the camel which is paramount.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Ali A, Derar R, Al-Sobayil F, Mehana S, Al-Hawas A. 2014. Impotentia generandi in male dromedary camels: clinical findings, semen characteristics, and testicular histopathology. Theriogenology. 82:890–896. doi: 10.1016/j.theriogenology.2014.07.001
- Allen MJ. 2003. Biochemical markers of bone metabolism in animals: uses and limitations. Vet Clin Pathol. 32:101–113. doi: 10.1111/j.1939-165X.2003.tb00323.x
- Alsemgeest SPM, Lambooy IE, Wierenga HK, Dieleman SJ, Meerkerk B, van Ederen AM, Niewold TA. 1995. Influence of physical stress on the plasma concentration of serum amyloid-a (SAA) and haptoglobin (HP) in calves. Vet Q. 17:9–12. doi: 10.1080/01652176.1995.9694521
- Al-Sobayil FA. 2010. Circadian rhythm of bone formation biomarkers in serum of dromedary camels. Res Vet Sci. 89:455–459. doi: 10.1016/j.rvsc.2010.03.024
- Baghshani H, Nazifi S, Saeb M, Saeb S. 2010. Influence of road transportation on plasma concentrations of acute phase proteins, including fibrinogen, haptoglobin, serum amyloid A, and ceruloplasmin, in dromedary camels (Camelus dromedarius). Comp Clin Pathol. 19:193–198. doi: 10.1007/s00580-009-0839-2
- Bauer NB, Er E, Moritz A. 2012. Effect of submaximal aerobic exercise on platelet function, platelet activation, and secondary and tertiary hemostasis in dogs. Am J Vet Res. 73:125–133. doi: 10.2460/ajvr.73.1.125
- Ceron JJ, Eckersall PD, Martinez-Subiela S. 2005. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol. 34:85–99. doi: 10.1111/j.1939-165X.2005.tb00019.x
- Damian JP, Ungerfeld R. 2011. The stress response of frequently electroejaculated rams to electroejaculation: hormonal, physiological, biochemical, haematological and behavioural parameters. Reprod Domest Anim. 46:646–650. doi: 10.1111/j.1439-0531.2010.01722.x
- DeLaurier A, Jackson B, Pfeiffer D, Ingham K, Horton MA, Price JS. 2004. A comparison of methods for measuring serum and urinary markers of bone metabolism in cats. Res Vet Sci. 77:29–39. doi: 10.1016/j.rvsc.2004.02.007
- Delmas PD, Price PA, Mann KG. 1990. Validation of the bone gla protein (osteocalcin) assay. J Bone Min Res. 5:3–4. doi: 10.1002/jbmr.5650050103
- Ebersole JL, Cappelli D. 2000. Acute-phase reactants in infections and inflammatory diseases. Periodontology. 23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x
- Eckersall PD. 2000. Acute phase proteins as markers of infection and inflammation: monitoring animal health, animal welfare and food safety. Ir Vet J. 53:307–311.
- Eckersall PD, Bell R. 2010. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J. 185:23–27. doi: 10.1016/j.tvjl.2010.04.009
- Eliakim A, Raisz LG, Brasel JA, Cooper DM. 1997. Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males. J Bone Min Res. 12:1708–1713. doi: 10.1359/jbmr.1997.12.10.1708
- Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. 2008. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage. 16:1196–1204. doi: 10.1016/j.joca.2008.03.008
- Frisbie DD, Mc Ilwraith CW, Arthur RM, Blea J, Baker VA, Billinghurst RC. 2010. Serum biomarker levels for musculoskeletal disease in two- and three-year-old racing thoroughbred horses: a prospective study of 130 horses. Equine Vet J. 42:643–651. doi: 10.1111/j.2042-3306.2010.00123.x
- Fumagalli F, Villagrán M, Damian JP, Ungerfeld R. 2012. Physiological and biochemical parameters in response to electroejaculation in adult and yearling anesthetized pampas deer (Ozotoceros bezoarticus) males. Reprod Domest Anim. 47:308–312. doi: 10.1111/j.1439-0531.2011.01859.x
- Ganesan K, Tiwari M, Balachandran C, Manohar BM, Puvanakrishnan R. 2008. Estrogen and testosterone attenuate extracellular matrix loss in collagen-induced arthritis in rats. Calcif Tissue Int. 83:354–364. doi: 10.1007/s00223-008-9183-9
- Gronlund U, Hulten C, Eckersall PD, Hogarth C, Waller KP. 2003. Haptoglobin and serum amyloid A in milk and serum during acute and chronic experimentally induced Staphylococcus aureus mastitis. J Dairy Res. 70:379–386. doi: 10.1017/S0022029903006484
- Huzzey JM, Nydam DV, Grant RJ, Overton TR. 2011. Associations of prepartum plasma cortisol, haptoglobin, fecal cortisol metabolites, and nonesterified fatty acids with postpartum health status in Holstein dairy cows. J Dairy Sci. 94:5878–5889. doi: 10.3168/jds.2010-3391
- Kanakis I, Nikoloau M, Pectasides D, Kiamouris C, Karamanos NK. 2004. Determination and biological relevance of serum cross-linked type I collagen Ntelopeptide and bone-specific alkaline phosphatase in breast metastatic cancer. J Pharm Biomed Anal. 34:827–832. doi: 10.1016/S0731-7085(03)00567-3
- Kress BC. 1998. Bone alkaline phosphatase: methods of quantitation and clinical utility. J Clin Lig. Ass. 21:139–148.
- Mosure W, Meyer R, Gudmundson J, Barth AD. 1998. Evaluation of possible methods to reduce pain associated with electroejaculation in bulls. Can Vet J. 39:1–3.
- Murata H. 2007. Stress and acute phase protein response: an inconspicuous but essential linkage. Vet J. 173:473–474. doi: 10.1016/j.tvjl.2006.05.008
- Murata H, Shimada N, Yoshioka M. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 168:28–40. doi: 10.1016/S1090-0233(03)00119-9
- Nazifi S, Oryan AM, Ansari-Lari M, Tabandeh MR, Mohammadalipour A, Gowharnia M. 2012. Evaluation of sialic acids and their correlation with acute-phase proteins (haptoglobin and serum amyloid A) in clinically healthy Iranian camels (Camelus dromedarius). Comp Clin Pathol. 21:383–387. doi: 10.1007/s00580-010-1102-6
- Ohl D. 1993. Electroejaculation. Urol Clin N Am. 20:181–188.
- Palmer CW. 2005. Welfare aspects of theriogenology: investigating alternatives to electroejaculation of bulls. Theriogenology. 64:469–479. doi: 10.1016/j.theriogenology.2005.05.032
- Petersen HH, Nielsen JP, Heegaard PMH. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 35:163–187. doi: 10.1051/vetres:2004002
- Pullig O, Weseloh G, Ronneberger D, Käkönen S, Swoboda B. 2000. Chondrocyte differentiation in human osteoarthritis: expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif Tissue Int. 67:230–240. doi: 10.1007/s002230001108
- Sabour H, Norouzi Javidan A, Latifi S, Larijani B, Shidfar F, Vafa MR, Heshmat R, Emami Razavi H. 2014. Bone biomarkers in patients with chronic traumatic spinal cord injury. Spine J. 14:1132–1138. doi: 10.1016/j.spinee.2013.07.475
- Schneider A, Corrêa MN, Butler WR. 2013. Short communication: acute phase proteins in Holstein cows diagnosed with uterine infection. Res Vet Sci. 95:269–271. doi: 10.1016/j.rvsc.2013.02.010
- Skidmore JA, Morton KM, Billah M. 2013. Artificial insemination in dromedary camels. Anim Reprod Sci. 136:178–186. doi: 10.1016/j.anireprosci.2012.10.008
- SPSS. 2009. Statistical package for social sciences. Chicago (IL): SPSS. Copyright for Windows, version 18.0.
- Steffens JP, Coimbra LS, Ramalho-Lucas PD, Rossa CJr, Spolidorio LC. 2012. The effect of supra- and subphysiologic testosterone levels on ligature-induced bone loss in rats — a radiographic and histologic pilot study. J Periodontol. 83:1432–1439. doi: 10.1902/jop.2012.110658
- Swaminathan R. 2001. Biochemical markers of bone turnover. Clin Chim Acta. 313:95–105. doi: 10.1016/S0009-8981(01)00656-8
- Tharwat M, Ali A, Al-Sobayil F. 2015. The impact of racing on serum concentrations of acute-phase proteins in racing dromedary camels. Comp Clin Pathol. 24:575–579. doi: 10.1007/s00580-014-1948-0
- Tharwat M, Ali A, Al-Sobayil F, Derar R, Al-Hawas A. 2014. Influence of stimulation by electroejaculation on myocardial function, acid–base and electrolyte status, and hematobiochemical profiles in male dromedary camels. Theriogenology. 82:800–806. doi: 10.1016/j.theriogenology.2014.06.023
- Tharwat M, Al-Sobayil F. 2015. Serum concentrations of acute phase proteins and bone biomarkers in female dromedary camels during the periparturient period. J Camel Pract Res. 22:271–278. doi: 10.5958/2277-8934.2015.00045.4
- Tharwat M, Al-Sobayil F, Buczinski S. 2014. Influence of racing on the serum concentrations of acute-phase proteins and bone metabolism biomarkers in racing greyhounds. Vet J. 202:372–377. doi: 10.1016/j.tvjl.2014.08.027
- Thompson PW, Spector TD, James IT, Henderson E, Hart DJ. 1992. Urinary collagen crosslinks reflect the radiographic severity of knee osteoarthritis. Br J Rheumatol. 31:759–761. doi: 10.1093/rheumatology/31.11.759
- Tingari MD, El Manna MMM, Rahim ATA, Ahmed AK, Hamad MH. 1986. Studies on camel semen. I. electroejaculation and some aspects of semen characteristics. Anim Reprod Sci. 12:213–222. doi: 10.1016/0378-4320(86)90042-4
- Tipton CM, Matthes RD, Maynard JA, Carey RA. 1975. The influence of physical activity on ligaments and tendons. Med Sci Sports. 7:165–175.
- Tizard IR. 2009. Veterinary immunology an introduction. 8th ed. St. Louis (MO): Saunders Elsevier.
- VerVoort SM, Donovan WH, Dykstra DD, Syers P. 1988. Increased current delivery and sperm collection using nifedipine during electroejaculation in men with high spinal cord injuries. Arch Phys Med Rehabil. 69:595–597.
- Watts NB, Jenkins DK, Visor JM, Casal DC, Geusens P. 2001. Comparison of bone and total alkaline phosphatase and bone mineral density in postmenopausal osteoporotic women treated with alendronate. Osteoporos Int. 12:279–288. doi: 10.1007/s001980170117
- Whitlock BK, Coffman EA, Coetzee JF, Daniel JA. 2012. Electroejaculation increased vocalization and plasma concentrations of cortisol and progesterone, but not substance P, in beef bulls. Theriogenology. 78:737–746. doi: 10.1016/j.theriogenology.2012.03.020
- Wiren KM, Zhang XW, Olson DA, Turner RT, Iwaniec UT. 2012. Androgen prevents hypogonadal bone loss via inhibition of resorption mediated by mature osteoblasts/osteocytes. Bone. 51:835–846. doi: 10.1016/j.bone.2012.08.111
- Yazwinski M, Milizio JG, Wakshlag JJ. 2013. Assessment of serum myokines and markers of inflammation associated with exercise in endurance racing sled dogs. J Vet Intern Med. 27:371–376. doi: 10.1111/jvim.12046
