ABSTRACT
The purpose of present study was to determine the quality and in vitro digestion parameters in silages of 100% pomegranate pomace (PPS), 100% apple pomace (APS) and 50% pomegranate pomace + 50% apple pomace (PAPS). The digestion parameters of 10% or 20% using of these silages in total mix ration (TMR) of dairy cattle were researched. The dry matter (DM), crude protein, flavonoids, tannins and anthocyanins of pomegranate pomace were higher than those of apple pomace. Fibrous and non-fibrous carbohydrates of pomegranate pomace were similar to those of apple pomace. The silage DM losses in APS decreased with pomegranate pomace (p = .004). The silage density, gas-methane production, and estimated digestion values of PPS and PAPS were higher than those of APS (p < .05). Lactic acid and pH value of APS and the PAPS were higher than the PPS (p < .01). The using of PPS up to 20% in TMR decreased in vitro estimated digestion of TMR (p < .05). The using of APS or PAPS up to 20% in TMR was appropriate for in vitro results. As a result, ensilaging of apple pomace with pomegranate pomace can increase silage density, silage DM; and can decrease silage DM losses; and do not affect silage quality-digestion parameters.
Introduction
Pomegranate (Punica granatum L.), which belong to the family Punicaceae, is a large shrub or small tree, an important fruit crop in native to semi-tropical Asia and naturalized in the Mediterranean region in very early times. According to the statistics of the Turkish Statistical Institute, Turkey was ranked important in pomegranate production in the world in 2013 after the Iranian and India (Gil et al. Citation2000; FAO Citation2013; TUIK Citation2018).
Pomegranate fruit consists of approximately 48% peel-flesh and 52% fruit, which is the edible part. Of the edible parts of pomegranate, 78% are fruit juice and 22% are seed (Sarica Citation2011; Zarei and Zeinolabedin Citation2011). In a previous study, it stated that pomegranate peel included 8.4% crude protein (CP), 55.4% non-fibrous carbohydrates (NFC), 34.5% neutral detergent fibre (NDF), 16.7% lignin and 0.84–1.0% total condensed tannins (TCT) (Kara Citation2016). Some researchers showed that pomegranate pomace contained 7.78% CP, 32.44% NDF, 27.76% ADF and 9.21% ash (Mirzaei and Maheri Citation2008; Ebrahimi Citation2012). Pomegranate juice includes specially 3-glucosides and delphinidin 3,5-diglucosides, anthocyanins such as cyanide and pelgorgonidine, and tannins such as punikalin, pedunculagin, punikalagin and ellagic acid (Kulkarni and Aradhya Citation2005; Zarei and Zeinolabedin Citation2011). The pomegranate skins are rich in ellagic-tannins such as punikalajin and its isomers 2,3-hexahydroxydiphenol-4,6-galloglucose, and it contains punicycline (4,6-galloglucose), gallic acid, ellagic acid and ellagic acid glycosides (hexycide, pentoside, rhamnoside) (Gil et al. Citation2000). The fruits of pomegranate used in many ways, including juice, dyes, inks, tannins for leather and a variety of remedies for various ailments. In addition, the pomegranate peels have widely used for traditional and medical herbal treatments against diarrhoea and dysentery in many countries (Ahmad and Beg Citation2001; Braga et al. Citation2005). Nevertheless, pomegranate fruits or by-products have used not commonly in animal diets. Shabtay et al. (Citation2008) stated that dietary supplementation with fresh pomegranate peel increased in feed intake and plasma alpha-tocopherol concentration, with a positive tendency toward increased weight gain of bull calves. Jami et al. (Citation2012) and Shabtay et al. (Citation2012) stated a significant increase in the digestibility of dry matter (DM), CP and NDF, as well as milk and energy-corrected milk yields in cows fed 4% pomegranate peel extract supplement.
The 30–40% of the total apple production in the world is damaged, and therefore not marketed, and 20–40% of this is processed for juice extraction (Kennedy et al. Citation1999; FAO Citation2013). It is estimated that only 20% of apple fruit weight is used and the remaining is discarded (Karling et al. Citation2017). The by-product left after extraction of the juice, called apple pomace, could be used as a livestock feed. Wet apple pomace consists of about 94% fruit and peel (flesh and peel) parts, about 4.5% seed, and about 1.5% hull around the seed (Kennedy et al. Citation1999). The dried apple pomace contains 7.7% CP and 5.0% ether extract (EE). It has 1.86 Mcal/kg DM metabolizable energy (ME) and 1.06–1.12 Mcal/kg DM net energy lactation (NEL) for dairy cows (NRC Citation2001). Abdollahzadeh et al. (Citation2010) noted that the using of up to 30% of mix tomato pomace and apple pomace silages in dairy cattle ration increased the digestibility of DM and organic matter, and the concentration of acetic, propionic, and total volatile fatty acids (VFA) in rumen fluid, and lowed rumen pH value.
Ruminants are an important source of methane emission, which stimulant global warming, in many countries because of their large population and high methane emission rate due to their ruminant digestive system. The dairy cattle constitute the most important herbivorous group in terms of anthropogenic methane emission due to over digestible nutrient content and a large amount of feed consumption (Edenhofer et al. Citation2014). When it view from this point, dairy cattle ration is important to include conversant or alternative feedstuffs containing an excess amount of phenolic compounds, which are demonstrated anti-methanogenic efficiency (Kara et al. Citation2015; Kim et al. Citation2015).
Agro-industrial biomasses, such as pomaces, are a rapidly degradable material (due to high soluble carbohydrate content and high moisture). The most important option for evaluating without cause to environmental pollution can use in animal diet as wet-dried pomace or silage. In the present study, determination of chemical compositions and in vitro digestion parameters (total gas and methane production and estimated digestion values) in pomegranate and apple pomaces and their silages were aimed. Besides, effects of 10–20% supplementation to total mix diet of these silages on in vitro gas-methane production and estimated digestion parameters were researched.
Materials and methods
Conservation of silages
In the study, pomegranate pomace and apple pomace were taken from a local fruit juice factory (Province of Kayseri, Turkey), and brought to the laboratory on the same day. The pomegranate pomace included approximately 50% peel and 50% seed and flesh. The pomaces were chopped into approximately 2.0 cm pieces and ensiled under laboratory conditions in glass silage, with about 1.2 L, jars with glass cap, which have clips and a rubber gasket. The silage jars have 1228.25 cm3 capacities (8.5 × 8.5 × 17 cm). The apple pomace also included skin, seed and pulp parts of fruit. The pomaces were ensilaged in three different groups as 100% pomegranate pomace (PPS), 100% apple pomace (APS) and 50% pomegranate pomace + 50% apple pomace (PAPS) (as wet weight basis) in glass silage jars as six replicates. The material was pressed tightly into the jars and sealed airtight. The about 1.9 kg wet pomace was pressed in silage jars. Silage jars were stored in a compartment of the laboratory at 20–25°C. All jars in treatment were opened on days 60 post-ensiling and were analysed.
The calculation of density and dry matter losses in silage jars
The ensiled wet pomace amount (g) in silage jar, which is with 1228.25 cm3 capacity, was recorded. The ensiled DM density was calculated using ensiled wet pomace amount and ensiled pomace DM according to following formula: [Density, g DM/cm3 = Ensiled pomace DM, g: The capacity of silage jar, cm3].
The silage DM losses were calculated using the ensiled pomace DM and the opened silage DM values according to following formula: Silage DM losses, % = [(Ensiled pomace DM, g – Opened silage DM, g):(Ensiled pomace DM, g)] × 100.
Chemical analysis
The DM levels of the pomace and silage samples were determined after waiting for 48 hours at 60°C. The dried samples were milled in an IKA A10 basic analytical grinder mill (IKA-Werke, Germany) to a maximum particle size of 1 mm for chemical analysis and in vitro gas production. The ash levels were determined after waiting at 525°C for 8 h. Nitrogen (N) content was analysed by the Kjeldahl method (AOAC Citation1995). The CP was calculated as Nx6.25. The EE and crude fibre (CF) levels were determined according to the method reported by the AOAC (Citation1995). The NDF, ADF and acid detergent lignin (ADL) contents, which form the cell wall components in the samples, were analysed according to the methods reported by Van-Soest et al. (Citation1991). The NDF was determined using sodium sulphite and heat stable amylase. Both NDF and ADF were not inclusive of residual ash. The level of NFC was calculated using the equation of NRC (Citation2001); NFC = 100 − (NDF mg/g + CP mg/g + EE mg/g + Ash mg/g). The hemicellulose (HC) contents of pomace and silages were calculated using the following formula; HC mg/g = NDF mg/g − ADF mg/g.
Determination of phenolic compounds and organic acids
The determination of total flavonoids was carried out according to the colorimetric assay reported by Kim et al. (Citation2003). A calibration curve was prepared with catechin and the results were expressed as mg of catechin equivalents (CatE)/g.
Total anthocyanins contents of pomegranate and apple pomaces were analysed on the spectrophotometer according to the procedures of AOAC (Citation2005).
Total condensed tannin levels of the pomaces were determined on the spectrophotometer according to the butane-HCI method reported by Makkar et al. (Citation1995).
For extraction of malic and citric acids, the pomace samples were mixed with deionized water (1:5), homogenized in ice bath for 1 minute and centrifuged at 5000 rpm for 10 min at 4°C. The supernatant which filtered, membrane filter with 0.45 µm, were analysed for the determination of organic acids by HPLC system (LC 20 AT, Shimadzu Co., Japan) with a diode array detector (DAD, SPD-M20A) (Artık et al. Citation1997). The separation of organic acids was performed on an Inertsil C18 column (5 μm, 250 mm × 4.6 mm). The HPLC elution was carried out at 30°C with isocratic flow of 2.0% KH2PO4, at pH 2.3 adjusted with o-phosphoric acid, as mobile phase at a 0.9 mL/min flow rate and 10 µL injection volumes (Toker et al. Citation2013).
The flavanols + flavonols contents of pomaces calculated according to following formula: Flavanols + flavonols = Total flavonoids − Total anthocyanins − Total condensed tannins.
Determination of silage pH, lactic acid and VFA in silage samples
The lactic acid, VFA and pH were determined in silage extracts, prepared by adding 450 mL of distilled water to 50 g of wet pomace silage, homogenizing for 5 min in a laboratory blender (Waring, USA) and filtered through four layers of cheesecloth. The silage pH value was determined in extraction liquid using a digital pH meter (Mettler Toledo S220, Switzerland).
For determination of concentrations of acetic, propionic, butyric, iso-butyric and iso-valeric acids in extraction liquid of silage samples, the liquid was decanted into centrifuge tubes and centrifuged at 26,000 g for 30 min (Tjardes et al. Citation2000). The supernatant was filtered and analysed with a high-performance liquid chromatography device (Agilent 1100 HPLC, Germany) equipped with a refractive index detector (HP 1047A). An Aminex Hpx 87H column (Germany) (300 × 7.8 mm column) was used. The flow rate of the mobile phase (0.005 M H2SO4) was 0.6 ml/min at 41°C (Canale et al. Citation1984).
The in vitro gas production technique
Rumen fluid inoculum
Rumen fluid, which required for in vitro gas production technique, was obtained from three Holstein dairy cows (at 54 months of age and 11th weeks of lactation, 620 kg live weight, and 30 L/day of milk production) fed with total mix ration (TMR) containing 5.0 kg/day maize silage, 3.7 kg/day wheat straw, 2.7 kg/day alfalfa hay and 9.8 kg/day concentre mix feed (with 21% CP and 2750 kcal/kg ME) as DM basis. Rumen fluid of approximately 1 L was collected using stomach cannel in a thermos including water at 39°C using CO2 gas, and filtered with four layers of cheesecloth in the laboratory.
Animal care procedures for the experiment were conducted under a research protocol approved (number: 14/01-2014) by the Local Ethics Committee for Animal Experiments in Erciyes University, Kayseri, Turkey.
Pomace silages and TMR samples for the in vitro gas production
The dairy cattle TMR, which includes maize silage, wheat straw, alfalfa hay and concentre mix feed, used as control TMR. The PPS, APS and PAPS used at 10% and 20% rates (in DM basis) instead of maize silage, wheat straw and alfalfa hay in control TMR. Totally seven TMR prepared as iso-caloric and iso-nitrogenic (). The TMR samples were milled through a 1-mm sieve (IKA MF10.1, Germany) for use in the in vitro gas production.
Table 1. Ingredients and chemical compositions of total mix rations used in study.
In vitro digestion procedure
The samples were incubated in rumen fluid and buffer mixture in glass fermenters with 100 ml volumes (Model Fortuna, Haberle Labortechnik, Germany) following the procedures of Menke and Steingass (Citation1988). One litre of buffer mixture included 474 mL of bi-distilled water, 237.33 mL of macro-mineral solution (5.7 g of Na2HPO4, 6.2 g of KH2PO4 and 0.6 g of MgSO4 in 1 L of bi-distilled water), 237.33 mL of buffer solution (35 g of NaHCO3 and 4 g of NH4HCO3 in 1 L of bi-distilled water), 0.12 mL of trace-mineral solution (13.2 g of CaCl2*2H2O, 10 g of MnCI2*4H2O, 1 g of CoCI2*6H2O and 0.8 g of FeCI3*6H2O in 100 mL of bi-distilled water), 1.22 mL of resazurine solution (0.1 g of resazurine in 100 mL of bi-distilled water) and 50 mL of reducing solution (0.285 g of Na2S*7H2O and 4 mL of 1 N NaOH in 96 mL of bi-distilled water). The dried samples (200 ± 10 mg) were weighed into fermenters. The fermenters were pre-warmed at 39°C in a thermostatically controlled cabinet (Incubator TC 256 G, The Tintometer Limited, Lovibond House, England), before 10 mL of rumen fluid and 20 mL of pre-warmed buffer mixture were dispensed anaerobically in each fermenter using an automatic dispenser (Isolab, Germany). The fermenters were closed using one position polypropylene clamps and incubated at 39 ± 0.5°C for 24 h. The initial volumes of fermenters were recorded. Each sample was studied in triplicate. In addition, three blank fermenters (no template; rumen fluid + buffer mixture) were used to calculate the total gas production (TGP).
Determination of in vitro total gas and methane production
After 24 h of incubation, the total gas volume was recorded from the calibrated scale on the fermenter. After measuring the total gas volume, the tubing of the plastic fermenter outlet was inserted into the inlet of the infrared methane analyser (Sensor, Europe GmbH, Erkrath, Germany) and the piston was pushed to insert the accumulated gas into the analyser. The methane as percent (%) of the total gas was displayed on a computer (Kara Citation2015).
Determination of estimated ME), NEL), OMd and short-chain fatty acids (SCFA) levels
The ME and OMd contents of the pomace silages and the 10% and 20% pomace silage supplementation to TMR were calculated using the equations of Menke and Steingass (Citation1988) as follows:
The NEL values of samples were calculated according to Blummel and Orskov (Citation1993):
SCFA were calculated using the equation:GP is the 24 h net gas production (mL/200 mg), CP is the crude protein (g/kg DM), A is the ash content (g/kg DM), EE is the aether extract (g/kg DM).
Statistical analysis
The statistical significances among pomaces for chemical contents and in vitro digestion values of were determined by the one-way variance analysis. The multivariate analyses were implemented for homogeneous variances by General Linear Model procedures to test treatment differences for pomace silages supplementation levels (0, 10 and 20) to TMR and pomace silage kind (PPS, APS or PAPS) in TMR. Data for in vitro digestion values were analysed using a randomized complete design with supplement levels x pomace silages.
Data were analysed based on the statistical model: Yijk = µ + Ei + Dj + EDij + eijk. Where, Yijk is the dependent variable; µ is the overall mean; E is the effect of i – supplement levels on the observed parameters; D is the effect of j – pomace silage on the observed parameters; ED interaction between the i – supplement levels and j – pomace silages; eijk is the standard error term.
The statistical analysis of data was performed, using SPSS 15.0 software. Significance was defined at p values of <.05
Results
The DM, ash, CP, EE, fibrous and NFC, phenolic compounds and acidity values for the pomaces of pomegranate and apple were given in the . The amount of ensiled pomace as fresh-wet (g) in silage jars, density of silage (g DM/cm3), the amount of opened silage as wet (g) in jars, opened silage DM (g) and silage DM losses (g) of PPS and PAPS were higher than those of APS (p < .001). The silage DM losses (%) of PPS was lower than that of APS (p = .004) (). The ash, CP, EE, CF, NDF, ADF, ADL, NFC and hemicellulose contents of the pomace silages were present in the . In the study the acetic, propionic, iso-butyric, butyric and iso-valeric acids concentrations of the silages (PPS, APS and PAPS) were similar (p > .05). The lactic acid concentration and pH value of the APS and the PAPS were higher than the PPS (p < .01) ().
Table 2. Chemical compositions of fresh pomegranate and apple pomace.
Table 3. The silage dry matter losses of pomegranate and apple pomace silages.
Table 4. The chemical compositions of pomegranate and apple pomace silages.
Table 5. The composition of organic acids and pH values of pomegranate and apple pomace silages.
The in vitro TGP, in vitro methane production, estimated digestion parameters (OMd, ME, NEL and SCFA) and ruminal pH determined at 24 h of ruminal in vitro digestion technique for the silages included pomegranate pomace (PPS and PAPS) were higher than silages included only apple pomace (APS) (p < .05; ).
Table 6. In vitro gas production and estimated digestion values for silages of pomegranate and apple pomace silages.
Both the 10% and 20% supplementations of PPS to the dairy cow TMR were decreased the TGP, NEL, ME, OMd and SCFA parameters according to supplementation in the same diets of both the APS and the PAPS (p < .05; ). In addition, methane production was not change the 10% and 20% supplementation of the PPS, APS and PAPS to the dairy cow TMR (p > .05; ).
Table 7. Effect of supplementations in different ratio of the PPS, APS and PAPS to total mix ration on in vitro gas production and estimated digestion parameters.
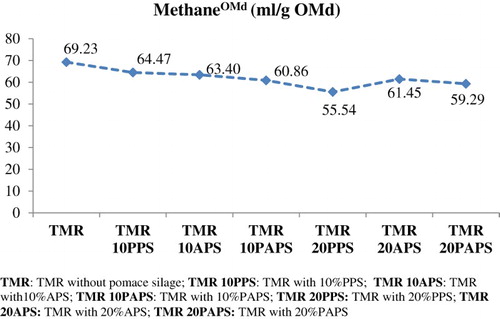
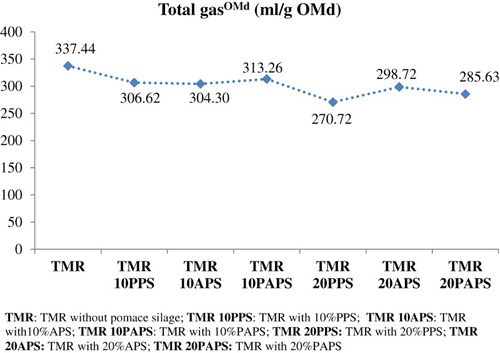
The in vitro methane production by digested organic matter (methaneOMd) of pomace silage in TMR were range 69.23 from 55.54 mL/g OMd and there are not different among TMR without pomace silage and TMR’s with pomace silage according to one-way variance analysis (p = .412) (). In addition, the in vitro TGP by digested organic matter of pomace silage (total gasOMd) in TMR was low by 20% PAPS using in TMR (337.44 vs. 270.72 mL/g OMd) (p = .027) ().
Discussion
The pomaces were obtained from the processing of fruit and vegetable crops for the production of some commercial products by the food processing industry and they are by-product feedstuffs. These by-products (or waste products) are rich in terms of fibrous compounds and easily soluble carbohydrates (mono- and di-saccharides) because of the fruits peel and edible parts. In addition, they may contain low/high amounts of other compounds (organic acids, phenolic compounds, flavonoids, lycopene, etc.) due to fruit’s seed and peel parts. The pomaces can be alternative/functional feedstuffs with high fibre, NFC and phenolic compounds contents. Therefore, the apple and pomegranate pomaces, which are by-products of apple and pomegranate fruits, would be discarded in outland (problem for environment condition) if they do not use as feedstuffs for ruminants. The by-product feedstuffs in the diets of ruminants can support growth and lactation as fibre, non-fiber carbohydrates, protein and functional compounds.
Chemical composition
The DM, CP, EE, citric acid, total flavonoids, TCT, total anthocyanins and total flavanols + total flavonols compositions of fresh pomegranate pomace were higher than that those of fresh apple pomace. In parallel with our study findings, Saleh et al. (Citation2017) stated that condensed tannins content of pomegranate pomace was 6.72 mg cyaniding equivalent/g in DM.
The nutrient and energy losses, which carry out by silage microorganisms, environmental conditions and silage moisture conditions, of ensilaging process can reduce nutritive value of ruminants. This loss is generally referred to as DM losses, and the most important problem is the high water level in the silage material. The DM losses are desirable to be as low as possible. Köhler et al. (Citation2013) demonstrated that DM losses of grass, lucerne and maize silages in bunker silos were 9–12%. The DM losses in the present study were 19.78% for PPS, 27.37% for APS and 22.46% for PAPS. The high DM losses of APS can be relation high water content of apple pomace material. The ensilaging of apple pomace with pomegranate pomace decreased the DM losses in silage. The density of DM in silage was showed a negative correlation to DM losses in maize silage by aforementioned researchers (Köhler et al. Citation2013).
The DM level of pomegranate pomace was ideal for desirable in silage conservation for maize silages (Baytok et al. Citation2005). Therefore, the DM level of APS was at a lower level according to the silage material (maize herbage, waste substances etc.) used to reach an ideal silage property (Kara Citation2015). In addition, DM content of APS was similar to results of fermented apple pomace which was ensilaged fresh apple pomace for two months in an anaerobic condition of Islam et al. (Citation2014). In the present study, the mixture silage of pomegranate pomace and apple pomace (50:50, as wet basis) (PAPS) reached DM value, which was higher than 20%. The lactic acid levels, which indicates ideal silage conservation, of APS elevated than the PPS, as well, this value of the PAPS was higher than the PPS. This result can be connected with an organic acid and easy soluble carbohydrates profile of the fresh-wet apple pomace. Therefore, the lactic acid and acetic acid values for apple pomace silages reported by Islam et al. (Citation2014) were higher than those of present study.
The flavonoids, which are the most important functional group from phenolic compounds in foods/feeds, comprise the anthocyanins (e.g. cyanidin, pelargonidin and petunidin), the flavonols (e.g. quercetin and kaempferol), flavones (e.g. luteolin and apigenin), flavanones (e.g. myricetin, naringin, hesperetin and naringenin) and flavan-3-ols (e.g. catechin, epicatechin and gallocatechin). Anthocyanins are pigments that are localized mainly in fruit peels, whereas flavonoids are localized in seed (Xia et al. Citation2010; Ali et al. Citation2014). In the total flavonoids and total anthocyanins of pomegranate pomace were about 20 and 10 times more than those of apple pomace can be relation with plant spices, peel and seed rate in pomace and environmental factors (e.g. geographical location, soil condition and climate) (Xu et al. Citation2016). Moreover, total flavonoid content of pomegranate pomace in present study corroborate with those reported by Ali et al. (Citation2014) demonstrated that total flavonoids contents in peel, flesh and seed of pomegranate fruit were 8.7–132 mg/g, 8.6–128 mg/g and 7.6–65 mg/g, respectively. On the other hand, Xu et al. (Citation2016) stated the extracts from different grape pomaces, which are rich phenolic substances, include 32–91 mg/g total flavonoids and 0.02–10.7 mg/g total anthocyanins.
The differences in chemical composition of these fruit pomaces may be due to a difference in starting materials, varieties, growing conditions (geographic, climatic and soil characteristics), amount of foreign materials and impurities as well as different processing and measuring methods. Besides, differences in peel:seed ratio in ingredient of pomegranate pomace can cause to be similar or different to the findings in previous studies. It is clear that, different chemical composition can result in different nutritive value. When we look at it from this point of view, it was conducted by a previous researcher that pomegranate peel contained 8.4% CP, 55.4% NFC, 34.5% NDF and 16.7% ADL (Kara Citation2016). The TCT of pomegranate pomace in the present study was low than that reported by Kara (Citation2016). The CP, NDF and ADF values for pomegranate pomace stated by previous researchers (Mirzaei-Aghsaghali et al. Citation2011; Ebrahimi Citation2012) were similar to those of present study. The ash content of pomegranate pomace was low than previous studies (Ebrahimi Citation2012; Mirzaei-Aghsaghali et al. Citation2011 ; Mirzaei and Maheri Citation2008) may be relation with seed rate in pomace. The CP, EE and NDF contents of APS in the present study were similar to the findings of Abdollahzadeh et al. (Citation2010).
The fresh-green plants or plant by-products, which have certain water content and soluble/structural nutrient composition, are undergoing some changes during fermentation when stored under the anaerobic condition. Some soluble and structural chemical compounds in ensilaged material degradable by microorganisms and reveal organic acids, water and gases (carbon dioxide, nitrogen gases) (Kara Citation2015, Citation2017). Appropriate silage fermentation can provide by lactic acid production by lactic acid bacteria, and increase lactic acid concentration in silage environment due to using and fermentation of non-fibrous compounds by these silage microorganisms (Podkowka and Podkowka Citation2011). As an agreement with this information, the NFC contents of fresh pomegranate and apple pomaces decreased by about 20–30% during silage fermentation. The highest concentration of lactic acid in fruit pomace silages in the current study were in APS can be relation to the APS in the highest lowing of NFC in silage fermentation. Kara (Citation2015) showed that increasing dose of maleic increased linearly lactic acid composition of maize silage, but linearly butyric acid composition. Besides, pH value also changes depending on end products of silage fermentation. The silage pH value of APS was parallel with a previous study (Islam et al. Citation2014). Desired pH value in appropriate silage for maize silage was range from 3.80 to 4.10 (Baytok et al. Citation2005; Dolezal et al. Citation2008). In the present study, low silage pH value of PPS was than those of silages including apple pomace may be associated with high NFC content. Besides, NDF, ADF and NFC contents of PPS, APS and PAPS were similar with the recommended values of National Research Council for dairy cattle forage and diets to provide the healthy rumen fermentation and rumination (NRC Citation2001).
In vitro gas production
The total gas volume produced as in vitro or in vivo demonstrates the digestibility capacity of feedstuff. Sommart et al. (Citation2000) reported that gas production of feedstuff in the in vitro systems was a good parameter for predict digestibility, and fermentation end products by rumen microbes. The soluble fraction of feedstuff can easily be connected by rumen microbes and leads to much gas production (Blummel and Becker Citation1997). In the present study, in vitro TGP, and estimated digestion parameters (ME, NEL, OMd and SCFA) of APS and PAPS were higher than those of PPS can be connection with low hemicellulose content or high phenolic compounds of pomegranate pomace compared to apple pomace. The in vitro total gas produced by PPS (29.29 ml/0.2 g DM) in the present study was higher than the values of pomegranate pomace (23.2 mL/0.2 g DM) and pomegranate pomace + urea (13.03 mL/0.2 g DM) reported by Ebrahimi (Citation2012). In other study, dried pomegranate seed and ensiled pomegranate seed produced about 18 and 26 mL in vitro total gas by 0.2 g DM at 24 h, respectively, (Taher-Maddah et al. Citation2012). The ME, NEL, OMd and SCFA values of PPS in the present study were similar about the findings obtained of ensiled pomegranate seed by Taher-Maddah et al. (Citation2012). Mirzaei-Aghsaghali et al. (Citation2011) showed that ME, NEL, OMd, SCFA parameters were determined as 6.20 MJ/kg DM, 2.35 MJ/kg DM, 42.34%, 0.504 mmol for pomegranate seeds, and 8.85 MJ/kg DM, 5.09 MJ/kg DM, 59.0%, 1.048 mmol for pomegranate peel, respectively. The in vitro fermentation values of PPS were compatible with the results of Agsaghali et al. (Citation2011) from this point the pomegranate pomace in present study included about 50:50 as % seed and peel. Khatooni et al. (Citation2014) showed that the in vitro gas production of 80% of apple pomace +20% of alfalfa meal mixture at 24 h incubation was carrying out as 67.17 mL/0.2 g DM. Differences in the in vitro digestion of pomegranate and apple pomace silages can be relation with varied processing (for produce fruit juice, shrub, ethanol, etc.) in pomace, water content of by-product, soluble/structural nutrient composition, concentrations of secondary metabolites (anthnocyanins, flavonoids, condensed tannins, etc.), duration and conditions of fermentation. Agsaghali et al. (Citation2011) indicated that in vitro TGP of apple pomace was about 59 mL for 0.2 g DM at 24 h of incubation. These levels of in vitro gas production can be accepted for feedstuffs, which are abundant from absorbable and easy-digestible carbohydrates, especially cellulose and lignin. The degradable of HC is very fast than those of cellulose and lignin.
The methane production of APS was lower than the PPS and PAPS may be relation with low total flavonoids, TCT or total flavanols + total flavonols contents, which are phytochemicals, a group predominant in some fruits, vegetables and cereals (Lila Citation2004; Lavelli et al. Citation2016). It is worth noticing that flavonoids were 11.60 and 6.59 mg catechin equivalent/g in pomegranate pomace and apple pomace, respectively. On the other hand, condensed tannins are 0.55 and 0.42%, i.e. 5.5 and 4.4 mg/g in pomegranate pomace and apple pomace, respectively. In a previous study, in vitro ruminal methane production decreased by flavonoid-rich plant extracts which was low number of ciliate protozoa up to about 60% in fermentation fluid (Kim et al. Citation2015). The results of in vitro research of Oskoueian et al. (Citation2013) demonstrated that 4.5% flavonoids such as naringin and quercetin in the substrate (DM basis) suppressed methane production, without negative effects on the in vitro gas production and digestion parameters. In addition, the effects on digestion activity (assimilation and absorption of feed substance) of phenolic compounds alterable depend on the level in the animal diet. In particular, the low levels (2–3%) of condensed tannins in diet have been reported prevent to breakdown of some proteins in the rumen and the realization by assimilation and absorption in the duodenum as by-pass (Barry Citation1987). This event is the positive effect of condensed tannins for ruminant. In contrast, abundant condensed tannin content of the feedstuff may lead to negative effects on assimilation and absorption on protein and other nutrients in the digestive tract (Kara et al. Citation2016). However, not all condensed tannins are the same. It should be considered that proanthocyanidins (tannins) strongly interact with proteins, resulting in the formation of insoluble protein-proanthocyanidin complexes. The abundant proanthocyanidin mass in substance, which will digest, can greater the binding energy with protein (Lavelli et al. Citation2016). In the present study, low in vitro gas production value and low estimated digestion parameters of PPS supplemented to TMR may be a relation with abundance of total flavonoids and anthocyanins in pomegranate pomace.
The results of the present study showed that the use of pomegranate and apple pomace silages in the ruminant diet is possible and have the following conclusions:
Ensiling of apple pomace with pomegranate pomace reached satisfactory silage quality values and in vitro digestion values
The using of APS or PAPS in dairy cow TMR instead of some forages (maize silage, straw, alfalfa hay) did not negatively effect in vitro gas production and other in vitro estimated digestion parameters,
The using of APS or PAPS up to 20% as DM in dairy cow TMR was appropriate in terms of in vitro results. However, there is a need to perform of in vivo experiments for studied pomace silages, especially PPS and PAPS.
In addition, pomegranate pomace can be silage and may use in ruminant diet, and then the environment pollution resulting from pomace will be used to prevent.
Acknowledgements
The abstract of this study presented in ‘VIII. Asian Buffalo Congress (21–25 April 2015; Istanbul, Turkey)’ and ‘32nd World Veterinary Congress, (13–17 September 2015; Istanbul, Turkey)’.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Kanber Kara http://orcid.org/0000-0001-9867-1344
Additional information
Funding
References
- Abdollahzadeh F, Pirmohammadi R, Farhoomand P, Fatehi F, Pazhoh FF. 2010. The effect of ensiled mixed tomato and apple pomace on Holstein dairy cow. Ital J Anim Sci. 9:212–216.
- Abdollahzadeh F, Pirmohammadi R, Fatehi F, Bernousi I. 2010. Effect of feeding ensiled mixed tomato and apple pomace on performance of Holstein dairy cows. Slovak J Anim Sci. 43:31–35.
- Aghsaghali AM, Maheri-Sis N, Mansouri H, Razeghi ME, Shayegh J, Aghajanzadeh-Golshani A. 2011. Evaluating nutritional value of apple pomace for ruminants using in vitro gas production technique. Ann Bio Res. 2(1):100–106.
- Ahmad I, Beg AZ. 2001. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 74:113–123. doi: 10.1016/S0378-8741(00)00335-4
- Ali SI, El-Baz FK, El-Emary GAE, Khan EA, Mohamed AA. 2014. HPLC-analysis of polyphenolic compounds and free radical scavenging activity of pomegranate fruit (Punica granatum L). Int J Pharm Clin Res. 6(4):348–355.
- AOAC. 1995. Association of official analytical chemists. Official methods of analysis. 15th ed., Vol. 1. Arlington (VA): AOAC.
- AOAC. 2005. Official methods of analysis of the A.O.A.C, 18th Ed. Washington, DC: Association of Official Analysis.
- Artik N, Poyrazoğlu E, Özkan G, Demirci T. 1997. Determination of organic acid profile in apple juices of Türkiye. 2nd Mediterranean Basin Conference on Analytical Chemistry. Rabat, Morocco. pp. 23–28.
- Barry TN. 1987. Secondary compounds of forages. In: Hacker JB, Ternouth JH., editors. Nutrition of Herbivores. Sydney, Australia: Academic Press; p. 91–120.
- Baytok E, Aksu T, Karslı MA, Muruz M. 2005. The effects of formic acid, molasses and ınoculant as silage additives on corn silage composition and ruminal fermentation characteristics in sheep. Turk J Vet Anim Sci. 29:469–474.
- Blummel M, Becker K. 1997. The degradability characteristics of fifty-four roughages and rough neutral detergent fiber as described in vitro gas production and their relation to voluntary intake. Brit J Nutr. 77:757–768. doi: 10.1079/BJN19970073
- Blummel M, Orskov ER. 1993. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim Feed Sci Tech. 40:109–119. doi: 10.1016/0377-8401(93)90150-I
- Braga L, Shupp J, Cummings C, Jett M, Takahashi J, Carmo L, Chartone-Souza E, Nascimento A. 2005. Pomegranate extract inhibits staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharm. 96:335–339. doi: 10.1016/j.jep.2004.08.034
- Canale A, Valente M, Ciotti A. 1984. Determination of volatile carboxylic acids (C1-C5) and lactic acid extracts of silage by high performance liguid chromatography. J Sci Agric. 35:1178–1182. doi: 10.1002/jsfa.2740351106
- Dolezal P, Zeman L, Skladanka J. 2008. Effect of supplementation of chemical preservative on fermentation process of lupine silage. Slovak J Anim Sci. 41:30–38.
- Ebrahimi B. 2012. Evaluation of pomegranate pomace using gas production technique. Euro J Exp Bio. 2:853–854.
- Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, et al. 2014. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, NY, USA: Cambridge University Press.
- FAO. 2013. Utilization of fruit and vegetable wastes as livestock feed and as substrates for generation of other value-added products. Food and Agriculture Organization of the United Nations. pp. 1–67.
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. 2000. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agr Food Chem. 48:4581–4589. doi: 10.1021/jf000404a
- Islam S, Islam MN, Matsuzaki M. 2014. Apple pomace silage ethanol intake and its effect on sheep. Bang J Anim Sci. 43:224–231.
- Jami E, Shabtay A, Nikbachat M, Yosef E, Miron J, Mizrahi I. 2012. Effects of adding a concentrated pomegranate-residue extract to the ration of lactating cows on in vivo digestibility and profile of rumen bacterial population. J Dairy Sci. 95:5996–6005. doi: 10.3168/jds.2012-5537
- Kara K. 2015. In vitro methane production and quality of corn silage treated with maleic acid. Ital J Anim Sci. 14:718–722. doi: 10.4081/ijas.2015.3994
- Kara K. 2016. Effect of dietary fibre and condensed tannins concentration from various fibrous feedstuffs on in vitro gas production kinetics with rabbit faecal inoculum. J Anim Feed Sci. 25:266–272. doi: 10.22358/jafs/65563/2016
- Kara K. 2017. Effect of maleic acid on nutritive value, carotenoids content and in vitro digestibility of maize silage. Anim Nutr Feed Tech. 17:245–254. doi: 10.5958/0974-181X.2017.00024.5
- Kara K, Aktuğ E, Özkaya S. 2016. Ruminal digestibility, microbial count, volatile fatty acids and gas kinetics of alternative forage sources for arid and semi-arid areas as in vitro. Ital J Anim Sci. 15:673–680. doi:10.1080/1828051X.2016.1249420.
- Kara K, Guclu BK, Baytok E. 2015. Comparison of nutrient composition and anti-methanogenic properties of different rosaceae species. J Anim Feed Sci. 24:308–314. doi: 10.22358/jafs/65613/2015
- Karling M, Bicas TC, Lima VA, Oldoni TLC. 2017. Grape and apple pomaces from southern Brazil: valorisation of by-products through investigation of their antioxidant potential. J Braz Chem Soc. 10:1857–1865.
- Kennedy M, List D, Lu Y, Foo LY, Newman RH, Sims IM, Bain PJS, Hamilton B, Fenton G. 1999. Apple pomace and products derived from apple pomace: uses, composition and analysis. In: Linskens HF, Jackson JF, editor. Analysis of plant waste materials. Heidelberg: Springer-Verlang; p. 75–101.
- Khatooni M, Nobar R, Cheraghi H. 2014. Evaluating possibility replacement of apple pomace with barley grain for ruminants with in vitro gas production technique. J Anim Sci Adv. 4(5):839–844. doi:10.5455/jasa doi: 10.5455/jasa.19691231040000
- Kim ET, Guan LL, Lee SJ, Lee SM, Lee SS, Lee ID, Lee SK, Lee SS. 2015. Effects of flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Aust J Anim Sci. 28:530–537. doi: 10.5713/ajas.14.0692
- Kim DO, Jeong SW, Lee CY. 2003. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81:321–326. doi: 10.1016/S0308-8146(02)00423-5
- Köhler B, Diepolder M, Ostertag J, Thurner S, Spiekers H. 2013. Dry matter losses of grass, lucerne and maize silages in bunker silos. Agr Food Sci. 22:145–150.
- Kulkarni AP, Aradhya SM. 2005. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chemist. 93:319–324. doi: 10.1016/j.foodchem.2004.09.029
- Lavelli V, Sri-Harsha PSC, Ferranti P, Scarafonia A, Iametti S. 2016. Grape skin phenolics as inhibitors of mammalian α-glucosidase and α-amylase – effect of food matrix and processing on efficacy. Food Funct. 7:1655–1663. doi: 10.1039/C6FO00073H
- Lila MA. 2004. Anthocyanins and human health: an in vitro investigative approach. J Biomed Biotechnol. 5:306–313. doi: 10.1155/S111072430440401X
- Makkar HPS, Blümmel M, Becker K. 1995. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and their implication in gas production and true digestibility in vitro techniques. Br J Nutr. 73:897–913. doi: 10.1079/BJN19950095
- Menke HH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 28:7–55.
- Mirzaei AA, Maheri SN. 2008. Nutritive value of some agro-industrial by-products for ruminants – a review. World J Zool. 3:40–46.
- Mirzaei-Aghsaghali A, Maheri-Sis N, Mansouri H, Razeghi ME, Mirza-Aghazadeh A, Cheraghi H, Aghajanzadeh-Golshani A. 2011. Evaluting potential nutiritive value of pomegranate processing by-products for ruminants using in vitro gas production technique. ARPN J Agr Biol Sci. 6:45–51. doi: 10.3844/ajabssp.2011.45.51
- NRC. 2001. Nutrient requirements of dairy cattle. 7th ed.Washington (DC): National Research Council, National Academy Press.
- Oskoueian E, Abdullah N, Oskoueian A. 2013. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res Int. 349129. doi:10.1155/2013/349129.
- Podkowka Z, Podkowka L. 2011. Chemical composition and quality of sweet sorghum and maize silages. J Cent Eur Agric. 12:294–303. doi: 10.5513/JCEA01/12.2.915
- Saleh H, Golian A, Kermanshahi H, Mirakzehi MT. 2017. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J Appl Anim Res. 45:629–636. doi: 10.1080/09712119.2016.1248841
- Sarica S. 2011. Using possibilities of pomegranate juice by-products in animal nutrition. GOU Ziraat Fak Derg. 28:97–101.
- Shabtay A, Eitam H, Tadmor Y, Orlov A, Meir A, Weinberg P, Weinberg ZG, Chen Y, Brosh A, Izhaki I, Kerem Z. 2008. Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. J Agr Food Chem. 56:10063–10070. doi: 10.1021/jf8016095
- Shabtay A, Nikbachat M, Zenou A, Yosef E, Arkin O, Sneer O, Shwimmer A, Yaari A, Budman E, Agmon G, Miron J. 2012. Effects of adding a concentrated pomegranate extract to the ration of lactating cows on performance and udder health parameters. Anim Feed Sci Technol. 175(1-2):24–32. doi:10.1016/j.anifeedsci.2012.04.004.
- Sommart K, Parker DS, Rowlinson P, Wanapat M. 2000. Fermentation characteristics and microbial protein synthesis in an in vitro system using cassava, rice straw and dried ruzi grass as substrates. Asian Aust J Anim Sci. 13:1084–1093. doi: 10.5713/ajas.2000.1084
- Taher-Maddah M, Maheri-Sis N, Salamatdoustnobar R, Ahmadzadeh A. 2012. Estimating fermentation characteristics and nutritive value of ensiled and dried pomegranate seeds for ruminants using in vitro gas production technique. Open Vet J. 2(1):40–45.
- Tjardes KE, Buskirk DD, Allen MS, Amest NK, Bourquin LD, Rust SR. 2000. Brown midrib-3 corn silage improves digestion but not performance of growing beef steers. J Anim Sci. 78:2957–2965. doi: 10.2527/2000.78112957x
- Toker R, Gölükcü M, Tokgöz H, Tepe S. 2013. Organic acids and sugar compositions of some loquat cultivars (Eriobotrya japonica L.) grown in Turkey. J Agr Sci. 19:121–128.
- TUIK. 2018. Turkish statistical institute. Crop production statistics. http://www.turkstat.gov.tr/PreTabloArama.do?metod=search&araType=vt.
- Van-Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber and non starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
- Xia EQ, Deng GF, Guo YJ, Li HB. 2010. Biological activities of polyphenols from grapes. Int J Mol Sci. 11:622–646. doi: 10.3390/ijms11020622
- Xu Y, Burton S, Kim C, Sismour E. 2016. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four virginia-grown grape varieties. Food Sci Nutr. 4:125–133. doi: 10.1002/fsn3.264
- Zarei MM, Zeinolabedin BS. 2011. Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening. Fruits. 66:121–129. doi: 10.1051/fruits/2011021