ABSTRACT
PGC-1α has been considered as an important mediator of functional capacity of muscle. Our previous study indicated that the mRNA level of PGC-1α increased in muscle during Schizothorax prenanti (S. prenanti) growth. To understand the biological significance of PGC-1α up-regulation, S. prenanti myosatellite cells were isolated and the function of PGC-1α in myoblast differentiation was further investigated. The results indicated that PGC-1α over-expressing transfectants fused to form myotubes with higher mRNA level of myosin heavy chain isoform I (MyHCI). No obvious differentiation was observed in PGC-1α-targeted shRNA-transfected cells with a marked decrement of MyHCI expression. Furthermore, S. prenanti PGC-1α increased the expression of MyoD and MyoG, which controlled the commitment of precursor cells to myotubes. In contrast, the levels of MyoD and MyoG mRNA were down-regulated with shRNA-targeting PGC-1α transfection. These investigations indicate that PGC-1α is associated with myoblast differentiation and it elevates MyoD and MyoG expression levels in S. prenanti myoblast cells.
Introduction
Muscle tissue is made up of muscle cells. Muscle cell differentiation is a highly regulated process. After a series of histological and molecular changes, muscle cells differentiate into myoblasts, then myoblasts fuse to form myotubes, and eventually, myotubes mature to form muscle fibres (Shi and Garry, Citation2006; Shadrach and Wagers, Citation2011). Muscle-specific genes such as myogenic differentiation antigen (MyoD) and myogenin (MyoG) are expressed during muscle cell differentiation (Buckingham, Citation1992; Gurung and Parnaik, Citation2012). MyoD regulates the commitment of muscle precursor cells to myoblasts (Buck, Citation2001), while MyoG controls the differentiation of myoblasts to myotubes (Brzóska et al., Citation2002). Additionally, myosin heavy chain isoform I (MyHCI), also known as slow-twitch oxidative type I, is considered as one of the most appropriate markers for fibre type delineation, which metabolizes lipids as fuel (Zhu et al., Citation2013).
Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), firstly was characterized as a functional activator of peroxisome proliferator-activated receptor γ in brown adipose tissue (Puigserver et al., Citation1999). The PGC-1α protein contains four main functional domains i.e. the activation domain, myocyte-specific enhancer factor 2C binding domain, nuclear respiratory factor-1 binding domain, and the RNA binding domain (LeMoine et al., Citation2010). Furthermore, PGC-1α interacts with various nuclear receptor super-families, including peroxisome proliferator-activated receptors, hepatocyte nuclear factor 4α, retinoid X receptor α, as well as many transcription factors such as Forkhead box 1, sirtuin 1 and myocyte enhancer factors 2 to activate transcription of specific target genes (Handschin, Citation2010). As a key regulator of mitochondrial content and oxidative metabolism, PGC-1α is critical in the maintenance of glucose, lipid, and energy homeostasis in muscle and other tissues (Ventura-Clapier et al., Citation2008). In muscle, it transforms type IIB muscle fibres into a more oxidative phenotype and thereby PGC-1α influents meat quality such as colour, juiciness, and taste (Lin et al., Citation2002; Ueda et al., Citation2005; Yamaguchi et al., Citation2010). We previously cloned PGC-1α gene from Schizothorax prenanti (S. prenanti), a well-known commercial cold-water fish species distributed in the upper reaches of the Yangtze River and its tributaries in China, and found that the mRNA level of PGC-1α in muscle increased during S. prenanti growth (Li et al., Citation2012). And now we investigated the function of PGC-1α in S. prenanti myoblast differentiation.
Materials and methods
Viral vector production
The FUGW-PGC-1α-RFP vector was constructed by subcloning S. prenanti PGC-1α cDNA (Genbank. accession number JN195738) into FUGW at the sites of HpaI and XbaI with primers shown in . Insertion of cDNA was verified by DNA sequencing. In addition, a gene specific shRNA () for S. prenanti PGC-1α was designed online (https://rnaidesigner.invitrogen.com/rnaiexpress/) and synthesized according to the S. prenanti PGC-1α cDNA sequence information. The shRNA was cloned into vector pSicoR-GFP at the sites of HpaI and XhoI. Negative control RNA () shares no sequence similarities with any reported S. prenanti gene sequences. Recombinant vectors were also verified by DNA sequencing. 293 T cells were grown on 10 cm plates to 70–80% confluence and co-transfected with these core plasmids and packaging plasmids pSPAx2, pMD2G, respectively (core plasmid: pSPAX2: pMD2G = 4:3:2). 48 h after transduction, RFP or GFP positive fluorescent cells were detected using an epifluorescent microscope (Nikon eclipse E600, Japan). The cell supernatants were collected and concentrated by ultracentrifugation.
Table 1. Primers for specific target genes.
Isolation of S. prenanti myosatellite cells
S. prenanti myosatellite cells were isolated as described by Fauconneau and Paboeuf (Fauconneau and Paboeuf, Citation2000). In brief, young S. prenanti (about 10 g) was anesthetized, slaughtered. Animal studies were approved by the Southwest University for Nationalities Institutional Committee for the Care and Use of Animals. The white myotomal muscle was excised under sterile conditions and minced. Fragments were centrifuged at 300 g for 5 min and washed twice in PBS supplemented with 1% penicillin/streptomycin. Enzymatic digestion was performed with 0.2% Type Ia collagenase (Sigma) in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, NY, USA) at 18 °C for 1 h with gentle agitation. The suspension was centrifuged at 750 g for 5 min, and the pellet was washed with DMEM, then given a second similar collagenase digestion, and centrifugation, the fragments were re-suspended in a trypsin solution (Sigma, 0.1% final concentration in DMEM) at 18 °C for 50 min with gentle agitation, then transferred to 2 volume of DMEM supplemented with 10% fetal bovine serum to block trypsin activity. Thereafter, the suspension was filtered on a 100 μm nylon cell strainer and centrifuged at 750 g for 2 min. The cells were re-suspended in DMEM supplemented with 10% fetal bovine serum and diluted to a final concentration of 106 cells/mL.
Cell culture, differentiation and transduction
5×105 cells/mL S. prenanti myosatellite cells were seeded and cultured at 18 °C in a humidified atmosphere containing 5% CO2. When the cells were about 70% confluence, they were transfected with serially diluted and concentrated viral vector stocks. For each transfection, 10 μg/mL of polybrene (Sigma) was used in the transducing inoculum. When the cells were grown to 90% confluence, the medium was switched to differentiation media containing 2% horse serum to induce myogenic differentiation.
Real-time RT–PCR
Total RNAs were extracted from myoblast cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNA concentration was determined with a spectrophotometer (BiowaveII, Biochrom, UK). cDNA was synthesized by reverse transcription from 2 μg RNA using a PrimeScript® RT reagent Kit (Fermentas Life Science, Hanover, MD, US). Real-time PCR analysis was performed in a fluorescence temperature icycler (Bio-Rad, Hercules, CA, USA) with the primers shown in under the following conditions: an initial denaturation at 95°C for 1 min followed by 40 cycles of 30 s denaturation at 95 °C, 30 s annealing at optimal primer temperature () and 30 s extension at 72 °C. S. prenanti β-actin was used as a loading control for normalization. The threshold cycle (CT) resulting from real-time PCR was analysed with a 2-ΔΔCt method (Livak and Schmittgen, Citation2001).
Statistical analysis
Data obtained from real-time RT–PCR were analysed statistically with SPSS Statistics V17.0 software and expressed as means ± SEM. The significance of the differences was determined by student’s t-test. Values of p < .05 were considered as statistical significance.
Results
PGC-1α over-expression and interference
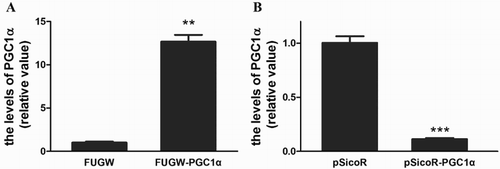
To assess the biological significance of PGC-1α up-regulation during S. prenanti growth, S. prenanti myosatellite cells were isolated and two myoblast cell lines were established. One over-expressed PGC-1α via transfection of PGC-1α gene into S. prenanti myoblast cells, and the other decreased the constitutive expression of PGC-1α via transfection of the vector containing shRNA-targeting PGC-1α. 72 h after transfection, the mRNA level of PGC-1α in S. prenanti myoblast cells was detected by real-time RT–PCR as shown in . The results revealed that the content of PGC-1α increased to 12.67 ± 0.79 fold in myoblast cells FUGW-PGC-1α compared with the cells transfected with FUGW (p = .0047, (A)). While the level of PGC-1α decreased to 0.11 ± 0.01 fold with pSicoR- shPGC1α viral infection (p = .0001, (B)).
Figure 1. PGC-1α over-expression and interference.
Notes: S. prenanti myosatellite cells were transfected with FUGW-PGC-1α, FUGW, PGC-1α targeted pSicoR-PGC-1α or pSicoR. 72 h later, the level of PGC-1α mRNA was tested by real-time RT–PCR in the cells transfected with FUGW-PGC-1α (A, **p < .01 vs. that transfected with FUGW) and pSicoR -PGC-1α (B, ***p < .001 vs. that transfected with pSicoR).
PGC-1a induces myoblast differentiation
Muscle differentiation is characterized by the alignment of the myoblasts into the myotubes (Phelps et al., Citation1998). The S. prenanti myoblast cells transfected with FUGW-PGC-1α differentiated to form myotube structures when cultured in differentiating medium on the 3rd day. The over-expressed PGC-1α cells formed typical myotubes with an elongated morphology compared with that observed in cells transfected with FUGW. In contrast, there was no obvious myotube formation in cells with decreased constitutive expression of PGC-1α via transfection of shRNA-targeting PGC-1α compared with those corresponding control cells. Therefore, PGC-1α regulated myogenic differentiation of S. prenanti myoblast cells.
Effect of PGC-1a increases on MyoD, MyoG and MyHCI expression
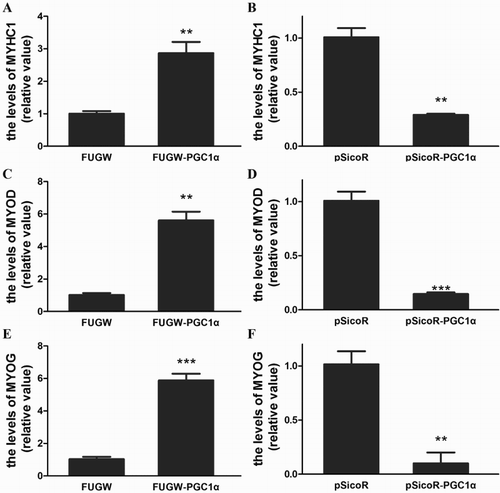
Specification of the myogenic lineage and differentiation of muscle cells are critically dependent on the basic helix–loop–helix transcription factors, MyoD and MyoG (Buckingham, Citation1992; Gurung and Parnaik, Citation2012). Furthermore, to understand the mechanism underlying the influence of PGC-1α on myoblast differentiation, the effect of PGC-1α on MyoD and MyoG expression was detected. As summarized in , the expression level of MyoD and MyoG were up-regulated in S. prenanti myoblast cells with FUGW-PGC-1α transfection. In contrast, the levels of MyoD and MyoG mRNA were down-regulated with shRNA-targeting PGC-1α transfection. These results indicated that PGC-1α enhanced expression of MyoD and MyoG in S. prenanti myoblast cells, which was paralleled with the activation of the muscle differentiation programme. Additionally, with the transfection of PGC-1α, the MyHCI gene expression level significantly increased, while shRNA-targeting PGC-1α decreased MyHCI compared with the corresponding control cells (). These results suggested that PGC-1α induced type I muscle fibre formation.
Figure 2. Role of PGC-1α in S. prenanti myoblast differentiation.
Notes: When induced by 2% horse serum, S. prenanti myogenic differentiation was observed on the 3th day. FUGW denoted FUGW transfectants, FUGW-PGC-1α denoted FUGW-PGC-1α transfectants, pSicoR denoted negative control shRNA transfectants, pSicoR R-PGC-1α denoted PGC-1α targeted shRNA transfectants.
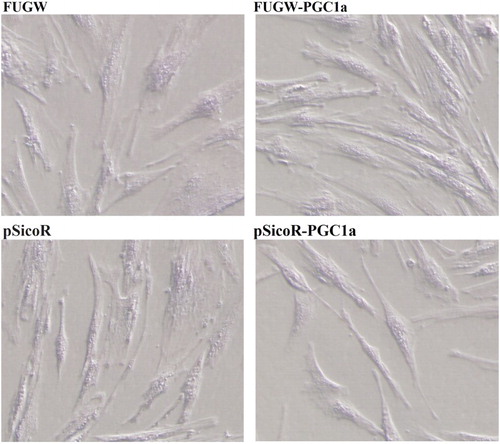
Figure 3. Infect of PGC-1α on expression of MyHCI, MyoD and MyoG.
Notes: The levels of MyHCI, MyoD and MyoG mRNA in S. prenanti myoblast transfectants were detected by real-time RT–PCR on the 3th day of myogenic differentiation. The level of MyHCI mRNA transfected with FUGW-PGC-1α (A) and PGC-1α targeted shRNA (pSicoR -PGC-1α, B), the level of MyoD mRNA transfected with FUGW-PGC-1α (C) and PGC-1α targeted shRNA (pSicoR-PGC-1α, D), the level of MyoG mRNA transfected with FUGW-PGC-1α (E) and PGC-1α targeted shRNA (F).(*p < .05, *p < .01, ***p < .001 vs. that corresponding controls). Data were the mean ± SEM of three independent experiments.
Discussion
In the past decade, some studies have focused on the function of PGC-1α in metabolic regulation of mitochondria, antioxidant defense and inflammatory response in muscle (Zhu et al., Citation2009; LeMoine et al., Citation2010; Summermatter et al., Citation2011), while the role of PGC-1α in regulating meat quality has been poorly documented. Our previous study indicated PGC-1α expression up-regulation in muscle during S. prenanti growth. In this study, we further investigated the function of PGC-1α in myoblast differentiation with over-expression and shRNA suppression strategies.
Firstly, S. prenanti myosatellite cells were isolated using type I collagenase and trypsin other than pronase digestion employed in isolation of pig myogenic satellite cells (Doumit and Merkel, Citation1992). In fact, a small quantity of S. prenanti myosatellite cells was obtained with pronase digestion in our study (data not shown). When PGC-1α transfected S. prenanti myosatellite cells were cultured in differentiating medium, the cells differentiated into typical myotubes. While the decreased constitutive expression of PGC-1α via transfection of shRNA-targeting PGC-1α leaded to no obvious myotube formation. The observation was parallel with our previous report that PGC-1α promoted typical myotube formation in murine C2C12 myoblast (Lin et al., Citation2014). These results suggested that PGC-1α was associated with myoblast differentiation.
Furthermore, the influence of PGC-1α on MyoD and MyoG, which controlled myoblast and myotube formation, were explored. PGC-1α increased both MyoD and MyoG expression. The up-regulation may accelerate myoblast differentiation. MyHCI, a marker for oxidative type I muscle fibre was also analysed and PGC-1α enhanced MyHCI expression. The detection indicated that PGC-1α induced type I muscle fibre formation, which was consistent with the report that PGC-1α can transform type IIB muscle fibres into a more oxidative phenotype (Lin et al., Citation2002). Our results revealed that PGC-1α was associated with S. prenanti myogenic differentiation. Since PGC-1α is a master regulator of mitochondrial biogenesis, the crosstalk between PGC-1α and myoblast differentiation may be related to mitochondria development.
Taken together, our study indicated that PGC-1α is closely associated with S. prenanti myogenic differentiation. The mechanisms may be attributed to increased expression of MyoD, MyoG and MyHCI.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brzóska E, Grabowska I, Wróbel E, Moraczewski J. 2002. Syndecan-4 distribution during the differentiation of satellite cells isolated from soleus muscle treated by phorbol ester and calphostin C. Cell Mol Biol Lett. 7:269–278.
- Buck IM. 2001. Skeletal muscle formation in vertebrates. Curr Opin Genet Develop. 11:440–448. doi: 10.1016/S0959-437X(00)00215-X
- Buckingham M. 1992. Making muscle in mammals. Trends Genet. 8:144–149. doi: 10.1016/0168-9525(92)90373-C
- Doumit ME, Merkel RA. 1992. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell. 24(2):253–262. doi: 10.1016/0040-8166(92)90098-R
- Fauconneau B, Paboeuf G. 2000. Effect of fasting and refeeding on in vitro muscle cell proliferation in rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 301:459–463. doi: 10.1007/s004419900168
- Gurung R, Parnaik VK. 2012. Cyclin D3 promotes myogenic differentiation and Pax7 transcription. J Cell Biochem. 113:209–219. doi: 10.1002/jcb.23346
- Handschin C. 2010. Regulation of skeletal muscle cell plasticity by the peroxisome proliferator-activated receptor γ coactivator 1α. J Recept Sig Transd. 30(6):376–384. doi: 10.3109/10799891003641074
- LeMoine CMR, Lougheed SC, Moyes CD. 2010. Modular evolution of PGC-1α in vertebrates. J Mol Evol. 70:492–505. doi: 10.1007/s00239-010-9347-x
- Li RW, Lin YQ, Zheng YC, Lu BM, Lin JC, Oku H, Huang L, Liu W, Liu ZX. 2012. Cloning, expression and polymorphism analyses of PGC-1a Gene of Schizothorax prenanti. Asian J Anim Vet Adv. 7:928–939. doi: 10.3923/ajava.2012.928.939
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. 2002. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 418:797–801. doi: 10.1038/nature00904
- Lin YQ, Zhao YY, Li RW, Gong JQ, Zheng YC, Wang Y. 2014. PGC-1α is associated with C2C12 myoblast differentiation. Cent Eur J Biol. 9:1030–1036.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262
- Phelps DE, Hsiao KM, Li Y, Hu N, Franklin DS, Westphal E, Lee EY, Xiong Y. 1998. Coupled transcriptional and translational control of cyclin-dependent kinase inhibitor p18INK4c expression during myogenesis. Mol Cell Biol. 18:2334–2343. doi: 10.1128/MCB.18.4.2334
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM. 1999. Activation of PPAR coactivator-1 through transcription factor docking. Science. 286:1368–1371. doi: 10.1126/science.286.5443.1368
- Shadrach JL, Wagers AJ. 2011. Stem cells for skeletal muscle repair. Philosophical Transactions B. 366:2297–2306. doi: 10.1098/rstb.2011.0027
- Shi XZ, Garry DJ. 2006. Muscle stem cells in development, regeneration, and disease. Genes & Dev. 20:1692–1708. doi: 10.1101/gad.1419406
- Summermatter S, Troxler H, Santos G, Handschin C. 2011. Coordinated balancing of muscle oxidative metabolism through PGC-1α increases metabolic flexibility and preserves insulin sensitivity. Biochem Biophys Res Commun. 408:180–185. doi: 10.1016/j.bbrc.2011.04.012
- Ueda M, Watanabe K, Sato K, Akiba Y, Toyomizu M. 2005. Possible role for avPGC-1 in the control of expression of fiber type, along with avUCP and avANT mRNAs in the skeletal muscles of cold-exposed chickens. FEBS let. 579:11–17. doi: 10.1016/j.febslet.2004.11.039
- Ventura-Clapier R, Garnier A, Veksler V. 2008. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1. Cardiovasc Res. 79:208–217. doi: 10.1093/cvr/cvn098
- Yamaguchi T, Suzuki T, Arai H, Tanabe S, Atomi Y. 2010. Continuous mild heat stress induces differentiation of mammalian myoblasts, shifting fiber type from fast to slow. Am J Physiol Cell Physiol. 298:C140–C148. doi: 10.1152/ajpcell.00050.2009
- Zhu LN, Ren Y, Chen JQ, Wang YZ. 2013. Effects of myogenin on muscle fiber types and key metabolic enzymes in gene transfer mice and C2C12 myoblasts. Gene. 532:246–252. doi: 10.1016/j.gene.2013.09.028
- Zhu L, Sun G, Zhang H, Zhang Y, Chen X, Jiang X, Krauss S, Zhang J, Xiang Y, Zhang CY. 2009. PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One. 4:e418.
