ABSTRACT
Summer-autumn tea leaf (SATL) is a waste tea plant resource which is not commonly used in tea industry due to its bitter and astringent taste. This study evaluated the effects of SATL-supplemented diet on broiler immune status. Accordingly, broilers were divided into control group and 3 SATL supplemented groups (0.5, 1.0, and 2.0%). The growth performance, immune organ index, serum antioxidant enzymes activity, sheep red blood cells (SRBC) Ab titers and Igs, as well as the Igs mRNA levels in the bursa of Fabricius were evaluated. The results showed that 1.0% and 2.0% SATL-supplemented diets significantly increased the relative thymus weight of broilers (P both < 0.05). 2.0% SATL treatment increased serum superoxide dismutase and glutathione peroxidase activities (P both < 0.01). Further, the serum SRBC Ab titers were significantly higher in the SATL-supplemented groups, meanwhile, IgY and IgA levels were remarkably elevated in the 1.0% and 2.0% SATL groups (P all < 0.05). In addition, an increasing of IgY and IgA mRNA levels in the bursa of Fabricius were observed in the 1.0% SATL group (P < 0.01). In summary, supplemental SATL can improve broiler immune status which indicates the potential application of SATL in broiler dietary supplement.
Introduction
Tea plant (Camellia. sinensis) has been grown in Southeast Asia for thousands of years and is currently cultivated in more than 30 countries worldwide (Butt and Sultan Citation2009). Due to the bitter and astringent taste of their products, summer-autumn tea plant leaves are considered to be of a lower grade than those plucked in spring, resulting in a sharp decline in their prices and consequently in massive wastage because considerable proportions of tea leaves are not harvested during summer and autumn (Dai et al. Citation2015). In the other aspect, the major secondary metabolites of tea plant, known as tea polyphenols, accounts approximately 20% of the dry weight of the leaf, which is considered to be the bioactive chemicals in tea. Notably, the tea polyphenols in the summer-autumn tea leaf are even more than those in the spring leaf (Erturk et al. Citation2010). It indicated the potential application of these wasted leaves.
Plant resources are favourable dietary supplements that are considered as efficient and safe immunity-improving agents (Khan et al. Citation2012; Khan et al. Citation2012; Sultan et al. Citation2014). Therefore, including these tea leaves into animal diets may effectively resolve the problem associated with the utilization of tea plant resources. Tea leaf has been used as a nutritional or functional dietary supplement for animals, and its immunity-enhancing function has received increasing attention. Tea leaf enhanced the growth performance and immune cell proliferation of a tea leaf-supplemented group compared with a control group in castrated male goats (Ahmed et al. Citation2015), showed immunomodulatory potential for use as feed additives for lambs and piglets (Deng et al. Citation2010; Zhong et al. Citation2015), modulated cell-mediated responses in fish (Harikrishnan et al. Citation2011) and also exert stimulatory effects on humoral immune responses to particulate and no particulate antigens, including increased antibody (Ab) titers in sheep red blood cells (SRBC)-immunized mice and elevated serum IgG and IgM levels in animals supplemented with tea leaf diets (Khan et al. Citation2015).
The beneficial effects of tea leaf as a dietary component in poultry diets are attracting increasing research interest (Khan Citation2014). Several researchers, including our research group, have investigated the effects of tea leaf on the growth performance and lipid metabolism of broilers (Yang et al. Citation2003) (Cao et al. Citation2005; Huang et al. Citation2013). In addition, studies have reported that tea leaf could be used as a novel antiviral resource for broilers (Lee et al. Citation2012) and an immune response enhancer against coccidiosis in broilers (Jang et al. Citation2007). It has been reported that diets containing different levels of fish oil and green tea can significantly increase antibody titers of Ross 308 broilers vaccinated in Influenza and Newcastle, which showing the immune-modulatory potential of green tea (Seidavi et al. Citation2015). Another recent study also showed that the specific Ab titers against newcastle disease virus vaccines increased significantly in broilers supplemented with tea leaf extract diets (Farahat et al. Citation2016). Increasing evidence supports the potential immunomodulatory effects of tea leaf on broilers; however, the underlying mechanisms require further investigation. Therefore, the present study was conducted to investigate a potential application of the summer-autumn tea leaf in the animal diet supplement. We studied the effects of SATL-supplemented diets on the immune indices, immune responses to particulate antigens, and serum Ig levels in broilers. Furthermore, we assessed IgY, IgA, and IgM mRNA levels in bursa of Fabricius.
Materials and methods
Tea leaf
The fresh tea leaf was harvested from a tea plant garden in Jinzhai, Anhui, China, August 2015. All leaves were soon baking dried, ground into powder, and sieved through a 60-mesh sieve.
HPLC analysis
The secondary metabolites were extracted using a previously described method. In brief, 0.1 g of SATL was mixed with 3 mL of 70% methanol in a water bath for 10 min at 70°C. After centrifugation at 6000 rpm for 10 min, the residues were re-extracted 2 times. The supernatants were combined, diluted with 70% methanol to make up a volume of 10 mL, and filtered through a 0.22-µm membrane before HPLC analysis. The catechins and caffeine contents in the extract were measured using a Waters 2695 HPLC system (Waters, Milford, MA, USA) equipped with a 2489 UV visible detector. Subsequently, 10 µL of the filtrate was injected into the HPLC system for analysis.
Crude protein and crude fibres analysis
The contents of protein in SATL were determined via Kjeldahl Method according to China National Standard (GB/T 5009.5-2010), and the total protein contents were calculated with the use of an adequate multiplier (6.25). The contents of crude fibre were determined according to China National Food Safety Standard (GB/T 8310–2002). All the analyses were repeated three times.
Broilers, diets, and experimental design
A total of 400 1-d-old Arbor Acres broilers were obtained from a local commercial company and randomly allocated into 4 groups. The broilers were fed in 4 separate rooms in a pen of 3.5 × 3.5 m covered with dry rice husk. The broilers were supplemented with the experimental basal diet () or 0.5, 1.0, or 2.0% SATL (w/w) for 42 consecutive days. Every room was equipped with infrared breeding bulbs, and the temperature was maintained at 31–32°C for 1–7 d and gradually decreased by 2–3°C every week until 25°C. Clean drinking water was provided to all broilers throughout the study period. All broilers were weighed weekly throughout the experimental period to record their average body weight (BW), average daily gain (ADG) and feed conversion ratio (FCR). In this study, 8 broilers were randomly assigned to each group and sacrificed by euthanasia at 14, 28, and 42 d. Peripheral blood was collected from the brachial veins of these broilers, and the serum was isolated by centrifugation at 3000 × g for 10 min and stored at −80°C. The thymus, spleen, and bursa of Fabricius tissues were excised at 28 and 42 d, weighed, and stored at −80°C. The immune organ index was calculated as the immune organs weights in relation to body weight (BW) (% of BW). The experiments were conducted in accordance with the guidelines of the Institutional Animal Ethics Committee of Anhui Agricultural University, and all efforts were made to minimize the suffering of the experiment broilers.
Table 1. Ingredient and nutrient composition of the experimental basal diet.
Antioxidant enzyme activity assay
Serum total superoxide dismutase (T-SOD) activity was assayed using the xanthine–xanthine oxidase and nitro blue tetrazolium system. One unit of T-SOD activity was defined as an inhibition of 50% nitro blue tetrazolium reduction rate/mL of serum. One unit of glutathione peroxidase (GSH-Px) activity was calculated as 1 µmol of GSH oxidized/min/mL of serum (Rotruck et al. Citation1973). And the level of GSH-Px was measured using spectrophotometric kits according to the manufacturer’s instructions (Jiancheng Bioengineering Institute), as explained in our previous study (Wang et al. Citation2015).
Serum Ig determination
Serum IgY and IgA levels were determined using ELISA kits (Bethyl Laboratories, Montgomery, TX, USA), according to the manufacturer’s instructions (Simon et al. Citation2014). In brief, precoated 96-well plates were coated with anti-chicken Ig Ab, and 100 μL of appropriately diluted samples (IgY dilution, 1:100000; IgA dilution, 1:10000) or standards was added to the wells in duplicate to start the reaction. The plates were covered and incubated at room temperature (20–25°C) for 60 min and washed 4 times with wash solution. All wash solutions were prepared by diluting the original wash solution with ultrapure water. Furthermore, 100 μL of detection Ab was added to each well and incubated as described above for 60 min. After washing, 100 μL of horseradish peroxidase detection Absolution was added to each well, followed by incubation for 30 min and plate washing. Subsequently, 100 μL of enzyme substrate (tetramethylbenzidine peroxidase substrate and peroxidase solution B) was added to each well, and the plate was placed in the dark for 30 min. The reaction was terminated by adding 100 μL of stop solution, and the absorbance values were measured at 450 nm using a plate reader.
SRBC assay
To assay the primary Abresponses, the SRBC method was followed as described previously (Leshchinsky and Klasing Citation2001), with minor modifications. At 28 d, 8 broilers of each group were immunized intramuscularly with 0.25 mL of 10% SRBC. At 7 d after vaccination, blood was collected in non-heparinized tubes by puncturing their brachial veins. Individual serum samples were analyzed for primary Ab responses against SRBC by using ELISA kits (Cellbio, Shanghai, China) and measured at 450 nm using an ELISA reader.
RNA isolation and real-time PCR
A portion of the bursa of Fabricius was collected from the selected broilers, flash-frozen in liquid nitrogen, and stored at −80°C. Total RNA isolation, cDNA preparation, and PCR-based target gene amplification were performed according to a previously described protocol (Wang et al. Citation2015). In brief, total RNA was isolated using TRIzol reagent, according to the manufacturer’s instructions (Takara Biotechnology, Shiga, Japan), and cDNA was prepared following the instructions provided in the Prime Script RT Reagent Kit (Takara Biotechnology). Real-time PCR was performed using a CFX System (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s protocol (Takara Biotechnology). Gene-specific primers () were designed using available gene sequences (www.ncbi.nlm.nih.goc/nuccore/).
Table 2. Real-time PCR primer sequences.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Differences between groups were examined by one-way ANOVA along with Duncan’s multiple range text, by SPSS software package (IBM SPSS Statistics 21 for Windows, SPSS, Inc., Chicago, IL, USA). Differences were considered significant at a P value of <0.05.
Results
Crude fibre and crude protein content
presents the content of crude protein (37.18 g/100 g) and crude fibre (15.03 g/100 g) in SATL as measured by the China National Standard method.
Table 3. Chemical components of the SATL (n = 3).
SATL major secondary metabolites analysis
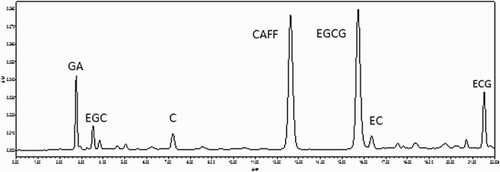
presents the results derived from the HPLC analyses of the SATL catechins contents, including epigellocatechin, epicatechin, epicatechin-3-gallate, epigallocatechins-3-gallate, the catechins contents of the SATL used in the present study are explained in .
Growth performance
This study observed that the food intake and mortality rate did not differ among the SATL-supplemented groups. At the end of the experiment, the BW showed no significant alteration compared to the control group except the high dose SATL treatment group. ADG and FCR showed no significant change by SATL treatments from 8 to 42 d ().
Table 4. Effects of SATL-supplemented diets on broiler growth performance (n = 8)1.
Immune organ weights and relative weights
The relative immune organ index (i.e. bursa of Fabricius, thymus, and spleen indices) was affected by SATL supplement (). The thymus index of the 1.0% and 2.0% SATL groups increased significantly at 28 and 42 d (P both < 0.05), respectively, thereby showing improvement in immune organ development. However, the spleen index of the 2.0% SATL group decreased significantly (P < 0.05).
Table 5. Effects of SATL-supplemented diets on broiler immune status (n = 8)1.
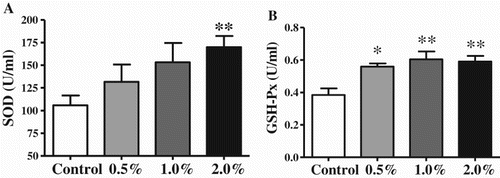
Antioxidant enzyme activity
SATL supplement increased the serum total antioxidant enzyme activity levels (). The T-SOD activity level increased significantly only in the 2.0% SATL group (P < 0.05). Meanwhile, the GSH-Px activity levels increased significantly in all SATL -supplemented groups (P < 0.05).
Serum Ig level
presents the results derived from the quantitative analyses of serum Ig levels. There is no difference of serum IgY levels in the 0.5% and 1.0% SATL groups were observed. Moreover, the 2.0% SATL group exhibited significantly increased serum IgY levels at 28 and 42 d (P both < 0.05). Similarly, the 2.0% SATL group showed remarkably increased IgA levels at 28 and 42 d (P both < 0.05).
Table 6. Effects of SATL-supplemented diets on serum Ig levels in broilers (n = 8)1
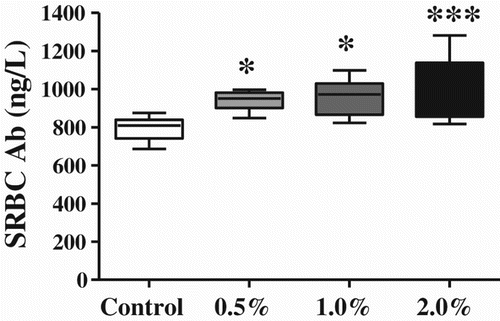
SRBC Ab titers
SATL improved the primary Ab responses against SRBC. The Ab titers of the 0.5, 1.0, and 2.0% SATL groups increased significantly by 18.33 (P < 0.05), 20.62 (P < 0.05), and 28.78% (P < 0.001), respectively, compared with the control group ().
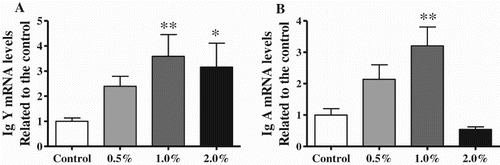
Ig mRNA level in the bursa of Fabricius
shows the relative Ig mRNA expression levels in the bursa of Fabricius of the SATL-supplemented groups at 42 d compared with the control group. The 1.0% and 2.0% SATL groups exhibited significantly increased IgY mRNA expression levels (P < 0.01, P < 0.05). The IgA mRNA expression levels increased significantly in the 1.0% SATL group (P < 0.01); however, IgM mRNA levels were not significantly influenced in all experimental groups (Data not shown).
Discussion
HPLC analysis revealed that the catechins and caffeine contents of the SATL sample used in the present study were comparable to those of common tea leaf products and tea leaf sample used in our previous study. (El-Shahawi et al. Citation2012; Huang et al. Citation2013). presents the BW, ADG and FCR of broilers. The present results indicated that the growth performance in the 0.5% and 1% SATL-supplemented groups was not adversely affected compared with the control group, which is consistent with previous report that 1% fine oolong tea powder did not affect growth performance of ducks (Wu et al. Citation2014), and the green tea feed supplementation does not induce any negative effects on immunological parameters (Alireza et al. Citation2017). In the present study, we observed the significant BW reduction (11.3%) in the 2.0% SATL group which may be attributed to the high tannin and fibre contents of the tea leaf used in the experiment (Yang et al. Citation2003). Furthermore, our previous study demonstrated that epigallocatechins-3-gallate could alleviate fat deposition by inhibiting fat anabolism and stimulating lipid catabolism in broilers (Huang et al. Citation2014). The findings from the study by David et al. showed that inclusion of tea leaf in broiler diets at 5 g/kg feed has positive influence on growth performance, carcass characteristics and fatty acids profiles of broiler meat (David et al. Citation2016). And another study showed that, plasma lipids of birds fed with green tea powder diet consisted of lower cholesterol, TG and higher HDL than normal ones (Afsharmanesh and Sadagh Citation2014). Therefore, we speculate that long-term use of high dose SATL-supplemented diets with rich catechins can achieve similar effects, and further relative dose-effect studies are needed.
The thymus, bursa of Fabricius, and spleen are the major immune organs of broilers, and the corresponding immune indices are associated immune cell growth, development, and division. Similarly, elevated immune indices indicate immune system maturation (Khan et al. Citation2012; Shijin et al. Citation2015). The organ weight in proportion to the broilers’ weight or the absolute organ weight is commonly used to qualify the immune status of the birds (Chen et al. Citation2013; Tong et al. Citation2014; Zhu et al. Citation2015). In this study, we observed that SATL supplement could significantly improve the thymus index in broilers, and the brusa index had increase tendency. Notably, the spleen index decreased significantly on SATL supplement, which may be attributed to the flavonoid content of tea leaf, and similar results were observed in a study on quercetin (Hager-Theodorides et al. Citation2014). Oxidative stress is recognized as redox imbalance, which leads to potential damage (Khan Citation2011). The primary antioxidant enzymes for reactive oxygen species (ROS) detoxification in allorganisms include T-SOD, catalase, and GSH-Px (Wang et al. Citation2016). ROS can cause biofilm system damage and oxidative phosphorylation defects. T-SOD and GSH-Px can effectively prevent ROS generation and lipid peroxidation. The present study observed that SATL could increase serum T-SOD and GSH-Px activity levels in broilers. A recent study reported similar results that single supplement of tea polyphenols could exert variable enhancing liver antioxidant stress in laying hens (Yuan et al. Citation2016), and another research showed that tea polyphenols can minimize growth inhibition, hyperlipidemia and oxidative stress induced by CTC treatment in broilers (Eid et al. Citation2003). Therefore, we suggest that tea leaf and its major component, polyphenols, could enhance the oxidative resistance of broilers. In addition, the 0.5% SATL group exhibited higher primary SRBC Ab titers than the control group (). The 1.0% and 2.0% SATL groups did not exhibit further increase in the Ab response to SRBC compared with the 0.5% SATL group (). By contrast, a study reported that Camellia L. plant extracts reduced primary Ab responses in broilers (Khalaji et al. Citation2011). Tea leaf did not exert significant effects on the humoral immune responses of broilers against Newcastle disease virus vaccines (El-Deek et al. Citation2012). Because SRBC is a T-dependent antigen, differences between primary and secondary responses warrant additional investigations.
In this study, the IgY mRNA expression levels increased with increasing SATL concentration from 0.5% to 1.0%, while a substantial increasing was not observed in 2.0% SATL group. IgY is the major Ig in broilers, and the 2.0% SATL group exhibited elevated serum IgY levels. By contrast, IgA showed a declining trend in the early period of treatment particularly at 14 d; the 1.0% and 2.0% SATL groups had significantly decreased serum IgA levels. However, after prolonged SATL supplement, serum IgA levels increased substantially, which may be attributed to B cell differentiation and IgM-to-IgA isotype-switching depending on the intestinal microbiota (Benveniste et al. Citation1971; Moreau et al. Citation1978; Tsuji et al. Citation2008). However, additional studies on the intestinal flora are warranted to elucidate the underlying Ig interactions. Several pieces of evidence indicate that the bursa is a vital lymphoid organ and is the key site of B cell development in the gut associated with lymphoid tissues such as the bursa of Fabricius. Consequently, cells that productively rearrange Ig genes express cell surface Igs and are selected for subsequent expansion in bursal follicles (Mccormack et al. Citation1989; Paramithiotis and Ratcliffe Citation1994; Ratcliffe Citation2006). In the present study, SATL affected the relative Ig expression levels in the bursa. Igs, are a crucial first line of defense (Ochsenbein and Zinkernagel Citation2000). IgA is the predominant Ig in broilers bile and intestinal secretions. The average serum IgA concentration in broilers is less than 4% of the total Ig concentration (Lebacq-Verheyden et al. Citation1974). In this study, we observed that SATL-supplemented diets could alter IgA mRNA levels; in particular, it resulted in significantly increased IgA levels in the 1.0% SATL group. We suggest that SATL could be applied for the activation of the adaptive immune system and Ig production, which is regarded as the principal component of serum and mucosal immunity. However, IgM mRNA level was not affected by SATL supplement.
Conclusions
In summary, the findings of the present study reveal that SATL -supplemented diets improve immune status (e.g. immune indices, serum antioxidant enzyme activity levels, Ig levels, and SRBC Ab titers) of commercial broilers. Our study results show that 1.0% SATL -supplemented diets had a significant positive effect on the growth performance, serum antioxidant enzyme activities, and Ig levels of broilers. Therefore, this study provides a dosage reference for practical applications of the summer-autumn tea leaf.
Acknowledgements
The financial support of this work was provided by the National Modern Agriculture Technology System (CARS-19), the Agricultural Science and Technology Achievements Transformation Project (2013GB2C300220, the Ministry of Science and Technology of P.R. China), and the Anhui Major Demonstration Project for the Leading Talent Team on Tea Chemistry and Health.
Disclosure statement
No potential conflict of interest was reported by the author.
ORCID
Dongxu Wang http://orcid.org/0000-0001-7377-6190
Additional information
Funding
References
- Afsharmanesh M, Sadagh B. 2014. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. J Clin Pathol. 23:711–724.
- Ahmed ST, Lee JW, Mun HS, Yang CJ. 2015. Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J Anim Physiol AN N. 99:1127–1137. doi: 10.1111/jpn.12279
- Alireza S, Mohammad D, Leila A, Hoven RVD, Mohammad HA-S, Mosè A, Antonello S. 2017. Dietary green tea powder affects the immunologic parameters of broiler chicks. Ital J Anim Sci. 16:1–7.
- Benveniste J, Lespinats G, Adam C, Salomon JC. 1971. Immunoglobulins in intact, immunized, and contaminated axenic mice: study of serum IgA. J Immunol. 107:1647–1655.
- Butt MS, Sultan MT. 2009. Green tea: nature’s defense against malignancies. Crit Rev Food Sci. 49:463–473. doi: 10.1080/10408390802145310
- Cao BH, Karasawa Y, Guo YM. 2005. Effects of green tea polyphenols and fructo-oligosaccharides in semi-purified diets on broilers’ performance and caecal microflora and their metabolites. Asian Austral J Anim. 18:85–89. doi: 10.5713/ajas.2005.85
- Chen K, Shu G, Peng X, Fang J, Cui H, Chen J, Wang F, Chen Z, Zuo Z, Deng J. 2013. Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B₁. Food Chem Toxicol. 59:446–454. doi: 10.1016/j.fct.2013.06.032
- Dai W, Qi D, Yang T, Lv H, Guo L, Zhang Y, Zhu Y, Peng Q, Xie D, Tan J. 2015. Nontargeted analysis using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (C. sinensis L.). J Agr Food Chem. 63:9869–9878. doi: 10.1021/acs.jafc.5b03967
- David AM, Upenyu M, Victor M, Arno H. 2016. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim Nutr. 2:160–167. doi: 10.1016/j.aninu.2016.05.003
- Deng Q, Xu J, Yu B, He J, Zhang K, Ding X, Chen D. 2010. Effect of dietary tea polyphenols on growth performance and cell-mediated immune response of post-weaning piglets under oxidative stress. Arch Anim Nutr. 64:12–21. doi: 10.1080/17450390903169138
- Eid YZ, Ohtsuka Z, Hayashi K. 2003. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Brit Poul Sci. 44:127–132. doi: 10.1080/0007166031000085427
- El-Deek AA, Al-Harthi MA, Osman M, Al-Jassas F, Nassar R. 2012. Effect of different levels of green tea (C. sinensis) as a substitute for oxytetracycline as a growth promoter in broilers diets containing two crude protein levels. Arch Geflugelkd. 76:88–98.
- El-Shahawi MS, Hamza A, Bahaffi SO, Al-Sibaai AA, Abduljabbar TN. 2012. Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem. 134:2268–2275. doi: 10.1016/j.foodchem.2012.03.039
- Erturk Y, Ercıslı S, Sengul M, Eser Z, Haznedar A, Turan M. 2010. Seasonal variation of total phenolic, antioxidant activity and minerals in fresh tea shoots (C. sinensis var. sinensis). Pak J Phama Sci. 23:69–74.
- Farahat M, Abdallah F, Abdel-Hamid T, Hernandez-Santana A. 2016. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Brit Poultry Sci. 57:714–722.
- Hager-Theodorides AL, Goliomytis M, Delis S, Deligeorgis S. 2014. Effects of dietary supplementation with quercetin on broiler immunological characteristics. Anim Feed Sci Tech. 198:224–230. doi: 10.1016/j.anifeedsci.2014.09.021
- Harikrishnan R, Balasundaram C, Heo MS. 2011. Influence of diet enriched with green tea on innate humoral and cellular immune response of kelp grouper (Epinephelus bruneus) to vibrio carchariae infection. Fish Shellfish Immu. 30:972–979. doi: 10.1016/j.fsi.2011.01.029
- Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. 2014. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 68:1075–1087. doi: 10.1038/ejcn.2014.143
- Huang J, Zhang Y, Zhou Y, Zhang Z, Xie Z, Zhang J, Wan X. 2013. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J Agr Food Chem. 61:8565–8572. doi: 10.1021/jf402004x
- Jang SI, Jun MH, Lillehoj HS, Dalloul RA, Kong IK, Kim S, Min W. 2007. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet Parasitol. 144:172–175. doi: 10.1016/j.vetpar.2006.09.005
- Khalaji S, Zaghari M, Hatami K, Hedari-Dastjerdi S, Lotfi L, Nazarian H. 2011. Black cumin seeds, Artemisia leaves (Artemisia sieberi), and Camellia L. plant extract as phytogenic products in broiler diets and their effects on performance, blood constituents, immunity, and cecal microbial population. Poult Sci. 90:2500–2510. doi: 10.3382/ps.2011-01393
- Khan RU. 2011. Antioxidants and poultry semen quality. World Poul Sci J. 67:297–308. doi: 10.1017/S0043933911000316
- Khan SH. 2014. The use of green tea (C. sinensis) as a phytogenic substance in poultry diets. Onderstepoort J Vet. 81:E1–E8. doi: 10.4102/ojvr.v81i1.706
- Khan A, Ali NH, Santercole V, Paglietti B, Rubino S, Kazmi SU, Farooqui A. 2015. C. sinensis mediated enhancement of humoral immunity to particulate and non-particulate antigens. Phytother Res. 30:41–48. doi: 10.1002/ptr.5498
- Khan RU, Naz S, Javadani M, Nikousefat Z, Selvaggi M, Tufarelli V, Laudadio V. 2012. The use of turmeric (curcuma longa) in poultry feed. World Poul Sci J. 68:97–103. doi: 10.1017/S0043933912000104
- Khan RU, Naz S, Tufarelli V, Laudadio V. 2012. Potential applications of ginger (zingiber officinale) in poultry diets. World Poul Sci J. 68:245–252. doi: 10.1017/S004393391200030X
- Khan RU, Rahman ZU, Nikousefat Z, Javdani M, Tufarelli V, Dario C, Selvaggi M, Laudadio V. 2012. Immunomodulating effects of vitamin E in broilers. World’s Poul Sci J. 68:31–40. doi: 10.1017/S0043933912000049
- Lebacq-Verheyden AM, Vaerman JP, Heremans JF. 1974. Quantification and distribution of chicken immunoglobulins IgA, IgM and IgG in serum and secretions. Immunology. 27:683.
- Lee HJ, Lee YN, Youn HN, Lee DH, Kwak JH, Seong BL, Lee JB, Park SY, Choi IS, Song CS. 2012. Anti-influenza virus activity of green tea by-products in vitro and efficacy against influenza virus infection in chickens. Poult Sci. 91:66–73. doi: 10.3382/ps.2011-01645
- Leshchinsky TV, Klasing KC. 2001. Relationship between the level of dietary vitamin E and the immune response of broiler chickens. Poult Sci. 80:1590–1599. doi: 10.1093/ps/80.11.1590
- Mccormack WT, Tjoelker LW, Barth CF, Carlson LM, Petryniak B, Humphries EH, Thompson CB. 1989. Selection for B cells with productive IgL gene rearrangements occurs in the bursa of Fabricius during chicken embryonic development. Gene Dev. 3:838–847. doi: 10.1101/gad.3.6.838
- Moreau MC, Ducluzeau R, Guy-Grand D, Muller MC. 1978. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Inect Immun. 21:532.
- Ochsenbein AF, Zinkernagel RM. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 21:624–630. doi: 10.1016/S0167-5699(00)01754-0
- Paramithiotis E, Ratcliffe MJ. 1994. Survivors of bursal B cell production and emigration. Poult Sci. 73:991–997. doi: 10.3382/ps.0730991
- Ratcliffe MJ. 2006. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev Comp Immuno. 30:101–118. doi: 10.1016/j.dci.2005.06.018
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. 1973. Selenium: biochemical role as a component of glutathione peroxidase. Nutr Rev. 179:588.
- Seidavi A, Asadpour L, Dadashbeiki M, Payancarreira R. 2015. Effects of dietary fish oil and green tea powder supplementation on broiler chickens immunity. Acta Sci Vet. 42:14–23.
- Shijin G, Shijun F, Qianqian X, Zhimei Z, Yanping W, Zhiqiang S. 2015. Immune function of Chinese formula Qingwen Baidu granule in broilers. Cent Eur J Immuno. 40:149–152.
- Simon K, de Vries Reilingh G, Kemp B, Lammers A. 2014. Development of ileal cytokine and immunoglobulin expression levels in response to early feeding in broilers and layers. Poult Sci. 93:3017–3027. doi: 10.3382/ps.2014-04225
- Sultan MT, Butt MS, Qayyum MM, Suleria HA. 2014. Immunity: plants as effective mediators. Crit Rev Food Sci. 54:1298–1308. doi: 10.1080/10408398.2011.633249
- Tong HB, Wang Q, Lu J, Zou JM, Chang LL, Fu SY. 2014. Effect of free-range days on a local chicken breed: growth performance, carcass yield, meat quality, and lymphoid organ index. Poult Sci. 93:1883–1889. doi: 10.3382/ps.2013-03470
- Tsuji M, Suzuki K, Kinoshita K, Fagarasan S. 2008. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin Immunol. 20:59–66. doi: 10.1016/j.smim.2007.12.003
- Wang Y, Jiang L, Li Y, Luo X, He J. 2016. Effect of different selenium supplementation levels on oxidative stress, cytokines, and immunotoxicity in chicken thymus. Bio Trace Elem Res. 172:1–36. doi: 10.1007/s12011-015-0550-x
- Wang D, Wang Y, Wan X, Yang CS, Zhang J. 2015. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol Appl Pham. 283:65–74. doi: 10.1016/j.taap.2014.12.018
- Wu P, Wen C, Leng ZX, Zhou YM. 2014. Effect of oolong tea (C. sinensis) powder particle size on growth performance, fat deposition, meat quality and antioxidant activity in meat ducks. Anim Feed Sci Tech. 194:131–135. doi: 10.1016/j.anifeedsci.2014.05.009
- Yang CJ, Yang IY, Oh DH, Bae IH, Cho SG, Kong IG, Uuganbayar D, Nou IS, Choi KS. 2003. Effect of green tea by-product on performance and body composition in broiler chicks. Asian Austral J Anim. 16:867–872. doi: 10.5713/ajas.2003.867
- Yuan ZH, Zhang KY, Ding XM, Luo YH, Bai SP, Zeng QF, Wang JP. 2016. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult Sci. 95:1709–1717. doi: 10.3382/ps/pew097
- Zhong RZ, Li HY, Fang Y, Sun HX, Zhou DW. 2015. Effects of dietary supplementation with green tea polyphenols on digestion and meat quality in lambs infected with Haemonchus contortus. Meat Sci. 105:1–7. doi: 10.1016/j.meatsci.2015.02.003
- Zhu W, Li D, Wang J, Wu H, Xia X, Bi W, Guan H, Zhang L. 2015. Effects of polymannuronate on performance, antioxidant capacity, immune status, cecal microflora, and volatile fatty acids in broiler chickens. Poult Sci. 94:345–352. doi: 10.3382/ps/pev006