ABSTRACT
Guinea pig represents a highly suitable animal model for study of Mycobacterium tuberculosis(Mtb)infection, as it demonstrates similarities to Mtb pulmonary infection in humans. It is known that guinea pigs can be efficiently infected by the respiratory or subcutaneous exposure to Mtb. However, information on Mtb infection through oral route is almost absent in the literature. Here, we examined whether guinea pigs can be infected by drinking Mtb containing water. Our findings confirmed that the guinea pigs could be infected with Mtb via drinking virulent water. The infected guinea pigs developed uniform oval-craterform ulcers at the 30th day after infection. Bacterial cultures showed Mtb growth in the lungs and spleens from the guinea pigs infected with high dose of Mtb. In addition, the infected animals had histopathological granulomatous lesions in lungs, spleens and mesenteric lymph nodes. We provide the compelling evidence that the guinea pigs could be infected by drinking Mtb containing water. The clinical and pathological observations in the infected animals were similar to those found in guinea pigs infected via the respiratory or subcutaneous routes.
Introduction
Tuberculosis (TB) caused by the intracellular pathogen Mycobacterium tuberculosis (Mtb) remains a life-threatening infectious disease of global burden with serious negative health and economic consequences (Devasundaram et al., Citation2016; Guo et al., Citation2016). Worldwide, about 9.6 million new cases of TB, including 1.0 million in children, occur in 2014 and one third of the world’s population, 2 billion people, is latently infected according to WHO (Global Tuberculosis Report Citation2015, WHO). Approximately 5% of these latently infected people will develop active TB during their lifetime. The lack of sufficient protection induced by Mycobacterium bovis BCG, the only registered TB vaccine, as well as the impact of HIV co-infection and the emergence of drug resistant Mtb strains all urge for improved vaccines and therapeutic drugs against TB. However, prior to new TB vaccine and drug candidates reaching human clinical trials stage, it is necessary to perform studies using animal models to examine efficiency of TB vaccine or drugs (Pirson et al., Citation2011). A large number of information pertaining especially to drugs and vaccine effects on the growth of Mtb in the lungs and spleens of infected animals has been generated (Khare et al., Citation2013). The small animal models of TB that are routinely employed to evaluate novel vaccines and drugs include the guinea pig.
Guinea pig is one of the most extensively used small animal models for TB research, as it displays similarities to Mtb pulmonary infection in humans. Guinea pig does not provide a direct comparison of the human response to Mtb infection. It does, however, provide a living, breathing lung on a small-scale with the possibility to carry out a large number of trials with definitive outcomes (Myllymäki et al., Citation2015). It is very known that guinea pigs can be efficiently infected by the respiratory or subcutaneous exposure to Mtb, this is also the most commonly used modelling method in our laboratory, however, now we are not sure if the animals can also infect Mtb through the digestive tract, which is an important factor in our another project, so we designed the current experiments.
Materials and methods
Mtb strain preparation
Mtb H37Rv strain was obtained from the Research Institute of Tuberculosis (Tokyo, Japan). The procedure of inoculation of Mtb H37Rv was achieved as described previously (Capuano et al., Citation2003). All the experiments with Mtb H37Rv were performed in the Animal Bio-Safety Level III facility of Wuhan University.
Animals and experimental design
Male specific pathogen-free Hartley guinea pigs (200–250 g in weight) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and held under barrier conditions in the Animal Bio-Safety Level III facility of Wuhan University. The animals were provided commercial guinea pigs chow in stainless-steel feeders and tap water ad libitum. Animals were housed in a temperature-and humidity-controlled environment and exposed to a 12 h light/dark cycle. After 7 days of acclimatization, 18 guinea pigs were randomly divided into 3 groups (6/group). The animals were deprived of drinking water for 4 h with free access to food before the experiment. The guinea pigs in control group were provided with normal drinking water; in the high-dose group, each animal was given 100 ml drinking water containing 2 × 107 colony forming units (CFU) Mtb H37Rv; in the low-dose group, each animal was given 100 ml drinking water containing 2 × 105 CFU Mtb H37Rv. After the animals used up the water (100 ml) within 5–8 h, they were given Mtb free water. According to our previous experience, Mtb can survive in water for more than 24 hours, this is highlighted by Velayati and colleagues confirmed the Mtb in contaminated water remained alive for a considerable period of time, i.e. up to 9 months (Velayati et al., Citation2015). Body weights of the animals were measured weekly during the study period of four months.
Tuberculin skin test
Tuberculin skin test (TST) is one of the most widely used diagnostic tests for the screening of Mtb infection in human and animals. TST was performed on the fourth week after Mtb challenged. 0.1 ml of PPD (purchased from Mycos Research LLC, Loveland, Colorado, USA) was injected intradermally into a shaved skin of the guinea pigs flank. The same volume of physiological saline was administrated as a negative control. The skin reactions were evaluated with a standard 1-5 scoring system at 24 h after PPD injection (Myllymäki et al., Citation2015).
Histopathologic analysis
Guinea pigs were humanely euthanized with an overdose of pentobarbital sodium at the end of the study. All findings associated with Mtb infection such as the swollen mesenteric lymph nodes, the visible granulomas in the lungs, spleens and other organs were recorded in the autopsy report. Tissue samples used for histopathological analysis were taken randomly from lung lobes, spleens and mesenteric lymph nodes of the guinea pigs. These tissue specimens were fixed in 10% formalin and embedded in paraffin. Standard sections were cut at 5 μm and processed for hematoxylin and eosin and examined by a light microscope.
Assessment of the bacterial load in target organs
To examine the bacterial load in target organs, the lung and spleen tissue specimens from individual guinea pigs were weighted and then minced and homogenized separately in PBS using a handheld tissue homogenizer (Pro Scientific, Inc., Monroe, Conn.). Serial dilutions of tissue homogenates were plated on 7H11 agar plates and incubated at 37°C with 5% CO2. The numbers of viable Mtb colonies on the agar plates were counted four weeks after the plating.
Statistical analysis
Data analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows software. Data are expressed as means ± SD. Statistical significance was determined by one-way ANOVA, followed by Duncan’s least significant difference test. P < 0.05 was considered to be significant.
Results
Tuberculin skin test
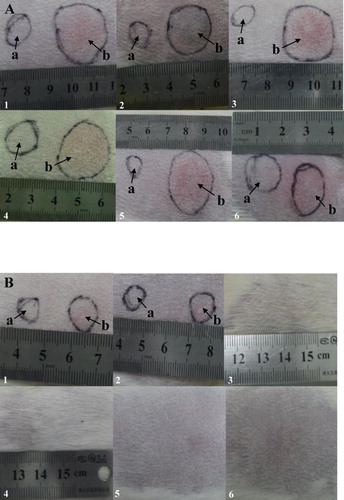
All guinea pigs in the high dose (2 × 107 CFU Mtb) group had positive TST, although the degree of positive reaction varied. At 24 h after PPD injected, the injection area became a bluish-red papule, which ulcerated and expanded, forming a typical oval lesion (A). The majority of guinea pigs displayed a skin lesion of about 1 cm in diameter, with some variation (range of 4–18 mm in diameter) and had grade 4+ or 5+ of TST. However, the animals in low-dose (2 × 105 CFU Mtb) group did not show visible skin lesion (B).
Figure 1. Skin lesion development of TST in the guinea pigs infected with Mtb by drinking water. A: Guinea pig receiving high dose of Mtb (2 × 107 CFU) group; B: Guinea pig receiving low dose of Mtb (2 × 105 CFU) group. Arrow a as a negative control and Arrow b representative images of lesions at 4 weeks post-infection.
Body weight changes
Each guinea pig was weighed during the experiment period of four months and compared with age-matched uninfected control animals. At the beginning of the experiment, the study guinea pigs had weight approximately 200 to 250 g. As shown in and , the body weight of the guinea pigs either uninfected or infected increased with time (P > 0. 05), but the increase was less in the animals in high-dose group compared to uninfected control animals (P < 0.05). The uninfected guinea pigs had a gradual weight increase with the time, gaining about 743 g by the end of the study, whereas the infected animals had only an increase of about 619 g. These results, together with the observation of abdominal respiration in the guinea pigs 2-month post-infection, suggesting that Mtb infection significantly affected the growth rate of the animals, resulting in growth restraint in TB infection.
Figure 2. Body weight of guinea pigs measured during the study period. After challenged with 2 × 107 CFU of Mtb, guinea pigs gained less weight compared to the uninfected control. Change in body weight is displayed as the mean of weights of two groups per time in this graph.
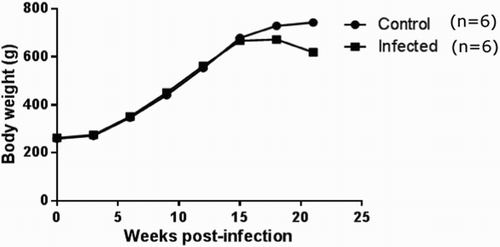
Table 1. Effects of Mtb infected on body weight gain in the guinea pigs.
Histopathological findings
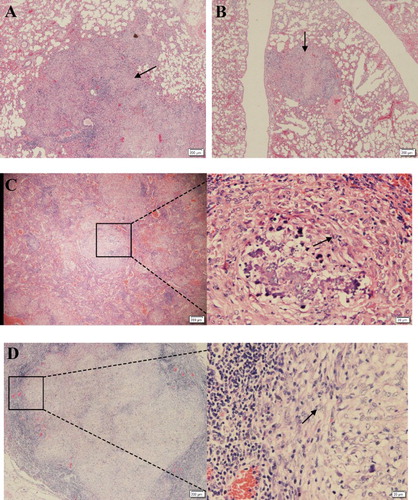
Guinea pigs infected through drinking water with the high-dose of Mtb developed circumscribed granuloma in lung lobes, spleens and mesenteric lymph glands (). Histopathology analysis of the tissues from infected animals demonstrated that multifocal granuloma consisted of large numbers of alveolar macrophages surrounded by a mantle of lymphocytes, monocytes and occasionally epithelioid giant cells. In contrast, pathologic changes associated with Mtb infection such as the visible granulomas in the lungs, spleens and mesenteric lymph glands were not found on histopathologic examination in the guinea pigs challenged with low-dose of Mtb, although the swollen mesenteric lymph nodes were commonly seen at autopsy, caseation granulomas were barely observed in these animals.
Figure 3. Histopathologic changes in selected tissues from infected guinea pigs. (A,B) Lung section of guinea pigs infection with 2 × 107 CFU of Mtb. Necrotic granulomas (arrow) were observed in the tissues from both right and left lung lobes at 20 weeks post-infection (×4). (C) Caseous necrosis in the centre of the lesions developed at 20 weeks post-infection, and the necrotic granuloma was confined by surrounding fibroblast (arrow) and fibrocyte in the spleen (×4). (D) Severe necrotizing fibrous granulomas were also appeared in the mesenteric lymph glands, also the necrotic granuloma was confined by surrounding fibroblast (arrow) and fibrocyte (×4).
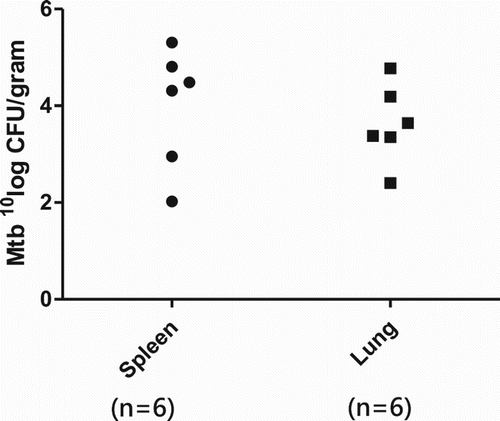
Bacterial burden in the organs
Bacterial cultures showed Mtb growth in the lung and spleen tissues from the guinea pigs infected with the high-dose Mtb. After four months of the infection, bacilli multiplied rapidly to about five logs in the spleens and lungs of the guinea pigs as illustrated in . The tissues from the spleens of the infected guinea pigs had higher numbers of Mtb than those of the lung tissues, which was the case for all the animals infected with the high-dose of Mtb. The highest numbers (105 CFU/g) were found in the spleen tissues, and near 105 CFU/g of Mtb were observed in the lungs. However, Mtb growth was not found in the lung and spleen tissues of the guinea pigs in the low-dose of Mtb.
Discussion
Although guinea pigs have been extensively used in TB research and a number of guinea pig models have been developed (Aiyaz et al., Citation2014; Clark et al., Citation2015; Sander et al., Citation2015; Sakthi et al., Citation2016), information on Mtb infection through oral route is almost absent in the literature. In the current study, we confirmed that the guinea pig could be infected with 2 × 107 CFU Mtb H37Rv strain via virulent water, which was evidenced by the development of a typical oval skin lesion, necrotic granulomas in the lungs, and Mtb growth in the infected tissues. The majority of guinea pigs in the high dose group displayed a skin lesion of about 10 mm in diameter, with some variation (range of 4–18 mm in diameter) and had grade 4+ or 5+ of TST, indicating infection with Mtb. In low dose (2 × 105 CFU) ones, however, we did not observe significant changes. Of note, in spite of their individual variability in terms of induration size and duration, the animal model displayed highly reproducible. Moreover, several studies have also demonstrated that injection of PPD in guinea pigs was highly sensitive to infection (Dharmadhikari et al., Citation2011; Moradi et al., Citation2015) and developed strong TST after infection with Mtb (Kalra et al., Citation2010).
The clinical and pathological changes observed in the infected guinea pigs were similar to those infected through the respiratory or subcutaneous routes in guinea pigs. We found that high-dose (2 × 107 CFU of Mtb) challenge of these animals by virulent water had acute and progressive TB which is featured by marked weight loss and cachexia occurring within four months after infection. The body weight of the guinea pigs either uninfected or infected increased with time, but the weight increase was less in animals in the high-dose group () at 15th week after Mtb challenge. These observations are in agreement with previous reports that Mtb infection significantly affected the growth rate of the animals, resulting in growth restraint in TB infection (Kashino et al., Citation2008). Similarly, the macroscopic and histopathologic changes that we found in the lungs, spleens and other tissues were similar those seen in human TB (O’Shea and McShane Citation2016). The development of the granulomatous lesions and its subsequent decline as cellular degeneration and necrosis, are the histologic hallmarks of Mtb infection (Fox et al., Citation2016). The granulomas developed in the infected guinea pigs share the features similar to those in humans (Montales et al., Citation2015), including centralized dystrophic mineralization, multicentric areas of caseous necrosis (A, B) and surrounding fibroblast and fibrocyte (C, D). During Mtb infection, the granuloma provides the microenvironment in which antigen-specific T cells colocate with and activate infected macrophages to inhibit the growth of Mtb. Our findings support the observations by others (Kashino et al., Citation2008; Clark et al., Citation2015) that guinea pigs are highly susceptible to Mtb infection, resulting in severe TB disease shortly after challenge. However, pathological changes associated with Mtb infection were not observed in the guinea pigs in low dose (2 × 105 CFU) of Mtb group during the course of this study. This may be due to a too low dose of Mtb, which will be removed by animal immunity. Also, our previous study demonstrates that dose of Mtb is a critical factor in determining the outcome of Mtb infection in animals (Zhang et al., Citation2014). Moreover, after having challenged guinea pigs orally with high-dose of Mtb, we observed that Mtb can be cultured from lungs and spleens of the animals, thus indicating that in these animals the Mtb were present in their tissues in this state.
It should be noted, however, that although swollen intestinal lymph nodes were often found in the guinea pig at necropsy, even in further pathological examination found necrotic granuloma, but pathologic changes (gross pathology and histopathology) associated with TB in the intestinal tract were not observed. These findings seem do not consistent with the observations of Villemin (Villemin, Citation1868). In 1868, Villemin fed 40 g of tuberculous sputum to guinea pigs and killed the animals 3 months later. Lesions were found in the small bowel and cecum. Later, in 1933 Mack and David conducted a series of similar experiments (McConkey and Smith Citation1933). Seventy-two adult guinea pigs were fed tuberculous sputum daily for periods ranging from 6 weeks to 4 months. Thirty-seven of these were maintained on a diet partially deficient in vitamin C; twenty-six developed ulcerative intestinal tuberculosis. In the remaining thirty-five animals whose diet was supplemented by an adequate amount of vitamin C only two developed tuberculous ulcers in the intestines. In our study, guinea pigs were supplied with commercial feed, no lack of vitamin C in the diet. This may be the reason for the absence of intestinal lesions in the infected animals.
In the current study, we provide the compelling evidence that the guinea pigs could be infected by drinking Mtb containing water. The clinical and pathological observations in the infected animals were similar to those found in guinea pigs infected via the respiratory or subcutaneous routes. A large number of Mtb enters the guinea pigs by drinking water, and further spreads to the intestinal lymph nodes and lungs with blood, eventually causing TB. Given the infection is through an alternative and practical means, this guinea pigs infection model has great potential for studying the pathogenesis of TB disease and for the development of new vaccines and drugs against Mtb infection. To our knowledge this is the first report of Mtb propagation in guinea pigs after exposure to the pathogenic bacteria by drinking Mtb containing water route.
Conflict of interest statement
The authors declare that they have no competing interests.
Acknowledgments
We thank Q. Yu for assistance with photography and Y. Wang for tissue procurement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aiyaz M, Bipin C, Pantulwar V, Mugasimangalam R, Shanley CA, Ordway DJ, Orme IM. 2014. Whole genome response in Guinea pigs infected with the high virulence strain Mycobacterium tuberculosis TT372. Tuberculosis (Edinb). 94:606–615. doi: 10.1016/j.tube.2014.10.001
- Capuano SV 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003
- Clark S, Hall Y, Williams A. 2015. Animal models of tuberculosis: Guinea pigs. Cold Spring Harb Perspect Med. a018572(5):1–9.
- Devasundaram S, Gopalan A, Das SD, Raja A. 2016. Proteomics analysis of three different strains of Mycobacterium tuberculosis under In vitro hypoxia and evaluation of hypoxia associated antigen’s specific memory T cells in healthy household contacts. Front. Microbiol. 1275(7):1–14.
- Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, et al. 2011. Natural infection of Guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb). 91:329–338. doi: 10.1016/j.tube.2011.03.002
- Fox GJ, Orlova M, Schurr E, Bliska JB. 2016. Tuberculosis in newborns: the lessons of the “lübeck disaster” (1929–1933). PLoS Pathog. e1005271(12):1–10.
- Global Tuberculosis Report 2015, WHO. http://www.who.int/tb/publications/global_report/en/.
- Guo M, Xian Q, Rao Y, Zhang J, Wang Y, Huang Z, Wang X, Bao R, Zhou L, Liu J, et al. 2016. SIV infection facilitates Mycobacterium tuberculosis infection of Rhesus Macaques. Front. Microbiol. 2174(7):1–10.
- Kalra M, Khuller GK, Sheikh JA, Verma I. 2010. Evaluation of Mycobacterium tuberculosis specific RD antigens for delayed type hypersensitivity responses in Guinea pig. Indian J. Exp. Biol. 48:117–123.
- Kashino SS, Napolitano DR, Skobe Z, Campos-Neto A. 2008. Guinea pig model of Mycobacterium tuberculosis latent/dormant infection. Microbes. Infect. 10:1469–1476. doi: 10.1016/j.micinf.2008.08.010
- Khare G, Reddy PV, Sidhwani P, Tyagi AK. 2013. Kefb inhibits phagosomal acidification but its role is unrelated to M. tuberculosis survival in host. Sci Rep. 3527(3):1–9.
- McConkey M, Smith DT. 1933. The relation of vitamin C deficiency to intestinal tuberculosis in the Guinea pig. J. Exp. Med. 58:503–512. doi: 10.1084/jem.58.4.503
- Montales MT, Chaudhury A, Beebe A, Patil S, Patil N. 2015. HIV-Associated TB syndemic: a growing clinical challenge worldwide. Front Public Health. 281(3):1–7.
- Moradi J, Mosavari N, Ebrahimi M, Arefpajohi R, Tebianian M. 2015. Evaluation of mycobacterium tuberculosis early secreted antigenic target 6 recombinant protein as a diagnostic marker in skin test. Osong Public Health Res. Perspect. 6:34–38. doi: 10.1016/j.phrp.2014.12.002
- Myllymäki H, Niskanen M, Oksanen KE, Rämet M. 2015. Animal models in tuberculosis research - where is the beef? Expert Opin Drug Discov. 10:871–883. doi: 10.1517/17460441.2015.1049529
- O’Shea MK, McShane H. 2016. A review of clinical models for the evaluation of human TB vaccines. Hum Vaccin Immunother. 5(12):1177–1187. doi: 10.1080/21645515.2015.1134407
- Pirson C, Vipond J, Hall Y, Williams A, Vordermeier HM. 2011. Vaccines designed to protect against mycobacterium tuberculosis infection may aid the identification of novel vaccine constructs and diagnostic antigens for bovine tuberculosis. Vet. Microbiol. 148:232–237. doi: 10.1016/j.vetmic.2010.08.019
- Sakthi S, Palaniyandi K, Gupta UD, Gupta P, Narayanan S. 2016. Lipoprotein LpqS deficient M. tuberculosis mutant is attenuated for virulence in vivo and shows protective efficacy better than BCG in Guinea pigs. Vaccine. 34:735–743. doi: 10.1016/j.vaccine.2015.12.059
- Sander P, Clark S, Petrera A, Vilaplana C, Meuli M, Selchow P, Zelmer A, Mohanan D, Andreu N, Rayner E, et al. 2015. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a Guinea pig model of tuberculosis. Vaccine. 33:1353–1359. doi: 10.1016/j.vaccine.2015.01.058
- Velayati AA, Farnia P, Mozafari M, Malekshahian D, Farahbod AM, Seif S, Rahideh S, Mirsaeidi M. 2015. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest. 147:1094–1102. doi: 10.1378/chest.14-0960
- Villemin JA. 1868. Etudes sur la tuberculose; preuves rationelles et experimentales de sa specificite et de son inoculabilite. Paris: Bailliere; p. 118.
- Zhang J, Xian Q, Guo M, Huang Z, Rao Y, Wang Y, Wang X, Bao R, Evans TG, Hokey D, et al. 2014. Mycobacterium tuberculosis Erdman infection of rhesus macaques of Chinese origin. Tuberculosis (Edinb). 94:634–643. doi: 10.1016/j.tube.2014.08.005