ABSTRACT
The purpose of the study was to determine the biochemical compositions and the leaching ratios according to different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of microdiets containing Spirulina sp., Chlorella sp. and Sargassum sp. in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm). The lowest and highest ash, lipid and protein values of algae tested were 3.29 ± 0.031% (Chlorella sp.)–27.94 ± 0.023% (Sargassum sp.), 0.91 ± 0.024% (Sargassum sp.)–8.91 ± 0.04% (Spirulina sp.) and 20.69 ± 0.07% (Sargassum sp.)–56.98 ± 0.216% (Chlorella sp.), respectively. The 2532 Da≥ leaching ratio of Chlorella sp. was lower than those of Sargassum sp. and Spirulina sp. (p < .05). The highest and lowest values for microdiets containing Spirulina sp. and Sargassum sp. were 2532 Da≥ and 2532–13,000 Da, respectively. However, the highest and lowest values for microdiets containing Chlorella sp. were 13,700–67,000 Da and 67,000 Da≤, respectively. In conclusion, the alginate method showed good performance because the biochemical compositions of microdiets produced reflected the ash, lipid and protein levels of algae tested. Results cautioned that the use of Spirulina sp. and Sargassum sp. in microdiets may yield the high leaching ratios containing 2532 Da≥ molecular weight. However, the use of Chlorella sp. results in the high leaching ratios containing 13,700–67,000 Da molecular weight.
1. Introduction
Aquaculture sector is highly dependent on fish meal and fish oil supply from wild fisheries. Because fish meal is regarded as the best dietary protein source due to the excellent balance of essential amino acids and essential fatty acids (Tacon Citation1993). For sustainable aquaculture as mentioned by Higgs et al. (Citation1995) replacing fish meal with more sustainable ingredients of either animal or vegetable sources is necessary. According to Lovell (Citation1998), feed ingredients containing 20% or more crude proteins are considered protein sources. The sustainability of the aquaculture sector may be threatened by its present over-dependence on fish meal and fish oil (FAO Citation2002). Naylor et al. (Citation2009) indicated that the aquafeed cost represents the variable cost of the highest fish production. The reducing of feed cost can be achieved by the use of cheap and sustainable feed ingredients. Therefore, trials have been done to evaluate cheap and sustainable plant-based protein sources. However, the main obstacles to the use of high amounts of plant protein sources in fish diets are low protein quality due to the amino acid imbalances, inadequate nutritional profile to meet fish requirements and the presence of antinutritional factors reducing the activity of fish digestive enzymes (Alexis Citation1997; Tacon Citation1997; Francis et al. Citation2001; Krogdahl et al. Citation2003). In addition, Drew et al. (Citation2007) stated that it should be paid attention to digestibility and fish development in the use of plant resources. However, algae with high protein content and high production rate have become the centre of attraction due to the possibility of these aquatic plants as an alternative protein source for aquaculture sector. The average protein level in macroalgae is around 8–15% dry matter, while the average lipid content is only 1–3%. Nakagawa and Montgomery (Citation2007) revealed that protein and lipid contents of microalgae were 30–50% and 40%, respectively. The addition of small amounts of algae meal to aquafeeds resulted in considerable effects on growth, feed utilization, lipid metabolism, body composition, stress responses, liver function, disease resistance, and carcass quality (Nakagawa et al. Citation1984; Nakagawa et al. Citation1986; Koh-Ichi Satoh et al. Citation1987; Nakagawa et al. Citation1987; Hashim and Maat-Saat Citation1992; Nakagawa et al. Citation1997).
Chlorella sp. is a single-celled alga that grows in fresh water. It contains 60% protein, essential amino acids, and various vitamins and minerals (Bengwayan et al. Citation2010). Tartiel (Citation2005) found that crude protein, fat and ash contents of Chlorella spp. were 46.7%, 14.8%, and 17.5%, respectively. Nakagawa and Montgomery (Citation2007) reported Spirulina sp. is one of the most frequently used microalgae in aquatic feeds due to its high contents of protein, vitamins, essential amino acids, minerals, essential fatty acids and antioxidant pigments such as carotenoids. Spirulina contains high protein contents (up to 70%) and lipids (7–16%) (Vonshak Citation1997). Spirulina replaced up to 40% of fish meal protein in tilapia (Oreochromis mossambicus) diet (Olvera-Novoa et al. Citation1998) and even higher replacement of fish meal was possible in common carp (Cyprinus carpio) (Nandeesha et al. Citation1998). Several studies have been conducted using dried Spirulina as a feed supplement (Jaime-Ceballos et al. Citation2005; Hanel et al. Citation2007; Dernekbasi et al. Citation2010; Ghaeni et al. Citation2011).
Seaweeds, classified as green, red and brown based on their pigmentation. Kumar et al. (Citation2008) indicated that seaweeds are excellent dietary sources of vitamins, proteins, carbohydrates, minerals and other bioactive compounds. Several studies on the nutritional compositions of seaweeds have been conducted (Wassef et al. Citation2001; Ma et al. Citation2005; Casas-Valdez et al. Citation2006; Valente et al. Citation2006; Ortiz et al. Citation2006; Marsham et al. Citation2007; Matanjun et al. Citation2009). Investigations carried out on seaweeds have shown promising results for the use of seaweeds as partial replacement of fishmeal or protein hydrolysate in aquafeeds. Seaweeds are rich in proteins, vitamins, carbohydrates, fibre, lipids and minerals. When fresh, they are 75–85% water and 15–25% organic components and minerals. Dry matter is 65–85% organic substances and 30–35% ash (FAO Citation2005). Sargassum is a good source of minerals, vitamins, carbohydrates and some amino acids. Yangthong et al. (Citation2014) revealed that Sargassum spp. could be supplemented to the sex-reversed tilapia diet at an optimum level of 5% to improve carcass quality without any adverse effect on growth performance.
But, an important issue concerning microdiets is leaching rates, which vary according to the method of microdiet binding and manufacture. Yufera et al. (Citation2002) determined the rate of different types of amino acid leaching from microbound diet (MBD) and microencapsulated diet (MED). Kvale et al. (Citation2006) reported leaching of protein molecules (9–18 kD) after 5 minutes of immersion in water at a rate of 80–98%, 43–54% and 4–6% for agglomerated, heat coagulated and protein encapsulated microdiet, respectively. Some studies on the leaching ratios of microdiets have been conducted (Heinen Citation1981; Langdon Citation1983; Alabi et al. Citation1999; Baskerville-Bridges and Kling Citation2000; Lopez-Alvarado et al. Citation1994; Ozkızılcık and Cahu Citation1996; Guthrie et al. Citation2000; Hamre Citation2006). Kuscu (Citation2017) showed that microdiets produced in different sizes with the alginate method had high leaching ratios, especially 2532 Da≥. Diken (Citation2017) revealed the molecular weight profiles of algae such as Spirulina sp., Sargassum sp. and Chlorella sp. used in the current study. However, a study on the leaching ratios of microdiets containing feed ingredients such as Spirulina sp., Sargassum sp. and Chlorella sp. is not available.
The purpose of this study was to determine the biochemical compositions and the leaching ratios according to four different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of microdiets containing Spirulina sp., Chlorella sp. and Sargassum sp. in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm).
2. Materials and methods
2.1. Feed ingredients and microdiet production
The individual behaviours of Spirulina sp. (Fuzhou Wonderful Biological Technology Co. Ltd.–China), Chlorella sp.(Akuamaks, Turkey) and Sargassum sp. (Fuzhou Wonderful Biological Technology Co. Ltd.–China) used as direct or indirect in aquaculture sector were tested in the formulations of microdiets produced with alginate methods in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm). Microdiets were produced according to Yufera et al. (Citation2005) ().
Table 1. Microdiet formulations used in the study (%).
2.2. Proximate compositions
Spirulina sp., Sargassum sp. and Chlorella sp. were tested in the current study as alternative feed ingredients and microdiets produced. Proximate compositions, such as ash and protein of feed ingredients and microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm), were determined according to the AOAC (Citation2000) procedures. Also, lipid analyses were performed according to the chloroform–methanol extraction method described by Bligh and Dyer (Citation1959).
2.3. Leaching ratios of feed ingredients and microdiets
The leaching ratios of feed ingredients and microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) were performed according to Boza et al. (Citation1994). Firstly, samples were stirred in the phosphate buffer (pH = 8;10 mg/ml), centrifuged and the supernatant was filtered with 0.22 μm syringe filter and analysed HPLC-Gel Filtration Chromatography for determination of the leaching ratios. Samples were injected in a chromatograph with a TSK-Gel G2000 SWXL column. The eluent was 0.1 mol/sodium sulphate in the 0.1 mol/l phosphate buffer at a flow rate of 1 ml/min, and column effluent was monitored for UV light absorption at 230 nm. Based on the retention time of molecular weight standards, four fractions were defined: 67,000 Da≤, 67,000–13,700 Da, 13,700–2532 Da and 2532 Da≥. The molecular weight standards (from SIGMA) were bovine albumin (67,000 Da), ribonuclease A (13,700 Da), insulin chain A (2532 Da), val-ala-ala-phe (407 Da), tyr-tyr-tyr (508 Da), tryptophan (204 Da), tyrosine (181 Da) and p-aminobenzoic acid (137 Da).
2.4. Statistical methods
In the present study, biochemical compositions and the leaching ratios in four different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of feed ingredients and microdiets (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) produced with the alginate method in the laboratory scale were given as mean ± standard error (SE). The measurements were carried out in triplicates. The experimental data were subjected to one-way (ANOVA) and mean ± standard error (SE) differences by using the SPSS 15.0 statistical package (SPSS Citation1993).
3. Results
shows the proximate compositions of feed ingredients and microdiets. According to , the significant differences between ash, lipid and protein values of feed ingredients and microdiets produced in laboratory scale were observed (p < .05). The lowest and highest ash, lipid and protein values of feed ingredients were 3.29 ± 0.031% (Chlorella sp.)–27.94 ± 0.023% (Sargassum sp.), 0.91 ± 0.024% (Sargassum sp.)–8.91 ± 0.04% (Spirulina sp.) and 20.69 ± 0.07% (Sargassum sp.)–56.98 ± 0.216% (Chlorella sp.), respectively. Sargassum sp. and microdiet groups (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) containing Sargassum sp. had the highest ash values. The lipid contents of microdiets containing Spirulina sp. and Chlorella sp. were higher than those of microdiet containing Sargassum sp. Protein contents of feed ingredients such as Spirulina sp., Chlorella sp. and Sargassum sp. used in the present study were 52.87 ± 0.193%, 56.98 ± 0.216% and 20.69 ± 0.078%, respectively.
Table 2. Proximate compositions of feed ingredients and microdiets (mean ± SE).
Based on the retention time of molecular weight standards, four fractions for the leaching ratios were defined: 67,000 Da≤, 67,000–13,700 Da, 13,700–2532 Da and 2532 Da≥. The differences between the leaching ratios observed in four different times of microdiet groups containing Spirulina sp., Chlorella sp. and Sargassum sp. produced with the alginate method in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) were statistically significant (p < .05).
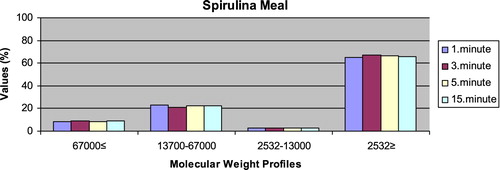
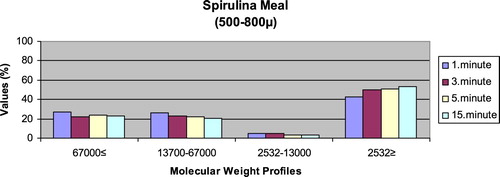
– reveal the leaching ratios of Spirulina sp. and microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) containing Spirulina sp.,. The highest and lowest leaching ratios in four different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of Spirulina sp. were observed in 2532 Da≥ and 2532–13,000 Da, respectively. The 67,000 Da≤ leaching ratios in different sizes of microdiets containing Spirulina sp. tended to increase. However, the 2532 Da≥ leaching ratios in different sizes of microdiets containing Spirulina sp. were decreased.
Figure 2. Leaching ratios in different times of microdiet (100–200 μm) containing Spirulina meal as feed ingredient (%).
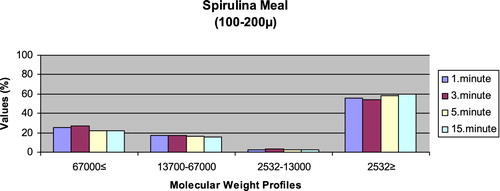
Figure 3. Leaching ratios in different times of microdiet (200–300 μm) containing Spirulina meal as feed ingredient (%).
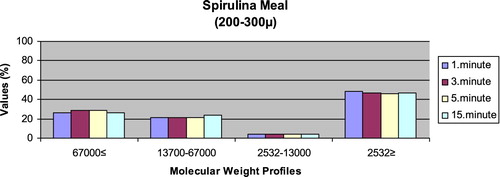
Figure 4. Leaching ratios in different times of microdiet (300–500 μm) containing Spirulina meal as feed ingredient (%).
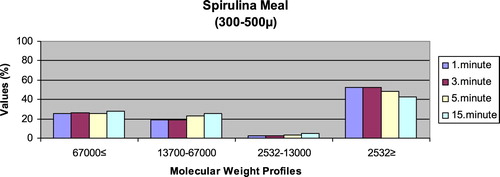
Figure 5. Leaching ratios in different times of microdiet (500–800 μm) containing Spirulina meal as feed ingredient (%).
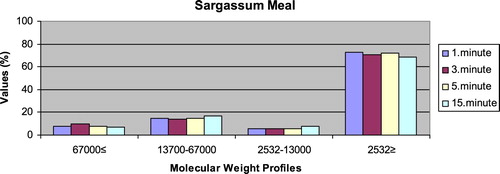
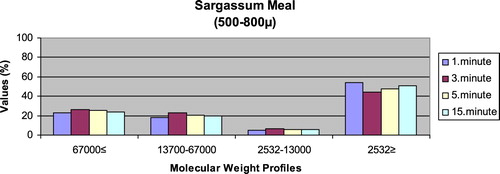
– show the leaching ratios of Sargassum sp. and microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) containing Sargassum sp. The highest and lowest leaching ratios in four different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of Sargassum sp. were observed in 2532 Da≥ and 2532–13,000 Da, respectively. The 67,000 Da≤ and 13,700–67,000 Da leaching ratios in different sizes of microdiets containing Sargassum sp. tended to increase. However, the 2532 Da≥ leaching ratios in different sizes of microdiets containing Sargassum sp. were decreased. According to the leaching ratios observed in four different times, the highest and lowest values for microdiets containing Spirulina sp. and Sargassum sp. produced in laboratory scale (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) were 2532 Da≥ and 2532–13,000 Da, respectively. The 67,000 Da≤ and 13,700–67,000 Da leaching ratios in different sizes of microdiets containing Sargassum sp. tended to increase. However, the 67,000 Da≤ leaching ratios in different sizes of microdiets containing Spirulina sp. tended to increase.
Figure 7. Leaching ratios in different times of microdiet (100–200 μm) containing Sargassum meal as feed ingredient (%).
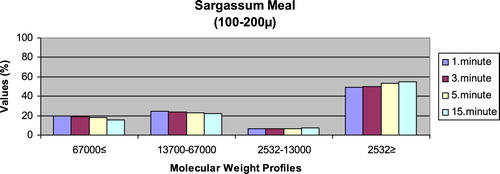
Figure 8. Leaching ratios in different times of microdiet (200–300 μm) containing Sargassum meal as feed ingredient (%).
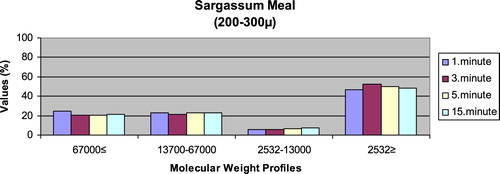
Figure 9. Leaching ratios in different times of microdiet (300–500 μm) containing Sargassum meal as feed ingredient (%).
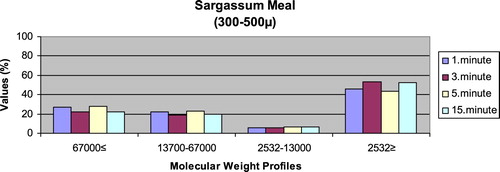
Figure 10. Leaching ratios in different times of microdiet (500–800 μm) containing Sargassum meal as feed ingredient (%).
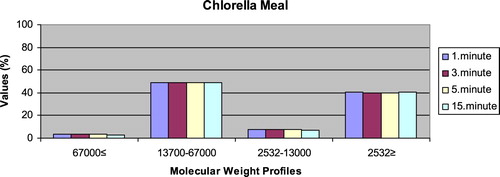
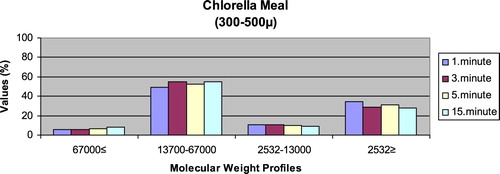
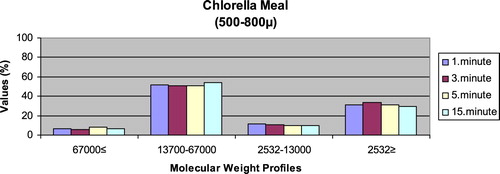
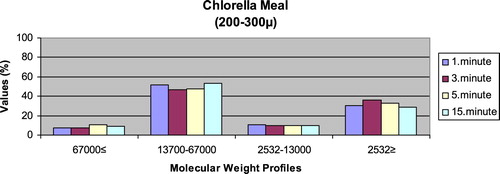
– give the leaching ratios of Chlorella sp. and microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) containing Chlorella sp.. The highest and lowest leaching ratios in four different times (1 minute, 3 minutes, 5 minutes and 15 minutes) of Chlorella sp. were observed in 13,700–67,000 Da and 67,000 Da≤, respectively. According to the leaching ratios observed in four different times, the highest and lowest values for microdiets containing Chlorella sp. produced in laboratory scale (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) were 13,700–67,000 Da and 67,000 Da≤, respectively. The 13,700–67,000 Da leaching ratios of microdiets containing Chlorella sp. tended to increase according to the leaching ratio of Chlorella sp. However, the 2532Da≥ leaching ratios of microdiets containing Chlorella sp. tended to decrease.
Figure 12. Leaching ratios in different times of microdiet (100–200 μm) containing Chlorella meal as feed ingredient (%).
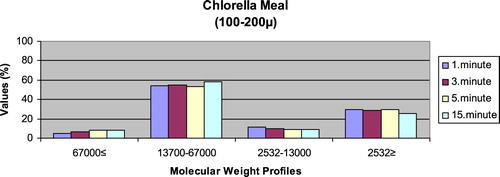
Figure 13. Leaching ratios in different times of microdiet (200–300 μm) containing Chlorella meal as feed ingredient (%).
4. Discussion
Until now, the knowledge about the leaching ratios of microdiets containing Spirulina sp., Chlorella sp. and Sargassum sp. is not available. In the present study, the leaching ratios of microdiets produced in different sizes with the alginate method were revealed by HPLC-Gel Filtration Chromatography. Also, we determined the ash, lipid and protein contents of microdiets and feed ingredients.
Results revealed that ash, lipid and protein levels of feed ingredients used in the ration reflected the biochemical compositions of microdiets produced with the alginate method in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm). Kuşcu (Citation2017) reported that the biochemical compositions of microdiets produced in the laboratory scale with the alginate method were similar to those of commercial microdiets tested. When the biochemical compositions of feed ingredients such as Spirulina sp., Sargassum sp. and Chlorella sp. were compared with microdiets produced with the alginate method, the alginate method was more successful in terms of reflecting the biochemical compositions of the individual feed ingredients tested. In addition, the study data showed that Chlorella sp. and Spirulina sp. provided important contributions to the high protein and lipid levels to microdiets produced with the alginate method, while Sargassum sp. used in the study provided important contributions with high ash levels to microdiets.
Kuşcu (Citation2017) revealed that the highest leaching ratios of microdiets produced with the alginate method were observed in 2532 Da≥. In the current study, the highest leaching ratios of microdiets containing Spirulina sp. and Sargassum sp. supported the results reported by Kuscu (Citation2017) but not the leaching ratios of microdiets containing Chlorella sp. Also, Kuşcu (Citation2017) showed that the highest and lowest molecular weight distributions of microdiets produced with the alginate method in the study reflected the leaching ratios at different times. Molecular weight distributions belonging to the algae such as Spirulina sp., Sargassum sp. and Chlorella sp. reported by Diken (Citation2017) were similar to the leaching ratios of feed ingredients such as Spirulina sp., Sargassum sp. and Chlorella sp. tested in the current study. According to the results obtained in the study, the highest leaching ratios of Chlorella sp. and microdiets containing Chlorella sp. were 13,700–67,000 Da. The 13,700–67,000 Da leaching ratio tended to increase in microdiets containing Chlorella sp. However, the 2532 Da≥ leaching ratios tended to decrease according to the leaching ratio of Chlorella sp. The results observed in algae tested and microdiets containing algae supported the relationships between the leaching ratios and molecular weight distributions of feed ingredients and microdiets.
Free amino acids (FAAs) seem to improve the performance of larvae when supplied at low levels in feeds, but not a surplus of FAAs (Szlaminska et al. Citation1993, Carvalho et al. Citation1997, Cahu et al. Citation1999, Cahu and Zambonino Infante Citation1995, Lopez-Alvarado and Kanazawa Citation1995). The faster absorption of FAAs compared to protein-bound amino acids may lead to amino acid (AA) imbalances in the larval intestine and followed a decrease in protein utilization (Ronnestad et al. Citation2000). Zambonino Infante et al. (Citation1997) showed that the partial substitution of whole protein by di- and tripeptides effectively improved larval development. Carvalho et al. (Citation2003) indicated that protein macromolecules are digested into peptides and AAs in the intestine and then, di- and tripeptides are rapidly converted into AAs for absorption, a balance among the mentioned peptide groups seems to be important to optimize the protein utilization. Carvalho et al. (Citation2003) suggested that the supplementation of diets with suitable levels of protein hydrolysates must be considered in order to achieve this purpose. In addition, it is suggested that artificial diets for fish larvae should have a molecular weight profile similar to that found in live food.
In conclusion, the alginate method had good performance because the biochemical compositions of microdiets produced in different sizes (100–200 μm, 200–300 μm, 300–500 μm and 500–800 μm) reflected the ash, lipid and protein levels of algae tested. The study data revealed that Chlorella sp. and Spirulina sp. provided important contributions with the high protein and lipid levels to microdiets produced with the alginate method, while Sargassum sp. used in the study provided important contributions with high ash levels to microdiets. Considering these data, it could be advised to use at moderate levels as ash, lipid and protein source instead of fish meal in aquafeeds. Results cautioned that the use of Spirulina sp. and Sargassum sp. in microdiet formulations may cause the high leaching ratios containing 2532 Da≥ molecular weight. However, the use of Chlorella sp. in ration results in the high leaching ratios containing 13,700–67,000 Da molecular weight. When such data are available, the knowledge about leaching ratios of microdiets could be used to optimize feeding time in the larvae tank.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alabi AO, Cob ZC, Jones DA, Latchford JW. 1999. Influence of algal exudates and bacteria on growth and survival of white shrimp larvae fed entirely on microencapsulated diets. Aquaculture Int. 7:137–158. doi: 10.1023/A:1009257902630
- Alexis MN. 1997. Fish meal and fish oil replacers in Mediterranean marine fish diets. In: Tacon AGJ, Basurco B, editors. Feeding tomorrow’s fish Cahiers options Mèditerranéennes, vol. 22. Zaragoza: Ciheam-IAMZ; p. 183–204.
- AOAC. 2000. Official methods of analysis of association of analytical chemist. 15th ed. Washington, DC.
- Baskerville-Bridges B, Kling LJ. 2000. Development and evaluation of microparticulate diets for early weaning of Atlantic cod (Gadus morhua) larvae. Aquaculture Nutr. 6:171–182. doi: 10.1046/j.1365-2095.2000.00149.x
- Bengwayan PT, Laygo JC, Pacio AE, Poyaoan JLZ, Rebugio JF, Yuson ALL. 2010. A comparative study on the antioxidant property of Chlorella (Chlorella sp.) tablet and glutathione tablet. E-Int Sci Res J. 2(1):25–35.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37:911–917. doi: 10.1139/y59-099
- Boza JJ, Jimenez J, Martínez O, Suarez MD, Gil A. 1994. Nutritional value and antigenicity of two milk protein hydrolysates in rats and Guinea pigs. J Nutr. 124:1978–1986. doi: 10.1093/jn/124.10.1978
- Cahu CL, Zambonino Infante JL. 1995. Effect of the molecular form of dietary nitrogen supply in Sea bass larvae: response of pancreatic enzymes and intestinal peptidases. Fish Physiol Biochem. 14:209–214. doi: 10.1007/BF00004311
- Cahu CL, Zambonino Infante JL, Quazuguel P, Le Gass MM. 1999. Protein hydrolysate vs. fish meal in compound diets for 10-day old sea bass (Dicentrarchus labrax) larvae. Aquaculture. 171:109–119. doi: 10.1016/S0044-8486(98)00428-1
- Carvalho AP, Escaffre AM, Oliva-Teles A, Bergot P. 1997. First feeding of common carp larvae on diets with high level of protein hydrolysates. Aquaculture Int. 5:361–367. doi: 10.1023/A:1018368208323
- Carvalho AP, Oliva-Teles A, Bergot P. 2003. A preliminary study on the molecular weight profile of soluble protein nitrogen in live food organisms for fish larvae. Aquaculture. 225:445–449. doi: 10.1016/S0044-8486(03)00308-9
- Casas-Valdez M, Portillo-Clark G, Aguila-Ramırez N, Rodrıguez- Astudillo S, Sanchez-Rodrıguez I, Carrillo-Domınguez S. 2006. Effect of the marine algae Sargassum spp. on the productive parameters and cholesterol content of the brown shrimp, Farfantepenaeus californiensis, (Holmes, 1900). Rev Biol Mar Oceanogr. 41:97–105. doi: 10.4067/S0718-19572006000100012
- Dernekbasi S, Unal H, Karavucel I, Aral O. 2010. Effect of dietary supplementation of different rates of Spirulina (Spirulina platensis) on growth and feed conversion in guppy (Poecilia reticulata Peters, 1860). J Anim Veter Adv. 9:1395–1399. doi: 10.3923/javaa.2010.1395.1399
- Diken G. 2017. The use of some animal and vegetable protein sources in the microdiets of meagre (Argyrosomus regius Asso, 1801) larvae, 454 pp. PhD Thesis. Isparta: Institute of Science.
- Drew MD, Borgeson TL, Thiessen DL. 2007. A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim Feed Sci Technol. 138:118–136. doi: 10.1016/j.anifeedsci.2007.06.019
- FAO. 2002. The state of the world fisheries and aquaculture. Rome: FAO.
- FAO. 2005. Aquaculture production statistics. Rome: FAO.
- Francis G, Makkar HPS, Becker K. 2001. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 199(3-4):197–227. doi: 10.1016/S0044-8486(01)00526-9
- Ghaeni M, Matinfar A, Soltani M, Rabbani M, Vosoughi A. 2011. Comparative effects of pure Spirulina powder and other diets on larval growth and survival of green tiger shrimp, Peneaus semisulcatus. Iranian J Fish Sci. 10:208–217.
- Guthrie KM, Rust MB, Langdon CJ, Barrows FT. 2000. Acceptability of various microparticulate diets to first-feeding walleye Stizostedion vitreum larvae. Aquaculture Nutr. 6:153–158. doi: 10.1046/j.1365-2095.2000.00104.x
- Hamre K. 2006. Nutrition in cod (Gadus morhua) larvae and juveniles. ICES J Mar Sci. 63(2):267–274. doi: 10.1016/j.icesjms.2005.11.011
- Hanel R, Broekman D, de Graaf S, Schnack D. 2007. Partial replacement of fishmeal by lyophilized powder of the microalgae Spirulina platensis in Pacific White Shrimp Diets. Open Mar Biol J. 1:1–5. doi: 10.2174/1874450800701010001
- Hashim R, Maat-Saat A. 1992. The utilizations of seaweed meal as binding agents in pelleted feeds for sneakhead (Channa striatus) fry and their effects on growth. Aquaculture. 108:299–308. doi: 10.1016/0044-8486(92)90114-Z
- Heinen JM. 1981. Evaluation of some binding agents for crustacean diets. Progressive Fish Culturist. 43(3):142–145. doi: 10.1577/1548-8659(1981)43[142:EOSBAF]2.0.CO;2
- Higgs DA, Dosanjh BS, Prendergast AF, Beams RM, Hardy RW, Riley W, Deacon G. 1995. Use of rapeseed/canola protein products in finfish diets. In: Sessa DJ, editor. Nutrition and utilization of technology in aquaculture. Champaign, IL: AOCS Press; p. 130–156.
- Jaime-Ceballos B, Villarreal H, Garcia T, Perez-Jar L, Alfonso E. 2005. Effect of Spirulina platensis meal as feed additive on growth, survival and development in Litopenaeus schmitti shrimp larvae. Rev Invest Mar. 26:235–241.
- Koh-ichi Satoh K, Nakagawa H, Kasahara S. 1987. Effect of Ulva supplementation on disease resistance of red sea bream (Pagrus major). Nippon Suisan Gakkaishi. 53:1115–1120. doi: 10.2331/suisan.53.1115
- Krogdahl A, Bakke-McKellep AM, Baevefjordi G. 2003. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L. Aquaculture Nutr. 9:361–371. doi: 10.1046/j.1365-2095.2003.00264.x
- Kumar CS, Ganesan P, Suresh PV, Bhaskar N. 2008. Seaweeds as a source of nutritionally beneficial compounds-a review. J Food Sci Technol. 45:1–13.
- Kuscu MC. 2017. The determination of nutritional losses of microdiets produced with two different manufacturing methods and commercial microdiets commonly used in the feeding of marine fish larvae, 69 pp. Master’s Thesis. Hatay: Institute of Engineering and Science.
- Kvale A, Yufera M, Nygard E, Aursland K, Harboe T, Hamre K. 2006. Leaching properties of three different micropaticulate diets and preference of the diets in cod (Gadus morhua L.) larvae. Aquaculture. 251:402–415. doi: 10.1016/j.aquaculture.2005.06.002
- Langdon CJ. 1983. New techniques and their application to studies of bivalve nutrition. In: Pruder GD, Langdon CJ, Conklin D, editors. Biochemical and physiological approaches to shellfish nutrition. Proceedings of the second international conference on aquaculture nutrition, October 1981, Spec. Publ. 2. Rehoboth Beach: World Mariculture Society; p. 305–320.
- Lopez-Alvarado L, Kanazawa A. 1995. Optimum levels of crystalline amino acids in diets for larval Red Sea Bream (Pagrus major). ICES Marine Science Symposia 201:100–105.
- Lopez-Alvarado J, Langdon CJ, Teshima S, Kanazawa A. 1994. Effects of coating and encapsulation of crystalline amino acids on leaching in larval feeds. Aquaculture. 122:335–346. doi: 10.1016/0044-8486(94)90342-5
- Lovell T. 1998. Nutrition and feeding of fish. Massachusetts: Kluwer Academic Publishers.
- Ma WCJ, Chund HY, Ang P, Kim PS. 2005. Enhancement of bromophenol levels in aquacultured silver seabream (Sparus sarba). J Agric Food Chem. 53:2133–2139. doi: 10.1021/jf048390m
- Marsham S, Scott GW, Tobin ML. 2007. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 100:1331–1336. doi: 10.1016/j.foodchem.2005.11.029
- Matanjun P, Mohamed S, Mustapha NM, Muhammad K. 2009. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol. 21:75–80. doi: 10.1007/s10811-008-9326-4
- Nakagawa H, Kasahara S, Sugiyama T. 1987. Effect of Ulva meal supplementation on lipid metabolism of black sea bream, Acanthopagrus schlegeli (Bleeker). Aquaculture. 62:109–121. doi: 10.1016/0044-8486(87)90315-2
- Nakagawa H, Kasahara S, Sugiyama T, Wada I. 1984. Usefulness of Ulva meal as feed supplementary in cultured Black Sea bream. Suisan Zoshoku. 32:20–27.
- Nakagawa H, Kumai H, Nakamura M, Nanba K, Kasahara S. 1986. Preventive effect of kelp meal supplement on nutritional disease due to sardine feeding in cultured yellow tail, (Seriola quinqueradiata) (Pisces). Proc. 4th Symp Trace Nutrients Res. Kyoto, Japan, 3:31–37.
- Nakagawa H, Montgomery WL. 2007. Algae. In: Nakagawa H, Sato S, Gatlin III D, editors. Dietary supplements for the health and quality of cultured fish. Cambridge, MA: CABI North American Office; p. 133–168.
- Nakagawa H, Umino T, Tasaka Y. 1997. Usefulness of Ascophyllum meal as a feed additive for red sea bream (Pagrus major). Aquaculture. 151:275–281. doi: 10.1016/S0044-8486(96)01488-3
- Nandeesha MC, Gangadhara B, Varghese TJ, Keshavanath P. 1998. Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio. Aquaculture Res. 29:305–312. doi: 10.1111/j.1365-2109.1998.tb01135.x
- Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K, Nichols PD. 2009. Feeding aquaculture in an era of finite resources. PNAS. 106(36):15103–15110. doi: 10.1073/pnas.0905235106
- Olvera-Novoa MA, Dominguez-Cen LJ, Olivera-Castillo L, Martínez-Palacios CA. 1998. Effect of the use of the microalgae Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters), fry. Aquaculture Res. 29:709–715. doi: 10.1046/j.1365-2109.1998.29100709.x
- Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernandez J, Bozzo C, Navarrete E, Osorio A, Rios A. 2006. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 99:98–104. doi: 10.1016/j.foodchem.2005.07.027
- Ozkizilcik S, Cahu FE. 1996. Preparation and characterization of a complex microencapsulated diet for striped bass Morone saxatilis larvae. J Microencapsul. 13:331–343. doi: 10.3109/02652049609026020
- Ronnestad I, Conceicao LEC, Arago C, Dinis MT. 2000. Free amino acids are absorbed faster and assimilated more efficiently than protein in postlarval Senegal sole (Solea senegalensis). J Nutr. 130:2809–2812. doi: 10.1093/jn/130.11.2809
- SPSS. 1993. SPSS for windows base system user’s guide, release 8.0.2. Chicago, IL.
- Szlaminska M, Escaffre AM, Charlon N, Bergot P. 1993. Preliminary data on semi synthetic diets for goldfish (Carassius auratus L.) larvae. In: Kaushik SJ, Luquet P, editors. Fish nutrition in practice. Paris: INRA; p. 607–612. Les colloques, 61.
- Tacon AGJ. 1993. Feed ingredients for warmwater fish: fish meal and other processed feedstuffs. FAO Fisheries Circular No. 856, 64 pp., Rome.
- Tacon AGJ. 1997. Fishmeal replacers: review of antinutrients within oilseeds and pulses. A limiting factor for the aquafeed green revolution? In: Tacon AGJ, Basurco B, editors. Feeding tomorrow’s fish. CHIEM-Cahiers Options Meditérranéennes, vol. 22; p. 153–182.
- Tartiel M B. 2005. Physiological studies on some green algae. Ph.D. thesis, Faculty of Agriculture, Cairo University, Egypt.
- Valente LMP, Gouveia A, Rema P, Matos J, Gomes EF, Pinto IS. 2006. Evaluation of three seaweeds Gracilaria bursapastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass Dicentrarchus labrax juveniles. Aquaculture. 252:85–91. doi: 10.1016/j.aquaculture.2005.11.052
- Vonshak A. 1997. Spirulina platensis (Arthospira): Physiology, Cell Biology and Biotechnology. London: Taylor and Francis, 540 pp.
- Wassef EA, El-Masry MH, Mikhail FR. 2001. Growth enhancement and muscle structure of striped mullet Mugil cephalus L. fingerlings by feeding algal meal-based diets. Aquaculture Res. 32:315–322. doi: 10.1046/j.1355-557x.2001.00043.x
- Yangthong M, Oncharoen S, Sripanomyom J. 2014. Effect of Sargassum Meal Supplementation on Growth Performance of Sex-Reversed Tilapia (Oreochromis niloticus Linn.) Agricultural sciences: leading Thailand to world class standards. Proceedings of the 52nd Kasetsart University Annual Conference, 4-7 February 2014, Kasetsart University, Thailand. Vol. 3: Fisheries, Agricultural Extension and Home Economics, pp. 234–241.
- Yufera M, Fernández-Díaz C, Pascual E. 2005. Food microparticles for larval fish prepared by internal gelation. Aquaculture. 248:253–262. doi: 10.1016/j.aquaculture.2005.04.026
- Yufera M, Kolkovski S, Fernandez-Diaz C, Dabrowski K. 2002. Free amino acid leaching from protein-walled microencapsulated diet for fish larvae. Aquaculture. 214:273–287. doi: 10.1016/S0044-8486(01)00902-4
- Zambonino Infante JL, Cahu CL, Peres A. 1997. Partial substitution of di-and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J Nutr. 127:608–614. doi: 10.1093/jn/127.4.608