ABSTRACT
Microbial contamination in shopping trolleys (eighty five) by considering different supermarkets (seven) from three major food companies in Las Palmas de Gran Canaria (Spain) was determined.
The two sampled areas were trolley handles and food trolley baskets-child seats. Samples were analyzed by selective and differential microbiological culture media.
E. coli four (2.4%) indicative of faecal contamination, Klebsiella pneumoniae twelve (6.5%) and Citrobacter freundii, six (5.1%), which have been isolated from human faecal samples, were isolated from trolleys; Pseudomonas rhodesiae, five (4.25%), and Pseudomonas fluorescens, three (2.55%), which both evidenced environmental contamination. Significant differences among the companies were found for the Enterobacteriaceae and coliforms. Regarding location, these differences (p < 0.003) were observed only for the coliform rates, which were higher in trolleys located outside.
The results of this study suggest the implementation of cleaning and disinfection programmes to improve trolley sanitation, and to reduce exposure to both potential pathogenic and transmitting bacterial infections.
Introduction
Inanimate objects (fomites) for public use, such as shopping trolley handles, lift buttons, handrails, etc, which come into direct contact with users’ hands, are a source of contamination of potential pathogenic microorganisms. They come into contact either directly by surface-to-mouth contact or indirectly by contaminated fingers and subsequent hand-to-mouth contact (Gerba and Maxwell Citation2012; Irshaid et al. Citation2014). Some studies have reported frequent exposure to pathogenic Staphylococcus aureus on shopping trolley handles, suggest that it is a hidden reservoir of this organism, and indicate a shopping basket/trolley sanitation necessity (Mizumachi et al. Citation2011). Shopping trolley contamination may occur from directly handling raw food products or trolleys contaminated by previous users (Gerba and Maxwell Citation2012). Nevertheless, cross-contamination in shopping trolley baskets occurs when disease-causing microorganisms are transferred from one food type to surfaces or, as in this case study, when dirty hands transfer microorganisms to trolley handles or baskets. For example, raw meat products are often contaminated with foodborne bacteria, such as Salmonella and Campylobacter (Bier et al. Citation2004), which may be transferred to surfaces. However, the level of bacterial contamination on shopping trolleys or shopping baskets is limiting making health assessment difficult (Mizumachi et al. Citation2011). It is believed that up to 80% of common infections can be spread through coming into contact with contaminated surfaces (Reynolds et al. Citation2005). Pathogenic organisms, i.e. viruses, bacteria and protozoa, may be excreted in large numbers in biological substances, including blood, mucus, saliva, faeces and urine (Hall and Douglas Citation1981; Hall et al. Citation1981; Feachem et al. Citation1983; Uhnoo et al. Citation1990; Weber et al. Citation1994; Islam et al. Citation2001). Some microbes are infectious at very low concentrations and can survive on different surfaces like countertops and telephone handpieces for hours, and even for weeks (Noskin et al. Citation1995; Bures et al. Citation2000; Abad et al. Citation2001).
Hands are frequently involved in such episodes, and act as vehicles that spread infections in both the community and hospitals (Pittet et al. Citation2000). Several studies have been published about microorganism contagion from contaminated fomites with pathogens to healthcare workers’ hands in hospitals (Kramer et al. Citation2006; Mizumachi et al. Citation2011). However, a correlation between the burden of contamination on hands and the likelihood of transmission to patients has not yet been established (Bellissimo-Rodrigues et al. Citation2017). Nonetheless, common infectious diseases still feature among the top 10 leading causes of death (Liu et al. Citation2012; Willmott et al. Citation2016), and healthcare-associated infections are recognized as a major cause of preventable death in healthcare settings (Willmott et al. Citation2016).
Regarding bacterial persistence on surfaces, enterococci species are able to survive for 24 h with no significant reduction in colony counts. Most gram-positive bacteria, such as Enterococcus spp. (including VRE), S. aureus (including MRSA) or Streptococcus pyogenes, survive for months on dry surfaces (Kramer et al. Citation2006). Many gram-negative species can also survive for months, such as Acinetobacter spp., Escherichia coli, Klebsiella spp., Pseudomonas aeruginosa, Serratia marcescens or Shigella spp. A few others, like Bordetella pertussis, Haemophilus influenzae, Proteus vulgaris or Vibrio cholerae, persist only for days (Kramer et al. Citation2006).
Interestingly, vancomycin-resistant enterococci are capable of prolonged survival on hands, gloves and environmental surfaces, they persist for 60 min on telephone handpieces and for 30 min on the diaphragmatic surface of a stethoscope (Noskin, et al. Citation1995). Other authors (Wade et al. Citation1991) have documented the survival of vancomycin-resistant E. faecium on hands for up to 30 min and bacteria such as S. aureus, Acinetobacter, Klebsiella aerogenes, Escherichia coli, Serratia marcescens, and Pseudomonas aeruginosa (Casewell and Desai Citation1983). Hence hand washing is considered an essential aspect of infection control to prevent microorganisms from being transmitted. A 30-second wash with soap and water is necessary to completely eradicate bacteria from hands (Noskin et al. Citation1995).
The increased availability of shopping trolleys in supermarkets, handled by numerous users on a daily basis, and the fact that trolleys are not routinely disinfected, are a potentially excellent opportunity for contaminating microorganisms to be transmitted (Anderson and Palombo Citation2009). This is particularly true for infants and children under the age of 5 years with reported 2- to 10-fold higher risk rates than for people aged 5 years or more (Jones et al. Citation2006; Ailes et al. Citation2008).
Riding infants in a shopping trolley next to packaged raw meat and poultry has been shown to be an important risk factor for Salmonella spp. and Campylobacter spp. infection, with attributable risks of 11% and 7%, respectively (Jones et al. Citation2006; Fullerton et al. Citation2007; Patrick et al. Citation2010) as these microorganism have been isolated from outer packages of meat and poultry products at retail outlets (Harrison et al. Citation2001; Wong et al. Citation2004; Burgess et al. Citation2005).
The aim of this study was to determine the microbial contamination and bacterial species on shopping trolley handles and baskets in different supermarkets on the Gran Canaria Island (Spain). In addition, the statistic relationship between metal and plastic shopping trolleys microbiological contamination was studied, as was the location of trolleys, either outside or inside supermarkets.
Material and methods
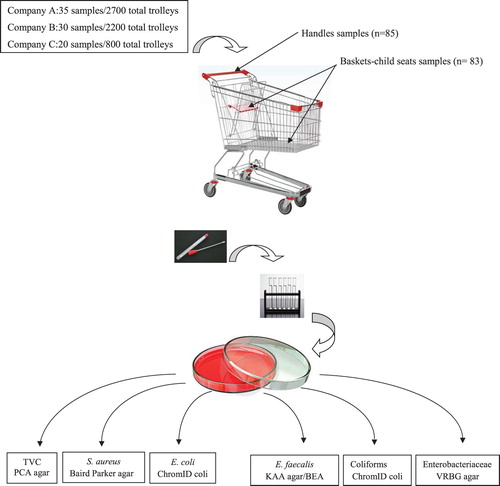
Sampling was performed in three different supermarket companies of Las Palmas de Gran Canaria (A, B, C), the Canary Islands (Spain), for 3 months. Eighty-five shopping trolleys from seven supermarkets were sampled to determine the microbiological contamination on trolleys. We collected 85 swabs from trolley handles and 83 other swabs from the food baskets-child seats (basket-seat). The latter samples were considered from a single site (Figure ).
Sampling the contact surfaces of trolleys
To this end, a maximum 100-cm2 area was sampled per swab. Shopping trolleys were sampled for microbial contamination, and were subsequently tested for the qualitative analyses of pathogenics (Gerba and Maxwell Citation2012). A sterile rayon-tipped swab (Copan Flock Technologies Srl., Brescia, Italy), moistened with sterile saline solution (preservative-free), was moved over the entire surface of trolley handles and a new swap was used over baskets-seats. Swabs were aseptically transferred to a tube that contained 10 ml of sterile 0.1% peptone water (adapted from Salo, et al. Citation2002), and were delivered 2 h after being packed on ice to be sent to the Hygiene Laboratory of Veterinary Faculty of Las Palmas de Gran Canaria University, where samples were processed before 24 h had elapsed.
The total number of shopping trolleys from each sampled company was determined by a statistical stratified analysis, where the following were sampled: 35 (2,700 in all) shopping trolleys in one supermarket of company A; 30 (2,200 in all) shopping trolleys in three supermarkets of company B; 20 (800 in all) shopping trolleys in three supermarkets of company C.
Microbiological analysis and identification
Decimal dilutions in peptone water solution (0.85% NaCl with 0.1% peptone; Cultimed, Barcelona, Spain) were used for microbial enumeration purposes. Tubes were shaken vigorously. Appropriate dilutions were prepared by using sterile 0.1% peptone water and were plated by the pour plate method on different bacteria selection agar (Figure ). After incubating the plate, the morphological characteristics of microorganisms were associated with each growth medium. Data are reported as colony-forming units (CFU/cm2). All the counts were taken in duplicate.
Total viable counts (TVCs) and mesophilic bacteria were determined using Plate Count Agar (PCA Cultimed, 413799), and were incubated at 31°C for 72 h (Pascual and Calderón Citation2002; Broekaert et al. Citation2011). Enterobacteriaceae were determined using Violet Red Bile Glucose Agar (VRBG), (Cultimed, 413745, Barcelona Spain). Incubation was done at 37°C for 24 h. Bacteria were represented as large colonies with purple haloes, as described by other authors (Pascual and Calderón Citation2002).
S. aureus was isolated by Baird Parker+Rabbit Plasma Fibronogen agar (bioMerieux, Marcy ĺEtoile, France; ISO 6888–2; ISO, 1999), and was incubated at 37°C for 24–48 h. Escherichia coli was identified by ChromID coli® (bioMerieux; AFNOR, 2014) and was incubated at 37°C for 24–48 h.
E. faecalis was determined in kanamycin-esculin-azide broth (KAA) (Canamicina Esculina Azida, Cultimed, 464695.0922), and was incubated at 35°C for 48 h following the manufacturer's instructions. Positive growth was considered if the tube changed to blackish-green. E. faecalis was spread on KAA agar and incubated at 35°C for 24–48 h. Finally, grown colonies were confirmed as E. faecalis when they were gram-positive cocci with a negative catalase test, and were able to grow on bile esculin agar (BEA) incubated at 42°C by esculin hydrolysis (Greenberg et al. Citation1992; Dionisio and Borrego Citation1995).
The microorganisms isolated from VRBG plates were identified using gram stain, colony counts, morphology, and catalase and oxidase reactions. Gram-negative bacteria were identified by API 20 E and mass spectrometry in a Bruker Biotyper matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDITOF MS) system (Bruker Daltonics, Germany) with a higher score level than 2,200 for species identification.
The Analytical Profile Index (API) 20 E test kit (BioMerieux®, Marcy ĺEtoile, France) was used following the manufacturer's instructions. Strips were examined after 24 and 48 h. Isolates were identified according to the API 20 E identification online instructions (http://apiweb.biomerieux.com).
There are no standards available for fomites surface counts. However, a general microbial target value of <2.5 CFU/cm2 after disinfection has been found to be attainable for a range of surfaces in food industries (Carrascosa et al. Citation2012). The preliminary data obtained in this study showed that the total viable counts in non-disinfected objects were considerably higher. Even so, were obtained an average of 753 CFU/cm2, a minimum of 80 CFU/cm2 and a maximum of 18,700 CFU/cm2.
Statistical analysis
The contamination rates for the considered microorganisms were summarized as frequencies and percentages, and were compared by the Chi-square (χ 2) or the exact Fisher test whenever appropriate. Odd ratios by means of 95% confidence intervals (95%CI) were used for the comparisons that showed statistical significance. Statistical significance was set at p < .05. Data were analyzed with the R package, version 3.3.1 (R Development Core Team Citation2016).
Results
Eighty-five shopping trolleys were sampled, and the enteric bacteria species on handles and baskets-child seats were isolated. Shopping trolley handles were found to be contaminated by enterobacteria on thirty five (41.17%) surfaces and on forty three (50.6%) baskets-child seats. Coliforms were growing on handles on twenty two (25.9%) trolleys and on thirty nine (45.9%) baskets-child seats. E. coli was identified only on three (2.55%) basket-child seats. Neither S. aureus nor E. faecalis was detected by specific agar medium.
Table contains the contamination rates of the sampled surfaces of the different supermarket companies (A, B, C). This table also summarizes the contamination rates according to supermarket companies, location and material (Table ). Company B showed significantly lower contamination rates than those obtained for Company A for enterobacteria on handles (23.3% vs. 62.9%), coliforms on handles (20% vs. 56%), enterobacteria on baskets (30% vs. 68.6%) and coliforms on baskets (33.3% vs. 60%). All the results obtained from Companies A, B and C showed significant differences for the coliforms and enterobacteria rates on both surfaces. E. coli on baskets was detected only on one Company A trolley, whereas S. aureus and E. faecalis were not detected on any analyzed trolley from companies A, B, C.
Table 1. Contamination rates of surfaces at different supermarket companies (A, B, C) locations and shopping trolley materials.
Table 2. Contamination rates of surfaces at different locations and shopping trolley materials.
For the relationship of the location (outside or inside) of shopping trolleys in supermarkets and their contamination, we found significant differences, but only for the coliforms rates (56.0% vs. 20.0%, p = .003) on handle surfaces. Contamination was higher outside than it was inside (Table ). Likewise, the comparison made of the plastic and metal material used to manufacture shopping trolleys showed no significant differences (Table ). The highest contamination rates on handles and on basket-child seats were on plastic material, except for coliforms contamination on handles, which was higher for metal trolleys (Table ).
In addition, E. coli and other potential pathogenic bacteria were also isolated from both surfaces, but showed different rates. The isolations of the bacteria that belonged to the Enterobactericeae family were particularly interesting, including Klebsiella pneumoniae isolated from 11 (6.5%) shopping trolleys, and Citrobacter freundii isolated from 6 (5.1%), which may be found in human faeces. Pseudomonas rhodesiae and P. fluorescens were isolated from five (4.25%) and three (2.55%) shopping trolleys, respectively, and these bacteria evidenced environmental contamination. Table shows other bacteria of special interest that were isolated from trolleys.
Table 3. Relation of the bacteria of special interest isolated from trolleys identified with MALDITOF.
Discussion
Microbiological counts in the analyzed shopping trolleys from different supermarkets showed a high contamination rate on both sampled surfaces, which was slightly higher on baskets-child seats. Most studies about fomites contamination have been undertaken on hospital equipment surfaces (Kramer et al. Citation2006; Bellissimo-Rodrigues et al. Citation2017), but very few studies have determined contaminated shopping trolleys (Al-Ghamdi et al. Citation2011; Ashgar and El-Said Citation2012; Gerba and Maxwell Citation2012; Irshaid et al. Citation2014), which indicates that consumers are exposed to enteric bacteria from grocery shopping trolleys on a regular basis. In our study, total bacterial levels were far higher than those found in public restrooms and other public places (airports, bus stations, public bathroom, shopping malls, etc.) reported by other authors (Gerba and Maxwell Citation2012). Those studies had sampled different surfaces with the same swab (handles-child seat) and obtained a single result for all the surfaces.
In the present study, we found up to 45.9% of coliforms and 2.45% of E. coli, whereas Gerba and Maxwell (Citation2012) found higher rates (72% and 21.17%, respectively). Similar results were shown by Reynolds et al. (Citation2005) and Al-Ghamdi et al. (Citation2011) and who determined 20% of coliform and 7% of faecal coliform contamination, respectively. Regarding contamination sources, Reynolds et al. (Citation2005) found no relationship to link biochemical markers, protein and bacterial contamination on public surfaces, including shopping trolley handles. While the presence of biochemical markers and protein provides information on the relative hygiene of various environments, very little is known about their correlation with infectious microbes (Reynolds et al. Citation2005). Nonetheless, other authors have described longer microbial persistence with higher inoculum in the presence of protein (Neely Citation2000), serum (Elmos Citation1977; Hirai Citation1991) or without dust (Wagenvoort and Penders Citation1997), but these studies were undertaken on fomites in hospitals.
Thus persistence of bacteria on surfaces can vary subject to intrinsic factors from microorganisms: gram-negative bacteria have been described to persist longer than gram-positive bacteria (Dickgiesser Citation1978; Hirai Citation1991). The latter are transmitted readily from environmental surfaces, followed by viruses and gram-negative bacteria (Rusin et al. Citation2002). Humid conditions and low temperatures, e.g. 4°C or 6°C, also improve the persistence of most bacteria types, such as Listeria monocytogenes (Helke and Wong Citation1994), Salmonella typhimurium (Helke and Wong Citation1994), Methicillin-resistant Staphylococcus aureus (MRSA) (Noyce et al. Citation2006), or Escherichia coli (Wilks et al. Citation2005; Williams et al. Citation2005). These risks can be minimized by applying a shopping trolley cleaning and disinfection programme using antimicrobial agents, according to the data reported in this field (Rutala and Weber Citation2001; Engelhart et al. Citation2002), and by devising efficient planning to rotate the periodical cleaning of all supermarket trolleys (between 800 and 2,700 trolleys per supermarket).
The potential pathological microorganism findings on the shopping trolleys included in this study agree with those reported by other authors (Reynolds et al. Citation2005; Al-Ghamdi et al. Citation2011; Ashgar and El-Said Citation2012; Gerba and Maxwell Citation2012). Our results revealed the importance of cleaning and disinfecting shopping trolleys to avoid the presence of K. pneumonia, which is an opportunistic pathogen responsible for a high proportion (4–8%) of nosocomial infections (Podschun and Ullmann Citation1998).
In addition, many studies have clearly shown that E. coli is the only coliform that is an undoubted inhabitant of the gastrointestinal tract. While Klebsiella spp., Citrobacter freundii and Enterobacter have been isolated from human faecal samples, they are in small numbers when present (Edberg et al. Citation2000). However, these opportunistic pathogens isolated herein such as C. freundii, which can cause systemic infections (Kim et al. Citation2003; Pereira et al. Citation2010; Chen et al. Citation2011). Moreover, C. freundii has been used to control good healthy measures, like periodical cleaning of tools and abattoir surfaces (Milhem et al. Citation2016). P. fluorescens has been reported to cause infections like blood transfusion-related septicaemia (Khabbaz et al. Citation1984). Thus, results of epidemiological studies have shown that a risk of infection from common enteric bacteria is related to the placing of small children in shopping carts (Fullerton et al. Citation2007; Patrick et al. Citation2010) and it increases the risk of coming into contact with a disease-causing organism.
When comparing plastic or metal shopping trolleys, plastic ones showed higher contamination, but differences were not significant. Nevertheless, larger sample sizes should be considered to obtain significant differences (p < 0.05). the tested material types gave no consistent results for nosocomial persistent pathogenics on inanimate surfaces. Although some researchers have reported that this type of material has no influence on persistence (Bale et al. Citation1993; Wendt et al. Citation1997), other authors have described longer persistence on plastic (Neely and Maley Citation2000). This topic can be clearly observed in food industries, where the ability of many bacteria to adhere to surfaces and to form biofilms has major implications. Properties like surface roughness, cleanability, disinfectability, wettability and vulnerability to wear influence the ability of cells to adhere to a particular surface, and thus determine the hygienic status of materials (Van Houdt and Michiels Citation2010). Other authors (Bellissimo-Rodrigues et al. Citation2017) have described a direct relationship between the bacterial load present on hands and the risk of cross transmission following a single hand-to-hand contact. Under the described experimental conditions, at least 1 log10 CFU of E. coli must be present on hands for it to be potentially transmitted to another person. This threshold may be useful to develop an evidence-based ‘safe hands’ microbiological concept that can be applied in the healthcare setting, and in the general community to prevent infections and antimicrobial resistance from spreading (Bellissimo-Rodrigues et al. Citation2017).
Conclusions
The obtained results suggest the need to establish adequate cleaning and disinfection programmes for shopping trolleys in order to avoid exposing shopping trolley users to infections. The most effective measure could be the use of an alkaline detergent and quaternary ammonium as a disinfectant, washing the shopping trolleys once a month and doing a proper rotation of them. The material (metal or plastic) and location (outside or inside) of the shopping trolleys in the supermarkets should be taken into consideration during the preparation of the cleaning and disinfection plan, to reduce microbial contamination more effectively. As additional measures, some studies have proposed using disinfecting wipes or disposable plastic barriers for handles. In the future, we hope to compare our contamination results and potential pathogenic rates with other studies done on supermarket trolleys, which have established a cleaning and disinfection programme to assess their efficacy and to finally answer the age-old debate about the relevance of environmental contamination.
Highlights
-
Determination of microbial contamination in shopping trolleys located in Las Palmas.
-
Isolation and identification of several pathogenic bacteria.
-
Environmental contamination was evidenced.
-
High microbiological contamination of supermarket trolleys was evidenced.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
António Raposo http://orcid.org/0000-0002-5286-2249
References
- Abad FX , Villena C , Guix S , Caballero S , Pinto RM , Bosch A. 2001. Potential role of fomites in the vehicular transmission of human astroviruses. Appl Environ Microbiol. 67:3904–3907. doi: 10.1128/AEM.67.9.3904-3907.2001
- Ailes EL , Demma S , Hurd J , Hatch TF , Jones D , Vugia A , Cronquist M , Tobin-D’Angelo K , Larson E , Laine K , et al. 2008. Continued decline in the incidence of Campylobacter infections, FoodNet, 1996–2006. Foodborne Pathog Dis. 5:329–337. doi: 10.1089/fpd.2008.0090
- Al-Ghamdi AK , Ashshi SAA , Faidah H , Shukri H , Jiman-Fatani AA. 2011. Bacterial contamination of computer keyboards and mice, elevator buttons and shopping carts. Afr J Microbiol Res. 5(23):3998–4003. doi: 10.5897/AJMR11.770
- Anderson G , Palombo EA. 2009. Microbial contamination of computer keyboards in a university setting. Am J Infect Control. 37(6):507–509. doi: 10.1016/j.ajic.2008.10.032
- Ashgar SS , El-Said HM. 2012. Pathogenic bacteria associated with different public environmental sites in Mecca city. Open J Med Microbiol. 2(04):133–137. doi: 10.4236/ojmm.2012.24020
- Bale MJ , Bennett PM , Beringer JE , Hinton M. 1993. The survival of bacteria exposed to desiccation on surfaces associated with farm buildings. J Appl Bacteriol. 75:519–528. doi: 10.1111/j.1365-2672.1993.tb01589.x
- Bellissimo-Rodrigues F , Pires D , Soule H , Gayet-Ageron A , Pittet D. 2017. Assessing the likelihood of hand-to-hand cross-transmission of bacteria: an experimental study. Infect Control Hosp Epidemiol. 38(5):553–558. doi: 10.1017/ice.2017.9
- Bier RC , Pillai SD , Phillips TD , Ziprin RL. 2004. Preharvest and postharvest food safety. Ames, IA : Blackwell Publishing.
- Broekaert K , Heyndrickx M , Herman L , Devlieghere F , Vlaemynck G. 2011. Seafood quality analysis: molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol. 28(6):1162–1169. doi: 10.1016/j.fm.2011.03.009
- Bures S , Fishbain JT , Uyehara CFT , Parker JM , Berg BW. 2000. Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control. 28:465–471. doi: 10.1067/mic.2000.107267
- Burgess F , Little C , Allen G , Williamson K , Mitchell RT. 2005. Prevalence of Campylobacter, Salmonella, and Escherichia coli on the external packaging of raw meat. J Food Prot. 68:469–475. doi: 10.4315/0362-028X-68.3.469
- Carrascosa C , Saavedra P , Millán R , Jaber JR , Pérez E , Grau R , Raposo A , Mauricio C , Sanjuán E. 2012. Monitoring of cleanliness and disinfection in dairies: comparison of traditional microbiological and ATP bioluminescence methods. Food Control. 28(2):368–373. doi: 10.1016/j.foodcont.2012.05.001
- Casewell MW , Desai N. 1983. Survival of multiply-resistant Klebsiella aerogenes and other gram-negative bacilli on finger-tips. J Hosp Infect. 4(4):350–360. doi: 10.1016/0195-6701(83)90005-1
- Chen S , Hu F , Liu Y , Zhu D , Wang H , Zhang Y. 2011. Detection and spread of carbapenem-resistant Citrobacter freundii in a teaching hospital in China. Am J Infect Control. 39(9):e55–e60. doi: 10.1016/j.ajic.2011.02.009
- Dickgiesser N. 1978. Untersuchungen über das Verhalten grampositiver und gramnegativer Bakterien in trockenem und feuchtem Milieu. Zbl Bakt Hyg I. Abt. 167:48–62.
- Dionisio LPC , Borrego JJ. 1995. Evaluation of media for the enumeration of faecal streptococci from natural water samples. J Microbiol Methods. 23:183–203. doi: 10.1016/0167-7012(95)00014-C
- Edberg SCL , Rice EW , Karlin RJ , Allen MJ. 2000. Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol. 88(S1):106S–116S. doi: 10.1111/j.1365-2672.2000.tb05338.x
- Elmos T. 1977. Survival of Neisseria gonorrhoeae on surfaces. Acta Derm Venereol. 57:177–180.
- Engelhart S , Krizek L , Glasmacher A , Fischnaller E , Marklein G , Exner M. 2002. Pseudomonas aeruginosa outbreak in a haematologyoncology unit associated with contaminated surface cleaning equipment. J Hosp Infect. 52:93–98. doi: 10.1053/jhin.2002.1279
- Feachem RG , Bradley DJ , Garelick H , Mara DD. 1983. Sanitation and disease. New York : Wiley.
- Fullerton KE , Ingram LA , Jones TF , Anderson BJ , McCarthy PV , Hurd S , Shiferaw B , Vugia D , Haubert N , Hayes T , et al. 2007. Sporadic Campylobacter infection in infants: a population-based case control study. Pediatr Infect Dis J. 26:19–24. doi: 10.1097/01.inf.0000247137.43495.34
- Gerba CP , Maxwell S. 2012. Bacterial contamination of shopping carts and approaches to control. Food Prot Trends. 32(12):747–749.
- Greenberg AE , Clesceri LS , Eaton AD , editors. 1992. Standard methods for the examination of water and wastewater, 18th ed. Washington, DC : American Public Health Association.
- Hall CB , Douglas RG. 1981. Modes of transmission of respiratory syncytial virus. J Pediatr. 99:100–103. doi: 10.1016/S0022-3476(81)80969-9
- Hall CB , Douglas RG , Schnabel KC , Geiman JM. 1981. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 33:779–783.
- Harrison WA , Griffith CJ , Tennant D , Peters AC. 2001. Incidence of Campylobacter and Salmonella isolated from retail chicken and associated packaging in South Wales. Lett Appl Microbiol. 33:450–454. doi: 10.1046/j.1472-765X.2001.01031.x
- Helke DM , Wong ACL. 1994. Survival and growth characteristics of Listeria monocytogenes and Salmonella typhimurium on stainless steel and Buna-N rubber. J Food Prot. 57:963–968. doi: 10.4315/0362-028X-57.11.963
- Hirai Y. 1991. Survival of bacteria under dry conditions; from a viewpoint of nosocomial infection. J Hosp Infect. 19:191–200. doi: 10.1016/0195-6701(91)90223-U
- Irshaid FI , Jacob JH , Khwaldh AS. 2014. Contamination of the handles and bases of shopping carts by pathogenic and multi-drug resistant bacteria. ESJ. 10(27):154–169.
- Islam MS , Hossain MA , Khan SI , Khan MN , Sack RB , Albert MJ , Huq A , Colwell RR. 2001. Survival of Shigella dysenteriae type 1 on fomites. J Health Popul Nutr. 19:177–182.
- Jones TF , Ingram LA , Fullerton KE , Marcus R , Anderson BJ , McCarthy PV , Vugia D , Shiferaw B , Haubert N , Wedel S , Angulo FJ. 2006. A case-control study of the epidemiology of sporadic Salmonella infections in infants. Pediatrics. 118:2380–2387. doi: 10.1542/peds.2006-1218
- Khabbaz RF , Arnow PM , Highsmith AK , Herwaldt LA , Chou T , Jarvis WR , Lerche NW , Allen JR. 1984. Pseudomonas fluorescens bacteremia from blood transfusion. Am J Med. 76(1):62–68. doi: 10.1016/0002-9343(84)90751-4
- Kim BN , Woo JH , Ryu J , Kim YS. 2003. Resistance to extended-spectrum cephalosporins and mortality in patients with Citrobacter freundii bacteremia. Infection. 31:202–207.
- Kramer A , Schwebke I , Kampf G. 2006. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 6(1):130. doi: 10.1186/1471-2334-6-130
- Liu L , Johnson HL , Cousens S , Perin J , Scott S , Lawn JE , Rudan I , Campbell H , Cibulskis R , Li M , et al. 2012. Child health epidemiology reference group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1
- Milhem H , Azizieh A , Younes AA. 2016. Prevalence of Escherichia coli and Citrobacter freundii in raw beef from major abattoirs located in damascus and countryside, Syria. Int J ChemTech Res. 9:290–296.
- Mizumachi E , Kato F , Hisatsune J , Tsuruda K , Uehara Y , Seo Y , Sugai M. 2011. Clonal distribution of enterotoxigenic Staphylococcus aureus on handles of handheld shopping baskets in supermarkets. J Appl Microbiol. 110:562–567. doi: 10.1111/j.1365-2672.2010.04910.x
- Neely AN. 2000. A survey of gram-negative bacteria survival on hospital fabrics and plastics. J Burn Care Rehabil. 21:523–527. doi: 10.1097/00004630-200021060-00009
- Neely AN , Maley MP. 2000. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 38(2):724–726.
- Noskin GA , Stosor V , Cooper I , Peterson LR. 1995. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 16(10):577–581. doi: 10.2307/30141097
- Noyce JO , Michels H , Keevil CW. 2006. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect. 63:289–297. doi: 10.1016/j.jhin.2005.12.008
- Pascual MR , Calderón V. 2002. Microbiologia alimentaria: Metodología analítica para alimentos y bebidas, 2nd ed. Madrid, Spain : Edt. Díaz de Santos.
- Patrick ME , Mahon BE , Zansky SM , Hurd S , Scallan E. 2010. Riding in shopping carts and exposure to raw meat and poultry products: prevalence of, and factors associated with, this risk factor for Salmonella and Campylobacter infection in children younger than 3 years. J Food Prot. 73:1097–1100. doi: 10.4315/0362-028X-73.6.1097
- Pereira AL , Silva TN , Gomes ACCM , Araújo ACG , Giugliano LG. 2010. Diarrhea-associated biofilm formed by enteroaggregative Escherichia coli and aggregative Citrobacter freundii: a consortium mediated by putative F pili. BMC Microbiol. 10:57–74. doi: 10.1186/1471-2180-10-57
- Pittet D , Hugonnet S , Harbarth S , Mourouga P , Sauvan V , Touveneau S , Perneger TV. 2000. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection control programme. Lancet. 356:1307–1312. doi: 10.1016/S0140-6736(00)02814-2
- Podschun R , Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 11:589–603. doi: 10.1128/CMR.11.4.589
- R Development Core Team . 2016. R: A language and environment for statistical computing. Vienna : R Foundation for Statistical Computing.
- Reynolds KA , Watt PM , Boone SA , Gerba CP. 2005. Occurrence of bacteria and biochemical markers on public surfaces. Int J Environ Health Res. 15:225–234. doi: 10.1080/09603120500115298
- Rusin P , Maxwell S , Gerba C. 2002. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 93(4):585–592. doi: 10.1046/j.1365-2672.2002.01734.x
- Rutala WA , Weber DJ. 2001. Surface disinfection: should we do it? J Hosp Infect. 48:S64–S68. doi: 10.1016/S0195-6701(01)90017-9
- Salo S , Alanko T , Sjöberg AM , Wirtanen G. 2002. Validation of the Hygicult® E dipslides method in surface hygiene control: a Nordic collaborative study. J AOAC Int. 85(2):388–394.
- Uhnoo I , Svenson L , Wadell G. 1990. Enteric adenoviruses. In: Farthing MJG , editor. Baillière’s clinical gastroenterology. London : Baillière Tindall, 4(3); p. 627–642.
- Van Houdt R , Michiels CW. 2010. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol. 109(4):1117–1131. doi: 10.1111/j.1365-2672.2010.04756.x
- Wade JJ , Desai N , Casewell MW. 1991. Hygienic hand disinfection for the removal of epidemic vancomycin-resistant Enterococcus faecium and gentamicin-resistant Enterobacter cloacae. J Hosp Infect. 18(3):211–218. doi: 10.1016/0195-6701(91)90145-X
- Wagenvoort JHT , Penders RJR. 1997. Long-term in-vitro survival of an epidemic MRSA phage-group III-29 strain. J Hosp Infect. 35:322–325. doi: 10.1016/S0195-6701(97)90229-2
- Weber R , Bryan R , Schwartz D , Owen R. 1994. Human microsporidial infections. Clin Microbiol Rev. 7(4):426–461. doi: 10.1128/CMR.7.4.426
- Wendt C , Dietze B , Dietz E , Rüden H. 1997. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 35:1394–1397.
- Wilks SA , Michels H , Keevil CW. 2005. The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol. 105:445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021
- Williams AP , Avery LM , Killham K , Jones DL. 2005. Persistence of Escherichia coli O157 on farm surfaces under different environmental conditions. J Appl Microbiol. 98:1075–1083. doi: 10.1111/j.1365-2672.2004.02530.x
- Willmott M , Nicholson A , Busse H , MacArthur GJ , Brookes S , Campbell R. 2016. Effectiveness of hand hygiene interventions in reducing illness absence among children in educational settings: a systematic review and meta-analysis. Arch Dis Childhood. 101:42–50. doi: 10.1136/archdischild-2015-308875
- Wong TL , Whyte RJ , Cornelius AJ , Hudson JA. 2004. Enumeration of Campylobacter and Salmonella on chicken packs. Br Food J. 106:651–662. doi: 10.1108/00070700410558184