ABSTRACT
Tracheal Wash (TW) and bronchoalveolar lavage (BAL) are not well documented in adult cattle and published studies were undertaken on diseased animals aiming at isolating the causative agent of respiratory diseases. This study was undertaken to establish the normal cytological and microbiological findings of TW and BAL in normal adult cattle and compare between the laboratory analysis findings of both techniques. Eighteen healthy adult cattle were divided into two groups. BAL was completed the first group (n = 10) and TW was completed the second group (n = 8). Normal cytological findings of TW in healthy adult cattle were found to be primarily neutrophils (42.7 ± 35.7)% intermixed with lower numbers of columnar epithelial cells (26.2 ± 34.8)%, alveolar macrophages (14.7 ± 15.4)%, squamous epithelial cells (10 ± 28.6)%, lymphocytes (6.8 ± 16.1)% and rarely seen eosinophils (0.1 ± 0.0)% with a total cell count of 457 ± 310.1 cells/µl. Cytological findings of BAL were found to be mainly alveolar macrophages (94.3 ± 3.9)%, intermixed with lower number of neutrophils (4.3 ± 3.9)% and lymphocytes (1.3 ± 1.2)% and rarely seen eosinophils (0 ± 0.1)% with a total cell count of 238 ± 169 cells/µl. There are remarkable differences in TW and BAL cytological findings and the degree of contamination was higher in TW samples than that seen in BAL lavage.
Introduction
Bovine respiratory disease complex is one of the most serious cattle diseases in the world because of the high economical loses that is associated with it in both beef (Moreno-Lopez Citation1990; Mahmoud and Allam Citation2013) and dairy cattle (Fathi et al. Citation2013).
Cattle are more susceptible to respiratory diseases among animals because of several anatomical and physiological factors (Veit and Farrell Citation1978). Identification of respiratory disease can be undertaken using the following diagnostic tools including clinical signs, auscultation and percussion, haematology, radiography, ultrasonography, laboratory analysis of tracheobronchial secretions including identification of the causative agent, which is collected using tracheal wash (TW), and bronchoalveolar lavage (BAL) procedures (Radostits et al. Citation2006) in addition to more invasive procedures such as thoracocentesis and lung biopsies.
TW and BAL are used to collect respiratory secretions from the trachea, and lower bronchioles and in alveoli, respectively. Both procedures are widely used in horses and small animals. However, it is not routinely used in cattle.
TW can be performed using endoscopy or traditionally by using the tras-tracheal procedure while BAL can be performed traditionally using BAL catheter, or using endoscopy. BAL is preferred for the diagnosis of diffuse respiratory diseases (Caldow Citation2001), while TW is preferred for the diagnosis of focal lung lesions.
BAL and TW samples are useful for evaluating the type of inflammatory reaction that occurs within the respiratory system, and revealing subclinical pulmonary diseases (Beech Citation1975). Both procedures give an insight on the severity, and stage of inflammatory response taking place (acute and chronic) (Caldow Citation2001). In addition, both are used for assessing the response to antimicrobial drugs treatment of various pulmonary diseases (Allen et al. Citation1992).
TW and BAL cytologic and microbiological finding are not well documented in normal adult cattle. Published studies about TW and BAL were undertaken on diseased adult animals where isolating the causative agent of respiratory diseases was the main objective.
The objectives of this study were to establish the normal cytological and microbiological values for TW and BAL in healthy adult cattle. Secondly to compare between TW and BAL cytological and microbiological findings.
Materials and methods
Study animals and design
During the spring (March–May) of 2012, our study was carried out at Jordan University of science and technology where we used 18 clinically healthy adult Holstein Friesian, mid-lactation, housed cattle. The animals were grouped into two groups; group 1 consisted of 10 adult Cattle from 3 to 8 years old for BAL samples collection. Group 2 consisted of 8 adult cattle 2.5–5 years old for TW samples collection. Study animals’ health status was evaluated using physical examination, with special focus on the respiratory system, and using complete blood count, total protein and fibrinogen measurements. Sick animals or animals that have values outside the reference values (not those that are related to stress as in animals having a stress leukogram) were excluded. Only animals with normal findings were enrolled in the study.
Experimental group 1
On day one, study animals underwent physical examination and blood samples were collected. The BAL samples were collected in suitable sterile containers on the same day after the physical examination was performed. BAL samples were then sent for the laboratory analysis and culture (Pringle et al. Citation1988).
Animals in this group were followed up for 4 days after the collection of BAL samples, using physical examination and hematology.
Experimental group 2
Study animals in this group were physically examined and blood samples were collected. After TW samples were taken, they were sent for the laboratory analysis and culture (Pringle et al. Citation1988).
Blood samples
Five ml of whole blood was collected from the tail coccygeal vein with and without EDTA at days 0, 1, 4, 5 and 6 post-BAL samples collection, and at day 1 before TW samples collection. EDTA tubes contain ethylenediaminetetraacetic acid (EDTA), were used for complete blood count (CBC) and fibrinogen concentration determination. Plain tubes were used for serum collection to determine the total serum protein concentration.
Blood smear
Thin blood films were made from the EDTA tubes and stained with diff-quick stain for white blood cells differential counts determination.
Complete blood counts, fibrinogen and total protein
The red blood cells count (RBCs), white blood cells count (WBCs), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and platelets count (Plt) were determined immediately using an automated hematology analyzer (ABC vet hematology analyzer, France).
Fibrinogen concentration was measured using the heat precipitation technique as described by Thrall et al. (Citation2012).
TW and BAL procedure
Brochoalveolar lavage and TW samples were collected from animals according to the following procedures:
Animal restraint
Animals were restrained using halter and a nose holder.
TW procedure
TW was completed using the endoscope. After the animal was restrained, a 20 ml of 2% Lidocaine hydrochloride was flushed in the animal nostrils. Then the scope was inserted until it reached the trachea. A double guarded catheter was inserted through the biopsy canal until it reached the trachea. At this time, 20 ml sterile normal saline was infused within the trachea and then immediately was aspirated.
The quantity of the retrieved fluids was recorded and collected into plain tubes for culture, and into suitable 125 ml sterile containers for cytology and other purposes. A direct slide smear was made immediately after the collection and the rest of the fluid samples were sent for laboratory analysis.
BAL procedure
After the animals were restrained, a 20 ml of 2% lidocaine hydrochloride was infused in the nose. Then the BAL catheter was passed gently through the nostril until it reached the pharynx, then passed into the trachea during inspiration while the head and neck were extended. The trachea was rattled to feel the catheter rattling within it (an indication that the catheter is present within the trachea). Then, a 20 ml of 2% lidocaine hydrochloride was injected into the catheter. After that catheter was passed deeper until it lodged into small bronchioles. At this point, the catheter balloon was inflated with air for the negative pressure formation then a 180 ml of sterile normal saline was injected into the catheter and aspirated immediately (Ishizaki et al. Citation2005).
The quantity of the retrieved fluids was recorded and collected into plain tubes for culture, and into suitable 125 ml sterile containers for cytology and other purposes. A direct slide smear was made immediately after the collection and the rest of the fluid samples were sent for laboratory analysis.
Brochoalveolar lavage and TW cytological sample analysis
The total cell counts of the TW and BAL were determined using an automated hematology analyzer (ABC veterinary hematology analyzer, ABX Diagnostic, France) and using the hemocytometer method as described previously (Johnson et al. Citation2002). The total protein was measured using the spectrophotometer using biuret method.
For cytological examination of the TW and BAL fluids, straight and cytospin smears were prepared. Slides were stained with diff-quick and Wright stain and examined for cellular morphology. A 500-cell differential cell count was performed under oil immersion field.
Brochoalveolar lavage and TW microbiological sample analysis
Microbiological culture of BAL and TW fluid was performed on samples collected in plain tubes using spread Platte technique. BAL samples were centrifuged for 10 min at 9000 × g then 100 µl from the sediment was taken and spread evenly over the entire surface of each agar media plates using glass spreader. Blood agar media plates were incubated for 48 h at 37°C in 10% CO2, chocolate agar media plates were incubated for 48 h at 37°C in 10% CO2, macConkey’s agar media plates were incubated on air at 37°C for 48 h and sabouraud dextrose agar media plates were kept at room temperature for 14 days (Pringle et al. Citation1988).
TW samples were directly cultured on agar plates without centrifugation using the same technique.
Statistical analysis
Descriptive statistical analysis was done for all the values of the different parameters obtained in the study. Those included physical examination parameters, hematology ical parameters, total protein and fibrinogen, BAL and TW differential cell counts, total cell counts and total protein. The obtained data were reported as mean ± SD.
Results
BAL
The BAL procedure was successful in 8 cows, out of 10 cows. The total instilled fluids were 180 ml, and the retrieved fluids were (81 ± 12.6) ml. This equal to (45 ± 11.6) per cent of the total instilled fluids.
Physical examination parameters
The physical examination parameters, heart rate (HR), respiratory rate (RR), temperature (Temp), and rumen motility (RM) were within normal limits, throughout the study (days 0,1,4,5 and 6), with no any abnormal respiratory sounds, or any signs of respiratory system disorders or infections. No significant clinical abnormality was recorded.
Complete blood counts, total protein, fibrinogen, and blood differential cell counts
Complete blood counts (CBC)
The complete blood counts of cattle in the BAL group are illustrated in . The means of the RBCs, WBCs, platelets, HCT and the RBC indicators (MCV, MCH) were all within normal limits.
Table 1. Mean ± SD of complete blood counts, total protein, fibrinogen, TP/F ratio, and blood differential cell counts (per cent and absolute) for 10 adult cattle at days 0, 1, 4, 5, and 6 post-BAL procedure.
Total protein and fibrinogen
The total protein concentration was within normal limits at days 0, 1 and 4. There was a slight hyperproteinemia at days 5 and 6. There was a mild hyperfibrinogenemia at days 0, 1, 2, 4, 5 and 6. Results are presented in .
White blood cells differential counts
The mean ± SD of the WBC differential counts were within normal limits ().
BAL cytological findings
Total cell counts and total protein
The total cell counts were measured manually (using hemocytometer), and automatically (using the ABC vet hematology analyzer). The results were illustrated in . The values obtained manually (211 ± 168 cells/µl) were lower than the values obtained using the automated method (260 ± 197 cells/µl). The total protein was less than 1 g/dl.
Table 2. Mean ± SD of BAL fluids differential cell counts, total cell counts (manual and automated), and total protein for 8 apparently healthy adult cattle.
Differential cell counts
The cytological findings of BAL consisted mainly of alveolar macrophages (94.3 ± 3.9) %, intermixed with lower number of neutrophils (4.3 ± 3.9)%, and lymphocytes (1.3 ± 1.2)%, and rarely seen eosinophils ().
Table 3. The microbiological finding of BAL fluids for 8 apparently healthy adult cattle.
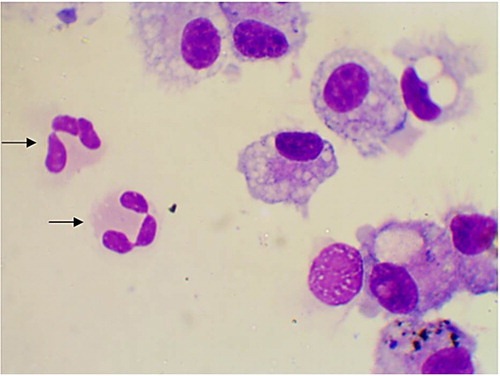
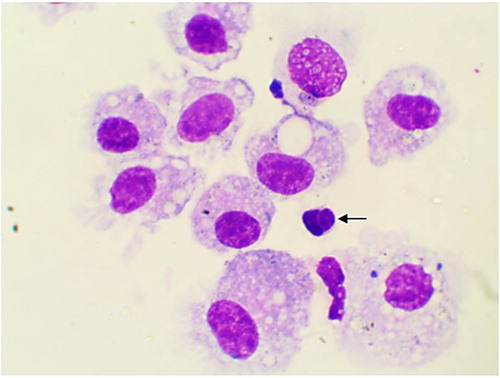
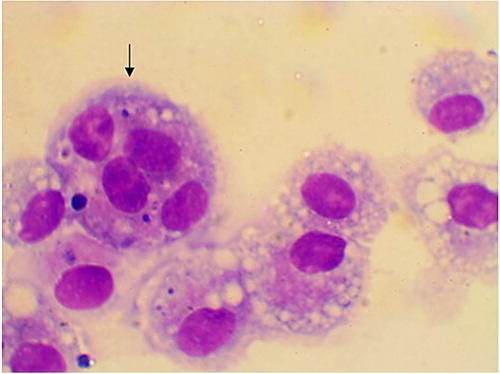
The alveolar macrophages were slightly variable in size with abundant vacuolated cytoplasm and irregular cell margins. The cytoplasmic vacuoles occasionally contained cellular debris or other material such as plant pollen grains (). In addition, few mitotic figures were occasionally seen and sometimes binucleated, trinucleated and multinucleated cells were observed (, and respectively). Neutrophils were normally segmented and non-degenerative similar to that seen in circulation (). Lymphocytes characterized by small round central to eccentric nuclei with dense clumped chromatin, and scant amounts of blue cytoplasm with smooth margins, were rarely seen (). In addition, eosinophils, characterized by prominent uniformly sized, small red orange in colour cytoplasmic granules.
Figure 1. Alveolar macrophages contain intracytoplasmic pollen grain (arrow) and cellular debris (two arrows) in TW from apparently healthy cattle (Diff-Quick stain, oil immersion).
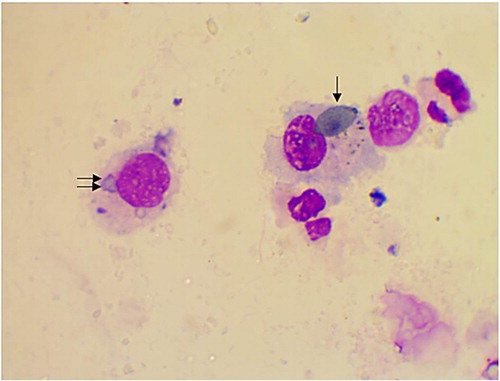
Figure 2. Binucleated alveolar macrophage in BAL from apparently healthy cattle, arrow (Diff-Quick stain, oil immersion).
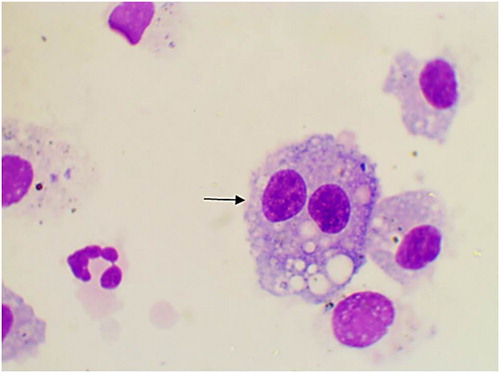
Figure 3: Trinucleated alveolar macrophage (arrow) in BAL from apparently healthy cattle, arrow (Diff-Quick stain, oil immersion).
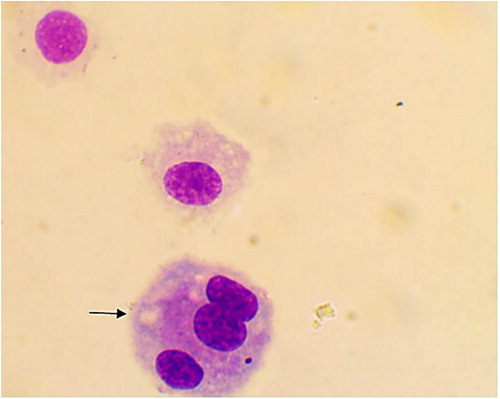
Figure 4. Multinucleated alveolar macrophage in BAL from apparently healthy cattle, arrow (Diff-Quick stain, oil immersion).
BAL microbiological findings
The culture results of BAL fluids consist of pleomorphic bacterial forms, and fungus, as shown in . All of these bacterial and fungus are not significant and were considered as a nasopharyngeal contamination.
TW
The TW procedure was successful in all the 7 cows. The total instilled fluid in each cow was 20 ml, except in one was 40 ml as the procedure failed at the first time. The total retrieved fluids were (9 ± 5) ml, which is equal to (45 ± 25) per cent of the instilled fluids.
Physical examination parameters
All cows were clinically normal except cow number 7 which was excluded from the study. No abnormal respiratory sounds were detected. Most of the cows showed a slight tachypnoea (5/7), other parameters were normal except two cows, which had showed tachycardia (2/7).
Complete blood counts (CBC), total protein, fibrinogen, and blood differential cell counts
Complete blood cells counts
The complete blood cells counts of all animals in group 2 were within the normal limits, as illustrated in .
Table 4. Mean ± SD of 8 adult cattle complete blood counts, total protein, fibrinogen, TP/F ratio, and blood differential cell counts (per cent and absolute), before TW procedure.
Total protein and fibrinogen
Total protein and fibrinogen concentration were within normal limits, with exception of animals numbers 5 and 6 showed a moderate increase in total protein measurements 9.5 g/dl, and 8.8 g/dl respectively, with a normal F/TP ratio of >10 ().
White blood cell differential counts
The white blood cells differential counts were within the normal ranges with moderate lymphocytosis in cow 7 (which was excluded from the study) and neutrophilia in cows 5, 6, and 8, as shown in .
TW cytological findings
Total cell counts and total protein
The total cell counts and the total protein concentration values were presented in .
Table 5. Mean ± SD of TW fluids differential cell counts, total protein and total cell counts for 8 apparently healthy adult cattle.
Differential cell counts
The differential cell counts of the TW fluids consist primarily of neutrophils (42.2 ± 35.7)% intermixed with lower numbers of columnar epithelial cells (26.2 ± 34.8)%, alveolar macrophages (14.7 ± 15.4)%, squamous epithelial cells (10.0 ± 26.6)%, lymphocytes (6.8 ± 16.1)% and rarely seen eosinophils (0.1 ± 0.0)%. The results were presented in . Most of those cells were found embedded in mucus. Many of the neutrophilic populations were segmented and non-degenerate. High numbers of mixed pleomorphic bacterial forms were seen adherent to squamous epithelial cells and in the background.
Alveolar macrophages were variable in size, and occasionally binucleated or multinucleated, with moderate amount of vacuolated cytoplasm. Some of the cytoplasmic vacuoles contained cellular debris and plants pollen grains, similar to that seen in .
Epithelial cells were commonly found as ciliated columnar epithelial cells with basally located nucleus. These cells were commonly seen in clustered and individually.
Intermediate size and superficial squamous epithelial cells with centrally located nuclei, were seen in clusters, and individually. Many of the squamous cells are associated with mixed pleomorphic bacterial forms.
Lymphocytes were characterized by small, round, central or eccentric nuclei with dense, clumped chromatin and scant amounts of blue cytoplasm with smooth margins. Few natural killer cells were noted and eosinophils were rarely seen.
TW microbiological findings
The microbiological results of the TW fluids consist primarily of pleomorphic bacterial forms and fungus (most likely Candida spp). These results were shown in . The most likely interpretation of these findings is a nasopharyngeal contamination.
Table 6. The microbiological findings of TW fluids from 7 apparently healthy cattle.
Discussion
The use of TW and BAL should be encouraged in cattle practice, providing valuable information about the lower respiratory tract (Beech Citation1975; Allen et al. Citation1992; Caldow Citation2001). Our study has found a difference in the composition of the cellular population of these two methods in healthy cattle.
In this study, all study animals appeared clinically normal and had no signs of respiratory system disorders or infections, except cow number 7 which was excluded from the study due to abnormal haematological findings includes a mild absolute lymphocytosis, which might indicate a true chronic antigenic stimulation. On physical examination this cow showed a mild elevation in the heart rate and respiratory rate, although no obvious clinical signs of respiratory problems were noted. Some animals showed a moderate increase in respiratory rate and heart rate. Those slightly abnormal findings were related to stress, hot weather and transport. In addition, no evidence of a respiratory disease was detected and all study animals considered apparently healthy.
Blood samples were taken for analysis one day before both TW and BAL procedures to make sure that experimental animals are healthy and systemically normal. Since it is likely that BAL is considered more invasive than TW, repeated blood analysis were done on experimental animals that have undergone BAL to watch for any possible adverse effect systemically.
BAL procedure is considered safe in cattle. As the entire physical examinations parameters were within normal limits during the follow-up examination and observation. The BAL procedure failed in two cows; perhaps the inflated balloon failed to form a tight seal at the entrance of the bronchiole.
The hematological parameters of BAL group were within normal range with some exceptions. The slight hyperproteinemia at days 5 and 6, and mild hyperfibrinogenemia at days 0–6 are most likely due to mild dehydration. This interpretation is supported by a ratio more than ten between the total plasma protein and fibrinogen. In the TW group, cows number 5, 6 and 8 showed mild absolute neutrophilia without left shift. The most likely interpretation of these leukogram changes is stress due to endogenous corticosteroid release (Thrall et al. Citation2012).
In the present study the total cell counts of the BAL group are in close agreement with that reported in horses (Derksen et al. Citation1989) and one study in calves (Khadom et al. Citation1985) but they are very low compared to values obtained from calves in other studies (Fox Citation1973; Maheswaran et al. Citation1980; Richards and Renshaw Citation1981; Rings and Muir Citation1982; Fogarty et al. Citation1983; Viso et al. Citation1985; Lay et al. Citation1986; Pringle et al. Citation1988). Those differences might be due to the variation in collection methods, the volume of the instilled and retrieved fluid in addition to the number of aliquots (Brain and Frank Citation1973; Pinsker et al. Citation1980; Pingleton et al. Citation1983; Lam et al. Citation1985; Pohunek et al. Citation1996). On the other hand, the validity of total cell counts in lower airway sampling is questioned due to variable dilution in the saline volume and cell clumping in mucus, especially considering the standard deviation for the cell counts in the TW samples is so high..
In the present study, the BAL consisted primarily of alveolar macrophages (94.3 ± 3.9)% intermixed with lower number of neutrophils (4.3 ± 3.9)% and lymphocytes (1.3 ± 1.2)% and rarely seen eosinophils (0 ± 0.1)%. These findings are very similar to that values reported in calves by fox (Fox Citation1973; Pringle et al. Citation1988; Lakritz et al. Citation2004), and slightly higher than those reported by Maheswaran et al. (Citation1980), Richards and Renshaw (Citation1981), Khadom et al. (Citation1985), Hagberg et al. (Citation2005), Ishizaki et al. (Citation2005). Our results are very high compared to the values reported in calves by wilkie (Wilkie and Markham Citation1981).
The total protein concentration of the BAL fluid was very scant in our study and this is similar to values reporeted in horses and cavles (Pringle et al. Citation1988).
Most of the BAL cultured microorganisms were found in a very low numbers, and all of them are not a significant pathogen of the cattle respiratory system and represent a nasopharyngeal contamination. This interpretation is supported by the lack of degenerate neutrophils with intracellular bacteria in the straight and cytospine preparation of The BAL fluids.
Our results differ from the previously isolated microorganisms from normal calves BAL, as most of them isolated Pasteurella and Mycoplasma from normal calves BAL fluids (Viso et al. Citation1985; Autio et al. Citation2007; Angen et al. Citation2009). However, it is closer to Pringles et al. (Citation1988), who isolated only Mycoplasma in addition to non-pathogenic Histophilus species from BAL fluids of normal calves.
The TW procedure was successful in all 8 cows. The amount of the retrieved fluids was 9 ± 5 ml about 45 ± 25% of the instilled fluids. The fluids were mucoid in consistancy, less foamy than that of BAL and more turbid. In three animals the retrieved fluids were very low when compared with that of other animals. This was expected since there was low volume fluid pool formed in those cows. Adult cows may lack similar regions to other animals (such as horses) where fluid pool is formed quickly after the fluid administration. Perhaps this is due to anatomical differences among species.
In this study, the differential cell counts of the TW fluid are in a close agreement with those values reported in horses (Larson and Busch Citation1985; Derksen et al. Citation1989). In both studies the TW cytological findings consists mainly of neutrophils and lower per cent of alveolar macrophages, lymphocytes and epithelial cells.
The cytologic findings of TW revealed a higher total cell counts compared to the BAL findings. The mature neutrophils were present in high numbers in the TW fluid in comparison with that seen in BAL fluid.
There are remarkable differences in TW and BAL cytological findings and those differences agree with those were found in horses (Derksen et al. Citation1989). In this study, TW fluids consisted mainly of neutrophils while those of BAL consisted mainly of alveolar macrophages. Those differences were due to the local migration of cells to and from the airways lumen, in addition, large airways may transport material originating from both conducting airways and the alveolar surface area, different areas contributing secretions in a non-homogeneous manner. Moreover, those differences could be explained by differences in cells turnover rates, as macrophages with long half-life would be discarded and transported up the mucociliary escalator to appear in tracheal secretions less frequently than cells with a shorter half-life that may be over represented in tracheal fluids. Also, those changes may be due to the different microscopic anatomy of the conducting air ways.
All the cultured microorganisms are non-significant, non-pathogenic bacteria and fungus, considered to be from nasopharyngeal contaminations. In our study, the nasopharyngeal contamination was very prominent in the TW fluid but not BAL. This interpretation is supported by the presence of high numbers of squamous epithelial cells associated with high numbers of mixed, pleomorphic bacterial forms and yeast like structures consistent with Candida species. Pollen grains were found in all TW samples as free grains or phagocytized by the macrophages unlike BAL in which pollen grains were rarely founds.
Although double guarded catheters were used for TW in this study, there was a high contamination, which is surprising. The contamination is thought to be a nasopharyngeal contamination from the endoscope. The reason of higher contamination in TW may be attributed to the degree of sterility of trachea that is less than that of bronchioles and the amounts of the instilled and retrieved TW fluids is 1/9 of that instilled in BAL fluids. So the bacterial contamination of BAL is 9 times diluted from that of TW.
The limitations of this study include the small number of experimental animals in each group, dairy cows in Jordan is considered expensive as an experimental unit and it is hard to convince the owner to use more animals. Perhaps, using more experimental animals would have helped in reducing the wide standard deviation in the TW results, which is also considered a limitation to this study. Another limitation is not screening experimental animals specifically for respiratory diseases other than visual appraisal, physical examination and thoracic auscultation, as well as hematology. Thoracic radiography, ultrasonography, and arterial blood gas analysis could have been used additionally.
Conclusions
-
(1) The normal cytological findings of TW in healthy adult cattle were found to consist primarily of neutrophils (42.7 ± 35.7)% intermixed with lower numbers of columnar epithelial cells (26.2 ± 34.8)%, alveolar macrophages (14.7 ± 15.4)%, squamous epithelial cells (10 ± 28.6)%, lymphocytes (6.8 ± 16.1)% and rarely seen eosinophils (0.1 ± 0.0)% with a total cell count of 450 ± 288 cells/µl.
-
(2) The cytological findings of BAL were found to be mainly alveolar macrophages (94.3 ± 3.9)% intermixed with lower number of neutrophils (4.3 ± 3.9)% and lymphocytes (1.3 ± 1.2)% and rarely seen eosinophils (0 ± 0.1)%, with a total cell count of 238 ± 169 cells/µl.
-
(3) Most of the cultivated bacteria in both TW and BAL samples were non-pathogenic and considered a nasopharyngeal contamination during the procedure.
Recommendations
Future studies about the cytological and microbiological findings of BAL and TW in adult healthy cattle to support or refuse our findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allen JW , Viel L , Bateman KG , Rosendal S. 1992. Changes in the bacterial flora of the upper and lower respiratory tracts and bronchoalveolar lavage differential cell counts in feedlot calves treated for respiratory diseases. Can J Vet Res. 56:177–183.
- Angen Ø , Thomsen J , Larsen LE , Larsen J , Kokotovic B , Heegaard PM , Enemark JM. 2009. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol. 137:165–171. doi: 10.1016/j.vetmic.2008.12.024
- Autio T , Pohjanvirta T , Holopainen R , Rikula U , Pentikäinen J , Huovilainen A , Rusanen H , Soveri T , Sihvonen L , Pelkonen S. 2007. Etiology of respiratory disease in non-vaccinated, non-medicated calves in rearing herds. Vet Microbiol. 119:256–265. doi: 10.1016/j.vetmic.2006.10.001
- Beech J. 1975. Cytology of tracheobronchial aspirates in horses. Vet Pathol. 12:157–164. doi: 10.1177/030098587501200301
- Brain JD , Frank R. 1973. Alveolar macrophage adhesion: wash electrolyte composition and free cell yield. J Appl Physiol. 34:75–80. doi: 10.1152/jappl.1973.34.1.75
- Caldow G. 2001. Bronchoalveolar Lavage in the investigation of bovine respiratory disease. In Pract. 23:41–43. doi: 10.1136/inpract.23.1.41
- Derksen F , Brown C , Sonea I , Darien B , Robinson N. 1989. Comparison of transtracheal aspirate and bronchoalveolar lavage cytology in 50 horses with chronic lung disease. Equine Vet J. 21:23–26. doi: 10.1111/j.2042-3306.1989.tb02084.x
- Fathi E , Farahzadi R , Imani M. 2013. Approach to treatment of bronchopneumonia by evaluation of selected acute-phase proteins in calf herds. Comp Clin Pathol. 22:125–129. doi: 10.1007/s00580-011-1377-2
- Fogarty U , Quinn P , Hannan J. 1983. Bronchopulmonary lavage in the calf–a new technique. Ir Vet J. 37:35–38.
- Fox M. 1973. The bovine alveolar macrophage. 1. Isolation, in vitro cultivation, ultrastructure, and phagocytosis. Can J Microbiol. 19:1207–1210. doi: 10.1139/m73-195
- Hagberg M , Wattrang E , Niskanen R , Tråvén M , Höglund J , Lundén A. 2005. Mononuclear cell subsets in bronchoalveolar lavage fluid during Dictyocaulus viviparus infection of calves: a potential role for γ/δ TCR-expressing cells in airway immune responses? Parasite Immunol. 27:151–161. doi: 10.1111/j.1365-3024.2005.00757.x
- Ishizaki H , Hanafusa Y , Kariya Y. 2005. Influence of truck-transportation on the function of bronchoalveolar lavage fluid cells in cattle. Vet Immunol Immunopathol. 105:67–74. doi: 10.1016/j.vetimm.2004.12.015
- Johnson CW , Timmons DL , Hall PE. 2002. Essential laboratory mathematics: concepts and applications for the chemical. 2nd ed.
- Khadom N , Dedieu J , Viso M. Bovine alveolar macrophage: a review. Proceedings of the Annales de Recherches Vétérinaires; 1985.
- Lakritz J , Marsh AE , Cockrell M , Smith MF , Tyler JW. 2004. Characterization of gelatinases in bronchoalveolar lavage fluid and gelatinases produced by alveolar macrophages isolated from healthy calves. Am J Vet Res. 65:163–172. doi: 10.2460/ajvr.2004.65.163
- Lam S , Leriche JC , Kijek K , Phillips D. 1985. Effect of bronchial lavage volume on cellular and protein recovery. Chest. 88:856–859. doi: 10.1378/chest.88.6.856
- Larson VL , Busch RH. 1985. Equine tracheobronchial lavage: comparison of lavage cytologic and pulmonary histopathologic findings. Am J Vet Res. 46:144–146.
- Lay J , Slauson D , Castleman W. 1986. Volume-controlled bronchopulmonary lavage of normal and pneumonic calves. Vet Pathol. 23:673–680. doi: 10.1177/030098588602300605
- Maheswaran S , Berggren K , Simonson R , Ward G , Muscoplat C. 1980. Kinetics of interaction and fate of Pasteurella hemolytica in bovine alveolar macrophages. Infect Immun. 30:254–262.
- Mahmoud M , Allam A. 2013. Seroprevalence of bovine viral diarrhea virus (BVDV), bovine herpes virus type 1 (BHV-1), parainfluenza type 3 virus (PI-3 V) and bovine respiratory syncytial virus (BRSV) among non vaccinated cattle. Global Veterinaria. 10:348–353.
- Moreno-Lopez J. 1990. Acute Respiratory disease. Cattle Amsterdam : Elsevier.
- Pingleton SK , Harrison GF , Stechschulte DJ , Wesselius LJ , Kerby GR , Ruth WE. 1983. Effect of location, pH, and temperature of instillate in bronchoalveolar lavage in normal volunteers 1, 2. Am Rev Respir Dis. 128:1035–1037.
- Pinsker K , Norin A , Kamholz S , Montefusco C , Schreiber K , Hagstrom J , Veith F. 1980. Cell content in repetitive canine bronchoalveolar lavage. Acta Cytol. 24:558–563.
- Pohunek P , Pokorna H , Striz I. 1996. Comparison of cell profiles in separately evaluated fractions of bronchoalveolar lavage (BAL) fluid in children. Thorax. 51:615–618. doi: 10.1136/thx.51.6.615
- Pringle J , Viel L , Shewen P , Willoughby R , Martin S , Valli V. 1988. Bronchoalveolar lavage of cranial and caudal lung regions in selected normal calves: cellular, microbiological, immunoglobulin, serological and histological variables. Can J Vet Res. 52:239.
- Radostits O , Gay C , Hinchcliff K , Constable P. 2006. Veterinary medicine a textbook of diseases of cattle sheep goats pigs and horses 10th ed. Elsevier.
- Richards A , Renshaw H. 1981. Bovine alveolar macrophages. Zoonoses Public Health. 28:603–611.
- Rings DM , Muir W. 1982. Cardiopulmonary effects of intramuscular xylazine-ketamine in calves. Can J Comp Med. 46:386.
- Thrall MA , Weiser G , Allison R , Campbell T. 2012. Veterinary hematology and clinical chemistry. Ames, Iowa, USA : Wiley.
- Veit HP , Farrell RL. 1978. The anatomy and physiology of the bovine respiratory system relating to pulmonary disease. Cornell Vet. 68:555–581.
- Viso M , Jaraki M , Espinasse J , Parodi A , Dedieu J. 1985. A sequential broncho-alveolar washing in non-anaesthetized normal bovines: method and preliminary results. Vet Res Commun. 9:213–219. doi: 10.1007/BF02215144
- Wilkie B , Markham R. 1981. Bronchoalveolar washing cells and immunoglobulins of clinically normal calves. Am J Vet Res. 42:241.