ABSTRACT
In this study, gonadal changes of red mullet (Mullus barbatus L., 1758) were investigated by histologically in Antalya Bay of Eastern Mediterranean in Turkey. A total of 890 specimens were collected monthly between May 2015 and October 2016 from fishing boats. The age of 890 red mullets was found to range from 0+ to VI+. Their gonads were dissected and fixed in 10% neutral buffered formaldehyde. They were embedded in paraffin blocks after histochemical processes. The sectioned tissue samples were stained with Haematoxylin-Eosin and Masson Trichrome. Two different stages in females and in males were observed according to gonadal development. Histologically, female individuals had in immature female (66 fish, 0+-IV+ ages), chromatin-nucleolus (67 fish, I+-VI+ ages), perinucleolus (63 fish, I+-IV+ ages), cortical alveoli (55 fish, I+-V+ ages), vitellogenesis (50 fish, I+-V+ ages), maturity (39 fish, I+-VI+ ages) and post-ovulation stages (59 fish, I+-VI+ ages). Male individuals had in spermatogenesis (96 fish, 0+-VI+ ages), spermiogenesis (97 fish, I+-IV+ ages), spermiation (85 fish, I+-VI+ ages), sperm release (84 fish, I+-VI+ ages) and resting stages (116 fish, I+-VI+ ages). Results show that the spawning season of M. barbatus is between May and September, and that egg diameters range from 31.00 ± 15 to 428.00 ± 40 µm.
Introduction
Red mullet (M. barbatus) is a fish of high economic value and a member of the Mullidae family, 7 species of which are prevalent in the Mediterranean Sea. Two of these species are native, and five are exotic species (Bilecenoğlu et al. Citation2014; Gökoğlu Citation2018). The number of species of Mullidae family found in the Gulf of Antalya are six. These species are M. barbatus, M. surmuletus (native), Upeneus pori, U. moluccensis, U. tragula and Parupeneus forskalli (Red Sea origin) (Golani et al. Citation2006; Bariche et al. Citation2013; Bilecenoğlu et al. Citation2014; Gökoğlu Citation2018). Including population dynamics, age and growth of M. barbatus have already been determined in the Mediterranean Sea (Mert et al. Citation1983; Türeli and Erdem Citation1997; Fiorentino et al. Citation1998; Voliani et al. Citation1998; Relini et al. Citation1999; Vrgoč Citation2000; İşmen and İşmen Citation2001; Özyurt Citation2003; Joksimovic Citation2005; Becer et al. Citation2006; Çiçek Citation2015) and bottom trawl selectivity parameters (Tokaç et al. Citation1995; Gurbet et al. Citation1997; Tosunoğlu and Tokaç Citation1997; Vrgoč Citation2000; Özyurt Citation2003; Tokaç et al. Citation2004; Joksimovic Citation2005). There are, however, no studies that histologically examine the gonadal development of Mullus barbatus L. in Antalya Bay of Turkey. Histological studies on reproductive biology always provide more accurate and precise results. The aim of this study is to histologically determine the onset of reproductive activity and analyze developmental stages of egg cells in ovaries and sperm in gonads of M. barbatus in the Bay of Antalya. This study will provide preliminary histological data on the species, and thus, pave the way for further research.
Materials and methods
The study was carried out in the Bay of Antalya at 36° 11′ N, 31° 02′ E; 36° 11′ N, 30° 26′ E coordinates and in the region between the depths of 20 and 100 m. Specimens (n = 890) were collected monthly (mean n = 56) from May 2015 to October 2016. Fish were caught by fishing boats that catch fish using gill-nets. Depending on the histochemical techniques employed, the tissues removed from the fish were kept in a Bouin’s and 10% neutral formaldehyde solution for at least 24–48 h. The tissues were detected in the neutral formaldehyde. They were washed for 1 h in order to eliminate the effect of the detection fluid. The tissues kept in different concentrations of alcohol for a certain period after the detection process were subjected to xylol and paraffin baths and blocked to prepare them for cutting with a microtome. Sections of 5 µ thickness cut from the tissues blocked for normal histology were kept in an oven at 40°C overnight. They were stained with hematoxylin–eosin (H + E) and Masson Trichrome (MT) for histological diagnosis. The slides stained and mounted in Canada balsam were examined under a binocular research microscope and their microphotographs were taken to identify histological features (Bancroft and Stevens Citation1996; Presnell and Schreibman Citation1997). The opaque and dark band of the scale and operculum of the fish were examined under a stereomicroscope for age estimation (Türkmen et al. Citation2010).
Results
Gonad development in females
Ovarian phase, gonad growth and gamete development in M. barbatus were first observed between November and December. The formation of an egg (oogenesis) is due to seasonal water temperature. The period of intelligentsias is short while that of oogenesis is long (October–February). Female ovaries were morphologically and histologically examined in 7 stages; Immature Female, Chromatin-nucleolus, Perinucleolus, Cortical alveoli, Vitellogenesis, Maturity and Ovulation.
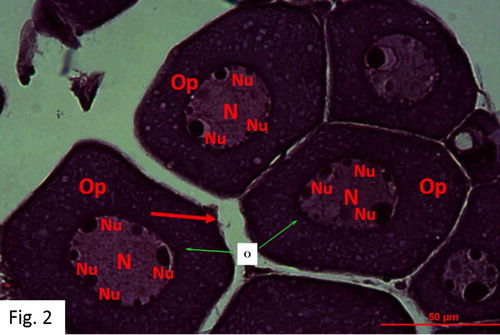
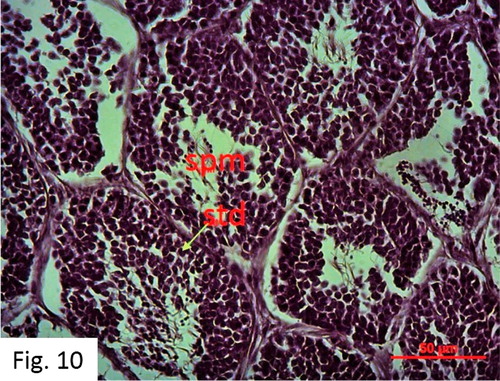
Immature Female: Mostly observed between August and April. 66 fish at 0+-IV+ ages were caught (). Gonads possess thin vein-like sections in the abdominal cavity, just below the spine. They are slightly purple-pink-yellowish and transparent, and look granular. Oocytes are spherical with a central nucleus. Oocytes with several nuclei in the peril-nucleolus stage are observed. Ovaries are not yet functional ().
Figure 1. Immature oocytes (H + E) Po: Primer oocytes; O: The small oocytes Oo: Oogonium Of: Ovarian folds.
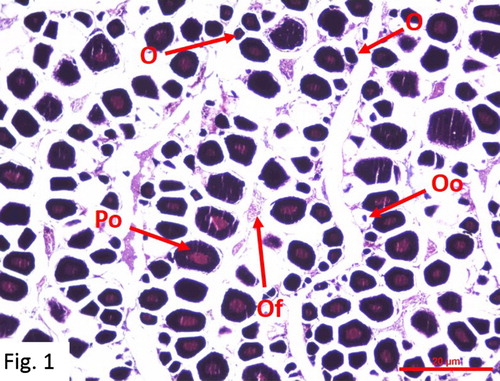
Table 1. Annual frequency of gonadal developmental stages of female and male individuals of M. barbatus.
Chromatin-nucleolus stage: Observed between November and August. 67 fish at I+-VI+ ages were caught (). The egg diameters range from 31 to 63 μm with a mean of 42 ± 12 μm. There are oocytes in the first stage of meiotic prophase. The oogonia are surrounded by connective tissues. They possess a light-coloured cytoplasm and a large nucleus. The oocyte has a nucleus containing nucleolus. The nucleoli are round ().
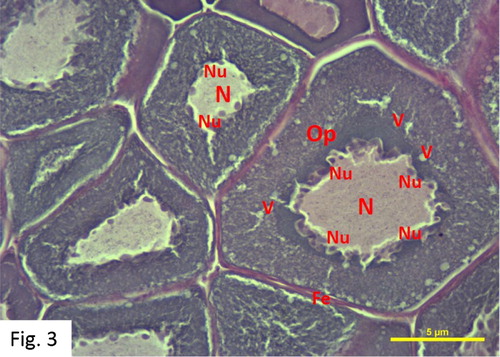
Perinucleolus stage: Observed between November and August. 63 fish at I+-IV+ ages were caught (). The egg diameters range from 46 to 106 μm with a mean of 77 ± 17 μm. The developing oocytes are centrally located. The oocytes contain a homogeneous cytoplasm, large nucleus and a large number of small elongated-looking peripheral nucleoli, which are more numerous, and peripheral in distribution in this stage than in the previous one. The cytoplasm is more basophilic in this stage than in the previous ones. Vacuoles form in the cytoplasm and begin to arrange themselves around the nucleus in the later phases of this stage ().
Cortical alveoli stage: Observed between February and August. 55 fish at I+-V+ ages were caught (). The egg diameters range from 56 to 219 μm with a mean of 168 ± 23 μm. The cytoplasm in the oocyte is basophilic and nonhomogeneous. The peripheral ooplasm contains cortical alveoli, which multiply and expand to fill the oocyte in the later phases of this stage. The egg yolk globules are mostly round and densely stained ().
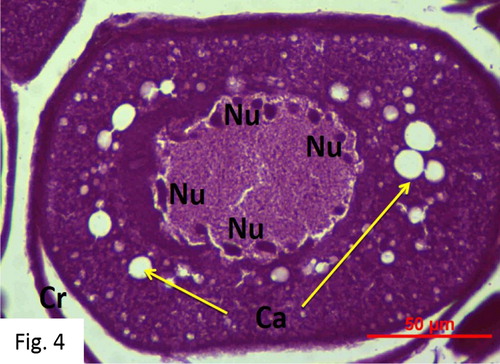
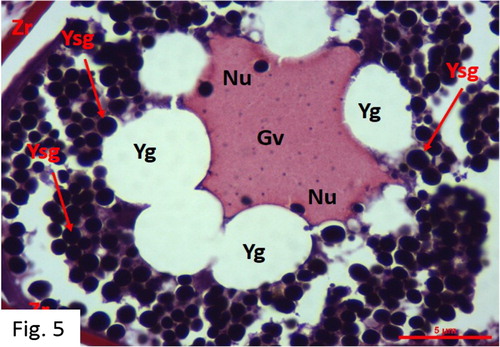
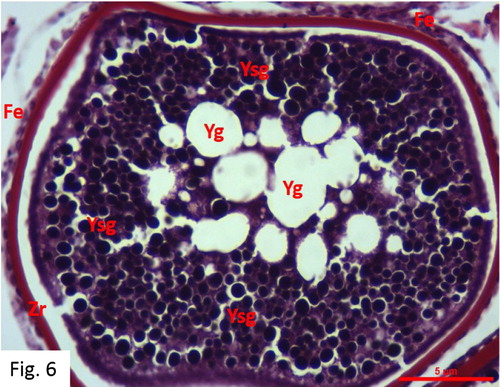
Vitellogenesis: Observed between March and October. 50 fish at I+-V+ ages were caught (). The egg diameters range from 188 to 305 μm with a mean of 246 ± 39 μm. Red-stained (acidophilic) yolk granules are widely apparent in the cytoplasm, starting from its periphery. The oocyte is larger in size and oil droplets are greater and more pronounced. The growth of the oocyte in size is compatible with the accumulation of numerous nutrient in the cytoplasm. Germinal vesicles, which are an indicator of oocyte maturation, are observed. The zona radiata is thickened. The nucleus membrane is dissolved and granular concentration increases. Oocyte membranes become thicker, and perivitelline space becomes wider in this stage than in the previous ones ().
Maturity: Observed between April and October. 39 fish at I+-VI+ ages were caught (). The egg diameters range from 229 to 428 μm with a mean of 344 ± 12 μm. Large hydrated oocytes and post-ovulatory follicles are observed. Oil droplets grow in number or merge together and become larger. At a later phase, the whole lipid and protein content of the egg mixes and becomes homogeneous, and the chorion becomes thinner due to the growth of the egg in size. The oocytes contain nutrient granules. The nucleus material changes in shape and position. The germinal vesicle in these oocytes migrates towards the animal pole and loses its shape completely, and the nucleoli completely disappear in the cytoplasm ().
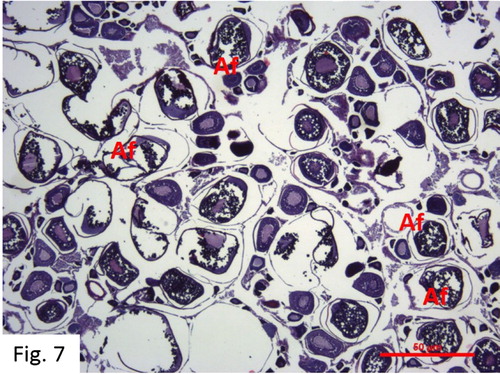
Post-ovulation: Observed between June and December. 59 fish at I+-VI+ ages were caught (). The ovaries are hemorrhagic. Free cells of unreleased eggs and undeveloped or underdeveloped oocytes can be observed together in the ovarian cavity at the end of the reproductive period. There are many athreptic follicles that were not released from the ovary ().
Gonad development in males
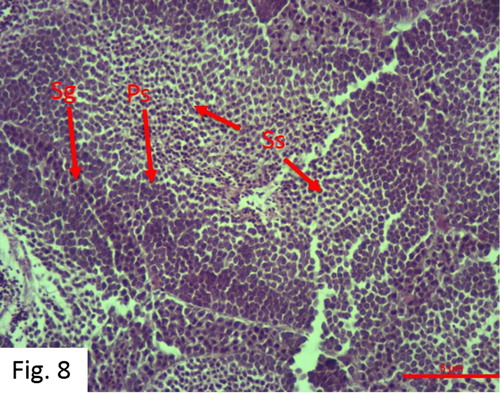
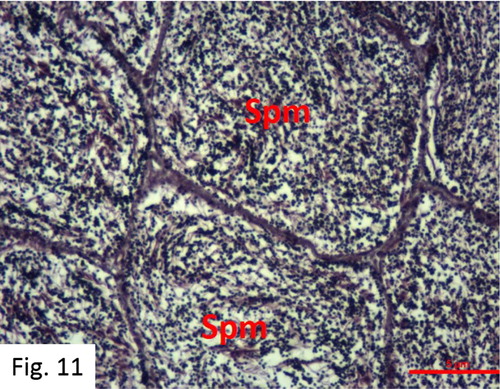
Spermatogenesis: Observed between September and July. 96 fish at 0+-VI+ ages were caught (). Centrally located spermatozoa have a round and dark-stained nucleus, which forms spermatogonial cells. In this inactive stage, testicular lobe dense spermatogonial cells and some primary spermatocyte mass are observed ().
Spermiogenesis: Observed between October and August. 97 fish at I+-IV+ ages were caught (). The highest rate (21.21%) was observed in February (). Spermatids and spermatozoa are more numerous than spermatogenesis, however, sperm channels are not well developed ().
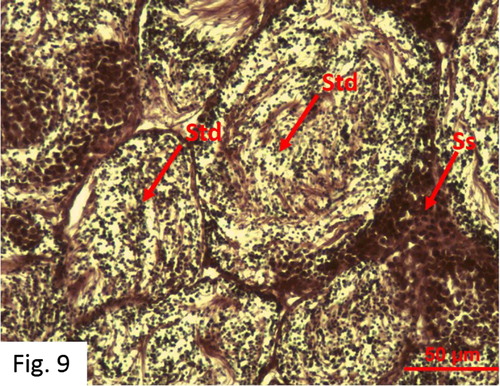
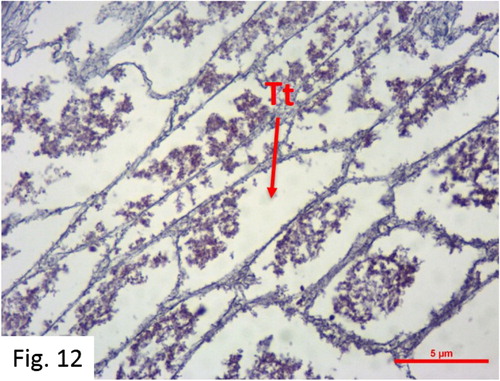
Spermiation: Observed between February and September. 85 fish at I+-VI+ ages were caught (). Numerous spermatozoa and spermatids are observed in the testis and deferens ducts. First, spermatogonium and then primary and secondary spermatocytes, and then spermatid, and finally spermatozoa stages were completed in the lobules separated by thick connective tissue compartments ().
Sperm release: Observed between March and September. 84 fish at I+-VI+ ages were caught (). Following maturation, a well-developed central lumen and partially hollow but a small number of spermatozoa are observed in the tubules in the cross-section of the testis (). Each fish, when the abdomen is squeezed, yields milk easily and the mature testis contains a very small amount of spermatogonial cells. The production of spermatogonial ceases, which results in a narrow spermatocyte.
Resting: Observed between May and January. 116 fish at I+-VI+ ages were caught (). After spawning, there is no sperm production, and regressed male gonad tissue and hollow tubules are observed. Some individuals possess residual sperms and blood cells in their tubules. Differentiated germ cells and spermatogonia groups are observed in the tubule walls with the reactivation of the testis ().
Discussion
The peak spawning period of red mullet in the Bay of Antalya is between May and September. M. barbatus, like most fish in the Mediterranean Sea, deposits eggs at the end of the spring and at the beginning of the summer. M. barbatus deposits eggs from May to September, depending on the water temperature. Becer et al. (Citation2006) report that red mullet starts its reproductive activities in the second half of May and continues until the end of June. Hekimoğlu (Citation1992) reports that the spawning season of red mullet in the Bay of Izmir is between the second half of April and the beginning of July. Akyol and Özekinci (Citation2000) and Metin (Citation2005) report that the spawning season of red mullet in the Bay of İzmir Bay is June. Papaconstantinou et al. (Citation1981) found that the spawning season of red mullet in the Saronic Bay and Thermaikos Bay (the Aegean Sea) is between April and July, while Kınıkarslan (Citation1972) and Toğulga (Citation1976) reported it to be between the middle of April and the beginning of July in the Aegean Sea. Though the results of this study are similar to those reported by previous ones, the spawning season of red mullet in the Mediterranean Sea is one month longer than that in the Aegean Sea. This may be due to environmental factors such as water temperature, salinity and nutrient quality in different geographical regions.
Metin (Citation2005) reports that the mature egg diameters of red mullet in the Bay of İzmir range from 0.61 to 0.72 mm while Mater and Çoker (Citation2002) state that they range from 0.65 to 0.77 mm, with no statistically significant difference between egg sizes. Aydın and Karadurmuş (Citation2013) found that the egg diameters of M. barbatus punticus in the Eastern Black Sea ranged from 157.6 to 611.8 μm. They stated that the biological parameters of M. barbatus punticus vary according to regional differences playing an important role in egg size. These values are definitely higher than those obtained in this study and suggest that regional differences play an important role. Kokokiris et al. (Citation2014) reported that the oocyte diameters of red mullet ranged from 56 to 363 μm. Becer et al. (Citation2006) determined that the egg diameters of M. barbatus varied between 0.32 and 0.58 mm in the Bay of Antalya. This study shows that the oocyte diameters of red mullet in the Bay of Antalya range from 31.00 ± 15 to 428.00 ± 40 µm. The egg diameters measured in this study are similar to those reported by Kokokiris et al. (Citation2014) and Becer et al. (Citation2006). The small differences may be due to the difference in methods, the effects of nutritional regimens on gonads, and the size of individuals.
Kokokiris et al. (Citation2014) examined the development of oocytes in M. barbatus in the Thermaikos Bay in five stages, which are oogonia proliferation, previtellogenesis, vitellogenesis, final oocyte maturation and ovulation. Glamuzina et al. (Citation1998), Valdés et al. (Citation2004) and Mackie et al. (Citation2009) morphologically examined mature females in 6 stages. Koç et al. (Citation2008) state that oogenesis in the majority of osteichthyes can be divided into five, six or eight stages. This study morphologically and histologically examined females in 7 stages; immature female, chromatin-nucleolus, perinucleolus, cortical alveoli, vitellogenesis, maturity and post-ovulation.
In the immature female stage, gonads possess thin vein-like sections in the abdominal cavity, just below the spine. They are slightly purple-pink-yellowish and transparent, and look granular. Oocytes are spherical with a central nucleus. Oocytes with several nuclei in the peril-nucleolus stage are observed. Ovaries are not yet functional. Kokokiris et al. (Citation2014) reported more space between follicles, a higher presence of interstitial tissue and a thicker ovarian wall in the immature female stage. Similar results were obtained in this study as well. Valdés et al. (Citation2004), Mackie et al. (Citation2009) and Kokokiris et al. (Citation2014) report the presence of light-stained cytoplasm and large nuclei in oogonia in the chromatin-nucleolus stage. Mahmoud (Citation2009) states that the oogonium and chromatin-nucleolus stage is characterized by ovary consisting of clusters of oogonium and oocytes in the chromatin-nucleolus and that more egg cells and perinucleolus oocytes are formed in the chromatin-nucleolus stage. In the chromatin-nucleolus stage, oocytes are small spherical cells with a thin indistinct peripheral zone referred to as ‘primordial follicle.’ The cytoplasm is strongly basophilic accepting all stains. The nucleus is spherical and large. A nucleolus covers the majority of the cell and is encapsulated by fibrous connective tissue. In this study, the oogonia are surrounded by connective tissues and possess a light-stained cytoplasm and a large nucleus. The oocyte possesses nucleus containing nucleolus and the nucleoli are round. Small previtrogenic oocytes are visible in the perinucleolus stage (Marino et al. Citation2001; Mc Millan Citation2007). Mahmoud (Citation2009) reports that, in the perinucleolus stage, the ovary consists mostly of polygonal and cytoplasmic cells, the nucleus volume of which increases, and that the nucleoli are smaller, greater in number and closer to the nucleus membrane, and that the cytoplasm is less basophilic. In the perinucleolus stage, the outer layer of follicular epithelial cells surrounding the oocyte thickens, but is not differentiated. Towards the end of this stage, the oocyte is characterized by the appearance of one or several small vacuoles in the cytoplasmic mass. These vacuoles begin to arrange themselves around the nucleus in a later phase of the stage. Similar to the results of other studies, the oocytes contain a homogeneous cytoplasm, large nucleus, and a large number of small elongated-looking peripheral nucleoli, which are more numerous and peripheral in distribution in this stage than in the previous one. The cytoplasm is more basophilic in this stage than in the previous ones. The developing oocytes are centrally located. Çelik (Citation2005), Mc Millan (Citation2007), Çakici and Üçüncü (Citation2007), Mackie et al. (Citation2009) and Kokokiris et al. (Citation2014) report that oocyte cytoplasm contains cortical alveoli near the outer surface in the glycoprotein structure. According to Koç et al. (Citation2008), granular structures in the ooplasm increase, cortical alveolar structures grow as the oocyte grows, the follicle size increases and the area surrounding the oocyte nucleus becomes opaque. During this stage, the nucleus grows. The nucleolus is pushed through the nucleus, the zona radiata begins to form and the follicle becomes thicker. The invaginations of the nucleus membrane are often accompanied by an irregular structure of the nucleus. This study also reports the presence of a nonhomogeneous basophilic cytoplasm containing cortical alveoli reflecting the cortical alveoli phase. Moreover, in a later phase of this stage, the cortical alveoli multiply and expand to fill the oocyte and the egg yolk globules are mostly round and densely stained. Koç et al. (Citation2008) state that, in the vitellogenesis stage, the oocyte grows in size, the egg yolk globules grow in size and number, the granular structures in the cortical alveoli stage become larger, and the nucleus becomes irregular in shape. According to Mahmoud (Citation2009), the ovary is often filled with previtogenic and vitellogenic oocytes at different stages of the egg yolk, most of the vacuoles are linked to each other and voids among egg yolk granules in the cytoplasm are formed. He also states that the cortical alveoli begin to liquefy with the accompanying growth in size, resulting in homogeneity in the egg yolk, beginning in the periphery of the oocyte. He states that the nucleus begins to migrate to the animal pole. This study shows that the oocyte membranes become thicker and perivitelline space becomes wider in this stage than in the previous ones. The red-stained (acidophilic) yolk granules become widely apparent in the cytoplasm, starting from its periphery and germinal vesicles, which are an indicator of oocyte maturation, are observed. Koç et al. (Citation2008) state that, in the maturity stage, the nucleus is not visible due to the fact that the granular structures fill the entire cytoplasm. The nucleus membrane is distorted. The lipid and protein particles are fused and become homogeneous. He also states that the vesicles fuse and grow in size. Mahmoud (Citation2009) defines this stage as the completion of the migration of the nucleus to the animal pole. The nucleolus loses its circular shape and migrates away from the nuclear membrane to the centre of the nucleus and completely loses its rigidity and its structure, and in the path of disintegration, it turns into a weak membrane and the cytoplasmic substances are released during this migration. He reports that the maturity stage appears oval and mostly pearl-shaped. As other researchers stated, as the egg progresses, the nucleus is directly behind the animal pole and when this occurs, the oocyte nucleus cannot be detected. The large egg yolk granules combine to form larger droplets towards the centre of the oocyte until the entire egg yolk area is fluidized. Oocytes that reach maturity permeate water and vitellogenic oocytes are known to be among them in different developmental stages. Cihangir (Citation1993) and Valdés et al. (Citation2004) state that the sheath at this stage is very thin and can explode with slight pressure. This study shows that the oocytes are full of nutrient granules and some of them become homogenous with the mixture of all lipids and proteins, and that the chorion becomes very thin due to the growth of the egg. The results also show that the nucleus material changes in shape and position, the germinal vesicle in the oocytes completes the migration to the animal pole and completely loses its shape, and the nucleoli disappear in the cytoplasm. Furthermore, the oocytes in this stage permeate water, the thickness of the membrane around the oocyte at the initial stages of egg development gradually decreases or even breaks, and the oocytes in different developmental stages are distributed among them. In the maturation stage, large oocytes and post-ovulatory follicles are visible. Valdés et al. (Citation2004) report the presence of atretic follicles in the post-ovulation stage. It is reported that most ovarian follicles are exposed to degeneration in the death of follicular cells and of the oocyte, and are removed by phagocytosis-mediated cells, and that atresia can be observed at every stage of follicle development (Junqueira et al. Citation1992). Mahmoud (Citation2009) reports that a large part of fully consumed ovaries contains a large number of empty nests and that those who do not lay eggs are exposed to atresia. He also characterizes ovaries in this stage with empty follicles and a small number of oogony stocks reserved for more laying. Koç et al. (Citation2008) state that this stage begins to disintegrate in accordance with the chromatin deformation of the nucleolus in the vitelline membrane structure. It is reported that the vesicles completely fuse and openings are formed in the outer regions of the vitelline membrane (breakdown of vitelline membrane and absorption of the yoke). The presence of eosinophilic cells (Valdés et al. Citation2004) and atretic follicles (Junqueira et al. Citation1992) in the post-ovulation stage of M. barbatus shows that they reached the latest stage. The nucleus starts to disintegrate in accordance with the chromatin deformation, and space is formed in the vitelline membrane (Koç et al. Citation2008; Mahmoud Citation2009).
Mackie et al. (Citation2009) and Abu El-Nasr (Citation2016) investigated testicular maturation in four and six stages, respectively. Fostier et al. (Citation1987), Kokokiris et al. (Citation2001), Valdés et al. (Citation2004) and Çek et al. (Citation2005) examined male gonadal development in five morphological stages. This study examined the development of male gonad in five morphological stages, which are spermatogenesis, spermiogenesis, spermiation, sperm release and resting. Mackie et al. (Citation2009) reported that the central sinus is smaller in the spermatogenesis stage than in the later ones, that the radial sperm sinuses are poorly developed and that spermatogonia, spermatocytes and connective tissue are dominant. As Valdés et al. (Citation2004) pointed out, the testicular tissue in the spermatogenesis stage shows parallelism with basophilic stained male reproductive cells in the lobules in various developmental stages. In this stage, mostly spermatogonia and a small amount of primary spermatocytes are present. This stage is usually observed in October, November and December. Valdés et al. (Citation2004) and Kokokiris et al. (Citation1999) report the presence of spermatids and spermatocytes in spermiogenesis. In this stage, radial sperm sinuses are more pronounced, however, fewer spermatozoa and dominant spermatocytes are observed (Mackie et al., Citation2009). The highest rates of spermatocytes and spermatozoa in the testicular tissue in the spermiogenesis stage are observed in February and March. The samples in this study reveal that though primary spermatocytes and fewer spermatozoa are present, sperm ducts are not well developed. Mackie et al. (Citation2009) report that the central sinus is larger in the spermiation stage than in the previous ones, that the radial sperm sinuses contain many sperms and spermatogonia, and that spermatocytes are dominant. Valdés et al. (Citation2004) report the presence of numerous spermatozoa in the testis and deferens ducts of male gonad in the spermiation stage. In this study, numerous spermatids as well as spermatozoa were observed in the testis and deferens ducts. First, spermatogonium and then primary and secondary spermatocytes, and then spermatid and finally spermatozoa stages were completed in the lobules separated by thick connective tissue compartments. The spermiation stage is observed between March and July but the highest rate is between April and June. Mackie et al. (Citation2009) report that, in the sperm release stage, the central and radial sperm sinuses grow and are filled with sperm but that spermatids and spermatozoa are dominant. Valdés et al. (Citation2004) state that although the tubules in the testis tissue are often hollow, a very small amount of spermatozoa is sometimes observed. In this study, a small number of spermatozoa were visible in the partially empty tubules after sperm release in the presence of a well-developed central lumen in a portion of the testis. As noted by Kokokiris et al. (Citation1999), Valdés et al. (Citation2004) and Mackie et al. (Citation2009), connective tissue is dominant and there is no production of spermatozoa in the testis tissue of male fish in the resting stage. They also report that there is little residual sperm and that sperm sinuses become smaller in the resting stage. The presence of regressed testicle tissue at the end of the spawning season is similar to the results reported by previous studies.
Conclusion
As a result of the histological examination, gonadal development of M. barbatus males was evaluated in five stages; spermatogenesis, spermiogenesis, spermiation, sperm release and resting, while gonadal development of M. barbatus females was addressed in seven stages; immature female, chromatin-nucleolus, perinucleolus, cortical alveoli, vitellogenesis, maturity and post-ovulation. In conclusion, M. barbatus, like most fish species in the Mediterranean Sea, spawn at the end of the spring and at the beginning of the summer. The spawning season of M. barbatus is between May and September in Antalya Bay of Eastern Mediterranean in Turkey.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Beytullah Ahmet BALCI http://orcid.org/0000-0002-6762-3259
Additional information
Funding
References
- Abu El-Nas TMA. 2016. Histological changes in the testes of the fish, Gerres filamentosus (Cuvier, 1829) during the reproductive cycle in the Hurghada Red Sea, Egypt. 5th International Conference on Agriculture, Environment and Biological Sciences (ICAEBS-16).
- Akyol O , Özekinci U. 2000. The effects of beach seine net on some economic fish species in the Aegean Sea. EÜ Su Ürünleri Dergisi. 17(1-2):185–199.
- Aydin M , Karadurmus U. 2013. An investigation on age, growth and biological characteristics of red mullet (Mullus barbatus ponticus, Essipov, 1927) in the Eastern Black Sea.
- Bancroft JD , Stevens A. 1996. Theory and practice of histological techniques. 4th ed. New York : Churchill Livingstone; 436 p.
- Bariche M , Bilecenoglu M , Azzurro E. 2013. Confirmed presence of the Red Sea goatfish Parupeneus forsskali (Fourmanoir & Guézé, 1976) in the Mediterranean Sea. BioInvasions Records. 2(2):173–175. doi: 10.3391/bir.2013.2.2.15
- Becer ZA , Balci BA , Özbaş M , Gökoğlu M , Gülyavuz H , Taşli A , Pehlivan M , Kaya Y. 2006. Antalya Körfezi’nden Avlanan Barbunya Balığı (Mullus barbatus L., 1758)’nın Büyüme Özellikleri Üzerine Bir Araştırma. EÜ Su Ürünleri Dergisi. 23(1):113–118.
- Bilecenoğlu M , Kaya M , Cihangir B , Çiçek E. 2014. An updated checklist of the marine fishes of Turkey. Turk J Zool. 38:901–929. TÜBİTAK. doi:10.3906/zoo-1405-60.
- Çakıcı Ö , Üçüncü Sİ . 2007. Oocyte development in the zebrafish, Danio rerio (Teleostei: Cyprinidae). EU J Fish Aquat Sci. 24 (1–2):137–141.
- Çek Ş , Turan F , Sarihan F. 2005. Zebra Çiklit (Cichlasoma nigrofasciatum (GÜNTHER, 1867))’ in Bazı Üreme Özelliklerinin Belirlenmesi. http://www.akuademi.net /USG/USG2005/Y/y19.pdf.
- Çelik F. 2005. Değişik Oranlarda Yeme Katılan E Vitaminin Oreochromis niloticus L., 1758 Türünün Büyüme Parametreleri ve Bazı Dokularının Histolojisi Üzerine Etkileri [master’s thesis]. Adana: Cukurova University, Graduate School of Natural and Applied Sciences, Department of Aquatic Products; p. 60.
- Çiçek E. 2015. Age, growth and mortality parameters of Mullus barbatus Linnaeus, 1758 (Perciformes: Mullidae) in Iskenderun Bay, northeastern Mediterranean. Iran J Ichthyol. 2(4):262–269.
- Cihangir B. 1993. Ege Denizi’nde Sardalya Balığı, Sardina pilchardus (Wabaum, 1792)’un Üremesi. Turk J Zool. 20:33–50.
- Fiorentino F , Zamboni A , Rossi M , Relini G. 1998. The growth of the red mullet (Mullus barbatus, L. 1758) during the first years of life in the Ligurian Sea (Mediterranean). CIHEAM-IAMZ. 35:65–78.
- Fostier A , Le Gac F , Loir M. 1987. Steroids in male reproduction. In: DR Idler , LW Crim , JM Walsh , editor. Proceeding 3rd international symposium reproductive physiology fish. St. Johns : Memorial University Press; p. 239–245.
- Glamuzina B , Skaramuca B , Kozul V. 1998. Oogenesis of the Dusky Grouper, Epinephelus marginatus (Lowe, 1834) in captivity. Aquaculture and Water Fish Culture, Shellfish Culture and Water Usage, European Aquaculture Society/Special Publication No: 26, Oosende, Belgium. p. 95–96.
- Gökoğlu M. 2018. A new goatfish species of the genus Upeneus (Mullidae) in the Gulf of Antalya. Acta Aquatica: Aquat Sci J. 5(2):56–58. doi: 10.29103/aa.v5i2.796
- Golani D , Öztürk B , Başusta N. 2006. Fishes of the Eastern Mediterranean. Istanbul : Turkish Marine Research Foundation. Pub. Number: 24, p. 259.
- Gurbet R , Hossucu H , Ilkyaz AT , Ozekinci U. 1997. The study on the comparison of selectivity of 40 and 44 mm mesh size in the trouser bottom trawls. Mediterranean Fisheries Congress, April 9–11, 1997, Izmir, Turkey. p. 77–91 (in Turkish).
- Hekimoğlu MA. 1992. İzmir Körfezi Barbunya Balığı (Mullus barbatus L., 1758) Populasyonu Üzerine Bir Çalışma [master’s thesis]. Bornova-İzmir: Ege University, Graduate School of Natural and Applied Sciences, Department of Aquaculture Engineering; 41 p.
- İşmen A , İşmen P. 2001. Reproduction biology, fecundity and population dynamics of species belonging to Mullidae family . Hatay : M.K.U. Research Foundation. No. 99/E-3702, 27p. (In Turkish).
- Joksimovic A. 2005. Population dynamic of red mullet (Mullus barbatus, L., 1758) in the Montenegrin shelf [PhD thesis]. Faculty of Biology, University of Belgrade.
- Junqueira LC , Carneiro J , Kelley RO. 1992. Basic histology. 7th ed. Baltimore : Appleton & Lange. 522 p.
- Kinikarslan N. 1972. Edremit Körfezi Barbunya (Mullus barbatus L., 1758)’ların Büyüme İndeksi ve Yıllık Büyümeleri Üzerine Araştırmalar. İÜ Fen Fak Hid Araş Ens Yay 8. İstanbul. 105s.
- Koç ND , Aytekin Y , Yüce R. 2008. Ovary maturation stages and histological investigation of ovary of the Zebrafish (Danio rerio). Braz Arch Biol Technol . 51(3). doi:10.1590/S1516-89132008000300010.
- Kokokiris L , Bruslé S , Kentouri M , Fostier A. 1999. Sexual maturity and hermaphroditism of the red porgy, Pagrus pagrus (Teleostei, Sparidae). Mar. Biol. 134:621–629. doi: 10.1007/s002270050577
- Kokokiris L , Le Menn F , Kentouri M , Kagara M. 2001. Seasonal cycle of gonadal development and serum levels of vitellogenin of red porgy, Pagrus pagrus (Teleostei: Sparidae). Mar. Biol. 139:549–559. doi: 10.1007/s002270100604
- Kokokiris L , Stamoulis A , Monokrousos N , Doulgeraki S. 2014. Oocytes development, maturity classification, maturity size and spawning season of the red mullet (Mullus barbatus barbatus Linnaeus, 1758). J Appl Ichthyol. 30:20–27. doi:10.1111/jai.12292.
- Mackie M , Jackson C , Tapp N , Norriss J , Thompson A. 2009. Macroscopic and Microscopik Description of Snapper (Pagrus auratus) Gonads from Shark Bay, Western Australia. Fisheries Research Division, Fisheries Research Report No: 184.
- Mahmoud HH. 2009. Gonadal maturation and histological observations of Epinephelus areolatus and Lethrinus nebulosus in Halaieb/Shalatien Area “Red Sea”, Egypt. Glob Vet. 3(5): 414-hut423.
- Marino G , Azzurro E , Massari A , Finoia MG , Mandich A. 2001. Reproduction in the dusky grouper from the southern Mediterranean. J Fish Biol. 58(4):909–927. doi: 10.1111/j.1095-8649.2001.tb00544.x
- Mater S , Çoker T. 2002. The Atlas of Ichtyoplankton of Turkey Marins (in Turkish). Ege University Faculty of Fisheries publications No: 71, Wordbook No: 12, 209 p.
- Mc Millan DB. 2007. Fish histology: female reproductive systems. Hardcover : Kluwer Academic Publishers; 598 p.
- Mert I , Doğan M , Türkmen M , Karataş B , Yavuzkan Y. 1983. Study on spawning period of economical important fish species caught from Antalya Bay. Republic of Turkey, Ministry of Agriculture and Rural Affairs, General Directorate of Fisheries, Project no. 82-A050050-7, Antalya, 34 p. (in Turkish).
- Metin G. 2005. İzmir Körfezi’nde Barbunya (Mullus brabatus L., 1758) Balığının Üreme Özellikleri. EU J Fish Aquat Sci. 22(1–2):225–228.
- Özyurt CE. 2003. The Determination of mesh size for some commercially important demersal fish species captured by deep trawl net at Babadıllimanı Bight (Silifke-Mersin) [PhD thesis]. Adana: Cukurova University; 124 p. (In Turkish).
- Papaconstantinou C , Tsimenidis N , Daoulas C. 1981. Age, growth and reproduction of red mullet (M. barbatus L., 1758) in the Bay of Saronikos and Thermaikos. Thalassographica Inst Oceanogr Fish Res. 4(1):39–66.
- Presnell JK , Schreibman MP. 1997. Humason’s animal tissue techniques. 5th ed. London : The Johns Hopkins University Press Baltimore. 572 p.
- Relini G , Bertrand J , Zamboni A. 1999. Synthesis of the knowledge on bottom fishery resources in Central Mediterranean (Italy and Corsica). Biol Mar Mediterr. 6(suppl. 1):314–322.
- Toğulga M. 1976. İzmir Körfezi’nde Barbunya Balığının Biyolojisi ve Populasyon Dinamiği Üzerine Araştırmalar. Yüksek Lisans Tezi, Ege Üniv. Fen Fak. Genel Zooloji Kürsüsü. İzmir. 46 s.
- Tokaç A , Lök A , Metin C , Tosunoğlu Z , Ulaş A. 1995. Selectivity research for demersal fish resource in trawl fishery. Tubitak, Project No: DEBAG-105, Izmir, 80 p. (in Turkish).
- Tokaç A , Özbilgin H , Tosunoğlu Z. 2004. Effect of PA and PE material on codend selectivity in Turkish bottom trawl. Fish Res. 67:317–327. doi: 10.1016/j.fishres.2003.10.001
- Tosunoğlu Z , Tokaç A. 1997. Improvement of deep trawl net selectivity. Fisheries Congress, 9–11 April 1997, Izmir, Turkey; p. 103–109. (In Turkish).
- Türeli C , Erdem Ü . 1997. The growth performance of red mullet (Mullus barbatus Linnaeus, 1758) and brushtooth lizardfish (Saurida undosquamis (Richardson, 1848)) from the coastal region of Adana Province (İskenderun Bay, Turkey). Turk J Zool. 21:329–334. (In Turkish).
- Türkmen M , Başusta N , Demirhan SA. 2010. Balıklarda yaş tayini (In Research Techniques in Fish Biology (Ed. By M Karataş)). Nobel Kitabevi. Ankara; p. 35–62 (In Turkish).
- Valdés P , García-Alcázar A , Abdel I , Arizcun M , Suárez C , Abellán E. 2004. Seasonal changes on gonadosomatic index and maturation stages in common pandora Pagellus erythrinus (L.). Aquac Int. 12 (4-5):333–343. doi: 10.1023/B:AQUI.0000042136.91952.9e
- Voliani A , Abella A , Auteri R. 1998. Some considerations on the growth performance of Mullus barbatus . Cah Options Méditerranéennes (CIHEAM). 2: 93–106.
- Vrgoč N. 2000. Struktura i dinamika pridnenih zajednica riba Jadranskog mora [Disertacija]. Sveučilište u Zagrebu.