ABSTRACT
The present study was designed to find the effect of ginger and garlic on the performance and integrity of gut in experimentally induced coccidiosis in broiler chickens. A total of two hundred and forty day-old Hubbard broiler chicks were divided into six equal groups as following: T1, Control (basal diet only); T2, Infected–untreated (positive control); T3, Infected and supplemented with garlic at 15 g/kg feed; T4, Infected and supplemented with gingers at 5 g/kg feed; T5, Infected and treated with amprolium hydrochloride at 1.25 g/liter drinking water; T6, Infected and supplemented with mixture of garlic and ginger at the rate of 2.5 and 7.5 g/kg feed. The results showed that feed intake, body weight and feed conversion ratio (FCR) was significantly (P < .05) high in ginger and garlic supplemented birds compared to the positive control. Similarly, oocysts shedding, lesion score and histopathology of the small intestines improved in ginger and garlic supplemented birds after induced-infection in broiler. The findings of the present study showed that ginger and garlic produced encouraging results in comparison to amprolium in broiler chickens infected with experimental coccidiosis.
Introduction
Coccidiosis is one of the major protozoan diseases of poultry caused by genus Emeria. This organism mostly affects the gastrointestinal tract causing reduced feed intake, weight gain, worsen feed efficiency and higher mortality (Tanweer et al. Citation2014; Chand et al. Citation2016). Massive economic losses are associated with the outbreak of coccidiosis in the form of morbidity and mortality of the birds (Abudabos et al. Citation2017). Eimeria species mostly infect the ceca and small intestines. During caecal coccidiosis, different types of lesions are present which depend upon the species of coccidia (Tanweer et al. Citation2014). The organisms proliferate in the intestinal cells and cause tissue destruction (Arczewska et al. Citation2012).
A large number of anticoccidial drugs are used for the control of coccidiosis, however, their excessive use has led to the development of drug resistance, residues in the tissues and organs and high economic cost. Therefore, scientists are searching to identify the efficacy of different herbs and herbal by-products to decrease the huge losses caused by coccidiosis in the poultry business. Herbal plants and their byproducts may serve as remedies for coccidiosis because of their low toxicity and reduced cost of production (Abbas et al. Citation2006). Garlic (Allium sativum) contains important alkaloids such as allin, ajoene, allicin and diallyl sulphide Sallylcysteine exhibiting antibacterial, anti-inflammatory, antiseptic, antiparasitic and immunomodulatory properties (Adibmoradi et al. Citation2006; Khan et al. Citation2012a). Ginger (Zingiber officinale) contains active ingredients such are gingerdoine, gengerdiol and gingerol (Khan et al. Citation2012b; Raza et al. Citation2016; Zia ur Rehman et al. Citation2018). Ginger has been reported to enhance the growth performance and digestibility in broilers and effective in treating and controlling coccidial infection (Zhang et al. Citation2009). The objective of the present study to find the effect of ginger and garlic in comparison with standard anticoccidial drug, amprolium against experimentally induced coccidiosis in broiler chickens.
Materials and methods
Collection and preparation of plant bulbs material
The herbal plant’s bulbs of garlic and ginger were purchased from the local market in fresh condition. After identification by the botanical section of Weed Science Department, The University of Agriculture Peshawar, the plants were used in the present study. The garlic and ginger bulbs were dried in hot air oven at the temperature of 70°C for three days. After drying, the plant materials were ground with the help of an electric grinding machine.
Experimental design
A total of 240 day-old Hubbard broiler chicks were purchased from the hatchery. After the adaptation period of one week, chicks of equal weight were randomly divided into six groups (designated as T1 to T6) with five replicates. The chicks were reared throughout the experimental period in an open-sided house with well-maintained cross ventilation. Sawdust was used as bedding material. Continuous light was provided throughout the experiment. During the grower stage, the birds were shifted to stainless steel cages (1.7× 1.5 m) till the end of the experiment. The temperature of the house was maintained at 35°C for the first week and then gradually decreased to 23°C till the end of the experiment. Manual feeders and drinkers were used. The diet was provided in two phases consisting of starter phase (0–21 days) and finisher (22–42 days) as shown in . The chicks had free access to feed and water. Birds were vaccinated against infectious bronchitis, Newcastle disease and infectious bursal disease according to the standard schedule.
Table 1. Basal composition of feed during the starter and finisher phase.
The groups were arranged as follows: T1, Control (basal diet only); T2: Infected–untreated (positive control); T3, Infected and supplemented with garlic at 15 g/kg feed; T4: Infected and supplemented with gingers at 5 g/kg feed; T5: Infected and treated with amprolium hydrochloride at 1.25 g/liter drinking water; T6: Infected and supplemented with mixture of garlic and ginger at the rate of 2.5 and 7.5 g/kg feed, respectively.
Preparation of Emeria infection
The oocysts of Emeria species were taken from infected caeca following the procedure of Chand et al. (Citation2016). Briefly, caecal contents were immersed in 2.5% potassium dichromate solution for overnight. The suspension was then sieved and the remaining portion was centrifuged at 1500 rpm for three minutes. The sediment was suspended and mixed with a saturated solution of NaCl. The supernatant was discarded and the remaining suspension was centrifuged at 1500 rpm for three minutes. The bottom containing oocysts were again placed in 2.5% solution of potassium dichromate. Potassium dichromate solution containing ooyst were incubated at 30Co for 24–72 h in petri dish and then stored at 4Co in the refrigerator. The number of oocysts was adjusted to 30,000 sporulated oocysts per 2 ml of inoculum. Except the negative control, all the groups were infected with 20,000–30,000 oocysts per chick orally on day 8 of the experiment.
Performance trait
The weighed feed was offered at libitum to all the experimental broilers. The refused feed was collected and weighed the next morning before offering feed. Body weight gain was recorded at the end of every week. Weight gain was calculated by subtracting the initial weight of birds from the final weight of the birds. The feed conversion ratio (FCR) was computed at the end of each week and the final FCR was calculated and note at the completion of experimental duration. The number of dead birds during the experiment was recorded throughout the experiment.
Number of oocysts per gram (OPG) of faeces
The faecal samples were collected on day 5, 7, 10 and 12 post coccidia infection (dpi). Samples were kept in the refrigerator for determination of OPG. The oocysts counting were done through Mc Master Techniques as described by Chand et al. (Citation2016). Faecal samples (2 g) were mixed in 10% (w/v) NaCl solution. This suspension was poured to McMaster chamber with the help of a micropipette and the numbers of oocysts were counted using microscope.
Lesion scoring
Lesion scoring of the ceca was done at the end of the experiment. One bird per replicate was randomly selected for lesion scoring. The lesion was comprised on petechial haemorrhages, thickness of intestinal wall and congestion based on severity. The lesions were scored as suggested by Tanweer et al. (Citation2014).
Histopathological examination
At the end of the experiment, three birds were selected randomly from each group and were slaughtered. Ceca were separated and processed for histopathological examination as described by Chand et al. (Citation2016). About 1 cm thick tissue of ceca was cut and placed in 10% buffer formalin. Tissue was passed in ascending grade of alcohol for dehydration. Tissue was then placed in paraffin and tissue block and sectioned by using microtome (Accu-Cut® SRM™ 200 Sakura) with a thickness of about 4 μm. Slide was stained with haematoxylin and eosin (H&E). Randomly, five views per slide per sample for a total of 50 readings per treatment were recorded.
Statistical analysis
Collected data were statistically analysed through the procedure of analysis of variance (ANOVA) by using randomized complete block design (RCBD). Means were compared for significance difference by least significant difference (LSD) using the method described by Steel and Torrie (Citation1997). Statistical package (STATISTIC-2010) was used to carry out above analysis on computer.
Results
Weekly and overall feed intake was significantly (P<.01) affected by supplementation of phytogenic (ginger and garlic) in broilers feed as shown in . Feed intake was significantly (P < .01) high in the negative control and significantly (P < .01) low in the positive group. During 2nd and 3rd week in the treated groups, the highest feed intake was recorded in amprolium group and the combination of ginger and garlic. During the 4th week, higher feed intake was noticed in all the treated groups. In the 5th week, significantly high feed intake was observed in the ginger and garlic supplemented groups. In the 6th week, highest feed intake was observed in the amprolium group, however, overall highest feed intake was recorded in the ginger and amprolium treated birds.
Table 2. Mean feed intake (g) of control and treated groups fed with ginger and garlic in coccidiosis induced birds.
Weekly and overall weight gain was significantly (p<.05) affected by the supplementation of phytogenic (ginger and garlic) in broilers feed as shown in . Except, 4th week, the highest (P < .01) weight gain was observed in the negative control. The worst weight gain was recorded in the positive group. Except, 4th week, the highest (P < .01) body weight was recorded in amprolium treated birds on weekly and overall basis. The ginger and garlic treated birds showed better body weight than positive control showing the effectiveness of these natural products.
Table 3. Mean body weight (g) of control and treated groups fed with ginger and garlic in coccidiosis induced birds.
Supplementation of phytogenic affected FCR at all stages except the second week of age (). In the 3rd and 4th weeks, better FCR was noticed in amprolium hydrochloride followed by ginger and garlic supplementation. In the 5th week, significantly (P < .01) high FCR was observed in amprolium, garlic, ginger supplementation or their combination. During 6th week and overall, significantly (P < .05) high body weight was observed in ginger supplemented birds.
Table 4. Mean FCR (g/g) of control and treated groups fed with ginger and garlic in coccidiosis induced birds.
Weekly and overall oocyst number was significantly (P<.01) affected by the supplementation of phytogenic (ginger and garlic) and amprolium hydrochloride in broilers as shown in . As expected, oocyst per gram was significantly (P < .01) low in the amprolium treated birds. In the ginger and garlic treated birds, significantly (P < .01) low oocysts was observed as compared to the positive control.
Table 5. Mean oocyst per gram of faeces in control and treated groups in coccidiosis induced birds.
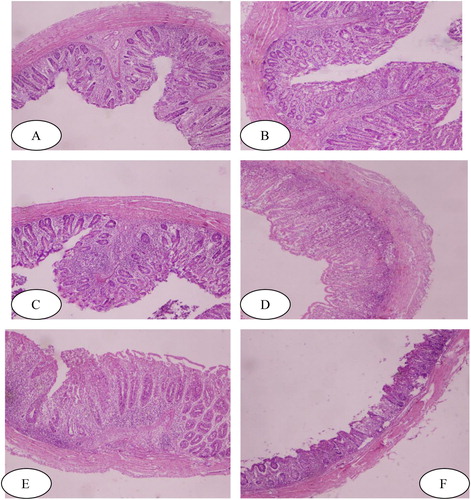
The effect of treatments on histopathology of ceca in control and treated groups on day 42 is given in . Negative control (uninfected untreated) revealed normal glandular structure, epithelium, and normal histomorphology (A). The infected and supplemented group with garlic revealed slight sloughing of villi (B). Infected and supplemented group with ginger showed hypotrophy of villi, hyperplasia of goblet cells and shortening of crypt (C), while infected and treated with amprolium hydrochloride group showed intact and strong normal histomorphology (D). Histopathology of the infected and supplemented group with ginger and garlic mixture showed sloughing of villi, shortening of crypt, glandular hypertrophy and hyperplasia of goblet cells (E). Histopathology of ceca of positive control (infected untreated) showed atrophy and sloughing of villi, shortening of crypt, necrotic glandular structures and glandular hypotrophy (F).
Figure 1. Negative control (A); Amprolium treated (B); Garlic treated (C); Ginger treated (D); Ginger and garlic treated (E); positive control (F) H & E stain (40 X) n = 100 µm.
Lesion score of ceca of different groups is presented in . Severe caecal haemorrhages were present in the positive control group. Moderate haemorrhages were seen in the ginger supplemented group and ginger-garlic mixed supplemented group. Mild haemorrhages and changes were revelled in garlic supplemented group while there was no lesion in the negative control. Moderate thickness of intestine was founded in positive control and ginger-garlic mixed supplemented group. Mild thickness of intestine was seen in garlic supplemented group, ginger supplemented group, and amprolium hydrochloride while no thickness of intestine was found in the negative control group. Congestion was moderate in the positive control while mild in garlic supplemented group, ginger supplemented group and ginger-garlic mixed supplemented group.
Table 6. Mean lesion score of control and treated groups fed with ginger and garlic in coccidiosis induced birds.
Mean mortality was significantly (P < .05) affected by phytogenic and amprolium hydrochloride in broilers challenged with Emeria (). The highest mortality was noted in the positive control, while lower and the same mortality was recorded for all other groups.
Table 7. Mean mortality (%) of control and treated groups fed with ginger and garlic in coccidiosis induced birds.
Discussion
In the current study, feed intake and body weight in broilers were significantly affected by phytogenic and amprolium hydrochloride in broiler. As expected, the highest feed intake, body weight and FCR were observed in the negative control, which severely decreased in the positive control. Amprolium treatment improved the feed intake and body weight in the infected birds. In addition, the supplementation of garlic and ginger improved the performance of the birds compared to the positive control although it was not as effective as amprolium. The higher feed intake was also recorded in ginger and garlic supplemented groups, while the lowest feed intake was recorded in the positive control. Ginger contains several important pharmacological compounds such as gingerdione, gingerdiol, shogaols and gingerol (Raza et al. Citation2016). The improved performance in broiler fed with ginger has been linked with the enhanced palatability and improved digestive process which increase the feed intake and hence weight gain (Khan et al. Citation2012a). In addition, ginger is also involved in the increased secretion of digestive enzymes and helps in the digestion process.
Similarly, garlic contains 17 amino acids, minerals, enzymes and sulpher containing compounds. The important alkaloids in ginger are s-allylcystein sulphoxide, s-methyl-cystein sulphoxide, diallydisulphide, s-allylcystein, diakyl polysulphides, ajoene and allicin (Khan et al. Citation2012b). The increase feed intake and body weight in garlic supplemented group may also be due to the presence of these compounds in garlic. Allicin has been reported to improve and regenerate the physiological structure of the intestinal epithilum layer, enhances crypt depth and villus height, which ultimately support the digestive capacity through increased absorption of nutrients and assimilation (Adibmoradi et al. Citation2006).
Oocyst count was significantly affected by supplementation of phytogenic (ginger and garlic) and amprolium hydrochloride during 5th, 7th, 9th and 11th day post infection. The decrease oocyst in the faeces of garlic supplemented group may be due to the presence of allicin in garlic. Allicin has antioxidant and antiparasitic activity and stimulates the immunity by enhancing profiline antibody response which directly kills the sporozoites (Khan et al. Citation2012b; Kim et al. Citation2013). The lower oocysts count may also be due to the presence of phenolic compounds in garlic, which act on the cytoplasmic membrane of Eimeria and make changes in their cation permeability, leading to the death of Eimirria (Tanweer et al. Citation2014). Garlic has been known for increased production of white blood cells, antibodies and enhanced phagocytosis of infected organisms (Khan et al. Citation2012b). These properties are probably responsible for the coccidiostatic effect of ginger. Kim et al. (Citation2013) reported that the supplementation of active ingredients of garlic (propyl thiosulphinate oxide and propyl thiosulphinate) had decreased faecal oocysts excretion and greater antibody response against Eimeria acervulina in broiler chickens. Important compounds derived from gingerdiol, shogaols, gingerol, gingerdione and other phenolic compounds show antioxidant properties (Khan et al. Citation2012b), which may be responsible for coccidiostatic effect in the broiler.
The present study showed that Eimeria species lead to the degenerative changes in the positive control group while garlic supplementation decreased the intestinal lesion. Findings of the present study supported the findings of Gotep et al. (Citation2016) who reported the highest capacity of crypt and villi of small intestines by the addition of garlic in the broilers feed infected with coccidiosis. The results obtained in groups treated with amprolium and garlic powders may be due to the presence of the chemical hydrochloride in amprolium hydrochloride and allicin and phenolic compounds in garlic, which alter the cytoplasmic penetrability and finally damage the Eimeria cells. The lower mortality (higher survival rate) in supplemented groups may be due to the antioxidant properties of garlic, which causes oxidative stress against parasites and neutralize oxygen reactive species (Allen and Danforth Citation1998).
Conclusion
It is concluded from the present study that ginger and garlic show improved performance and coccidiostatic effect in broilers.
Conflict of interest
Authors have no potential conflict of interest
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbas RZ , Iqbal Z , Akhtar MS , Khan MN , Jabbar A , Sandhu ZU. 2006. Anticoccidial Screening of Azedarach Indica (Neem) in broilers. Pharmacol Online. 3:365–371.
- Abudabos AM , Alyemni AH , Swilam EO , Al-Ghadi MA. 2017. Comparative anticoccidial effect of some natural products against eimeria spp. infection on performance traits, intestinal lesion and occyte number in broiler. Pak J Zool. 49. doi:10.17582/journal.pjz/2017.49.6.1989.1995.
- Adibmoradi M , Navidshad B , Seifdavati J , Royan M. 2006. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J Poultry Sci. 43:378–383. doi:10.2141/jpsa.43.378.
- Allen PC , Danforth HD , Augustine PC. 1998. Diet modulation of avian coccidiosis. Int. J. Parasitol. 28:1131–1140. doi: 10.1016/S0020-7519(98)00029-0
- Arczewska-Wlosek A , Swiatkiewicz S. 2012. The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J Anim Feed Sci. 21:133–142. doi: 10.22358/jafs/66058/2012
- Chand N , Faheem H , Khan RU , Qureshi MS , Alhidary IA , Abudabos AM. 2016. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ. Sci. Poll. Res. 23:14414–14421. doi:10.1007/s11356-016-6600-x.
- Gotep JG , Tanko JT , Forcados GE , Muraina IA , Ozele N , Dogonyaro BB , Oladipo OO , Makoshi MS , Akanbi OB , Kinjir H , Samuel AL. 2016. Therapeutic and safety evaluation of combined aqueous extracts of Azadirachta indica and Khaya senegalensis in chickens experimentally infected with Eimeria oocysts. J. Parasitol. Res. Article ID 4692424, 9 pages. doi:10.1155/2016/4692424.
- Khan RU , Naz S , Nikousefat Z , Tufarelli V , Javdani M , Qureshi MS , Laudadio V. 2012b. Potential applications of ginger (Zingiber officinale) in poultry diet. World’s Poultry Sci J. 68:245–252. doi:10.1017/S004393391200030X.
- Khan RU , Nikousefat Z , Tufarelli V , Naz S , Javdani M , Laudadio V. 2012a. Garlic (Allium sativa) supplementation in poultry diet: effect on production and physiology. World’s Poultry Sci J. 68:417–424. doi:10.1017/S0043933912000530.
- Kim DK , Lillehoj HS , Lee SH , Lillehoj EP , Bravo D. 2013. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br J Nutr. 109:76–88. doi:10.1017/S0007114512000530.
- Raza T , Chand N , Khan RU , Shahid MS , Abudabos AM. 2016. Improving the fatty acid profile in egg yolk through the use of hempseed (Cannabis sativa), ginger (Zingiber officinale), and turmeric (Curcuma longa) in the diet of Hy-Line White Leghorns. Arch Anim Breed. 68:183–190. doi:10.5194/aab-59-183-2016.
- Steel RGD , Torrie JH , Diekey DA. 1997. Principles and procedures of statistics: A biometrical approach. New York, NY : Mc Graw HillBook.
- Tanweer AJ , Saddique U , Bailey CA , Khan RU. 2014. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol Res. 113:2951–2960. doi:10.1007/s00436-014-3957-y.
- ur Rehman Z , Chand N , Khan RU , Naz S , Alhidary IA. 2018. Serum biochemical profile of two broilers strains supplemented with vitamin E, raw ginger (Zingiber officinale) and L-carnitine under high ambient temperatures. South Afr J Anim Sci. 48:935–942. doi: 10.4314/sajas.v48i5.13
- Zhang GF , Yang ZB , Wang Y , Jiang SZ , Gai GS. 2009. Effects of ginger root processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poultry Sci. 88:2159–2166. doi:10.3382/ps.2009-00165.
