ABSTRACT
Imprinted genes play important roles on embryonic development. Aiming at the stillbirths in fat-1 transgenic foetuses of sheep, we detected the DNA methylation levels of candidate CpG regions in five imprinted genes (h19, igf2, igf2r, dlk1 and gtl2) of diverse tissues in the sheep foetuses, including both male and female fat-1 transgenic stillbirths of sheep and the wild live-births of sheep. Imprinted gene igf2, igf2r and dlk1 exhibited gender-neutral methylation except h19, which implies that the deregulated DNA methylation influenced by exogenous gene fat-1 in imprinted gene h19 more likely differs with gender. Besides, we found that exogenous gene fat-1 could tend to influence DNA methylation of imprinted gene igf2r in kidney of sheep male-foetus by comparing number of differentially methylated CpG sites. Since the bio-function of fat-1 on growth is consistent with h19 and igf2r, but opposite with igf2 and dlk1, we suggest that the stillbirth risk factors on sheep foetus with exogenous gene fat-1 should be focused on abnormal development (of organs or cells) that probably caused by deregulated methylation status of imprinted genes, and the deregulated methylation may be driven by conflicting and overlapped bio-function between exogenous gene and imprinted genes, rather than extrinsic source of gene.
Abbreviations: CSF: control sheep female-foetuses; CSM: control sheep male-foetuses; HTLA: Humane Treatment of Laboratory Animals; n-3 PUFA(s): omega-3 polyunsaturated fatty acid(s); TSF: fat-1 transgenic sheep-stillbirths of female; TSM: fat-1 transgenic sheep-stillbirths of male
Introduction
Transgene has been the considerable controversy all around the world. In terms of fat-1 transgenic animals, the expression of exogenous gene fat-1 can be detected in tissues and upregulates omega-3 polyunsaturated fatty acid (n-3 PUFA) (Yang et al. Citation2017). N-3 PUFAs, as essential fatty acids, cannot be synthesized in mammals (van West and Maes Citation2003). The intakes of n-3 PUFAs are conducive to improve some human diseases, such as diabetes and cardiovascular disease (Psota et al. Citation2006; White et al. Citation2010; Algamas-Dimantov et al. Citation2014; Liu et al. Citation2015). By mediating the inflammatory response, n-3 PUFAs perform positive functions in the immune system (Harbige Citation2003; Gravaghi et al. Citation2011; Delpech et al. Citation2015). In the fat-1 transgenic pig, as one kind of widely researched animals, more attention is given to the level of n-3 PUFAs and the nutritional value of pork (Lai et al. Citation2006; Liu et al. Citation2016; Li et al. Citation2018). The live fat-1 transgenic offspring of pigs had been presented without any stillbirth statements (Lai et al. Citation2006; Pan et al. Citation2010; Zhang et al. Citation2012), which means the issue of stillbirth risk are evaded generally on fat-1 transgenic pig. Actually, the negative influence of reproduction had been studied in transgenic animals. Fat-1 transgenic female mice had elevated levels of n-3 polyunsaturated fatty acids (PUFA) while females from this line were observed to have impaired reproductive performance characterized by a smaller litter size than wildtype controls (Pohlmeier et al. Citation2011). Furthermore, for fat-1 transgenic mice, researchers were unable to obtain homozygous fat-1 transgenic offspring from the two highest expressing lines, while they suggested that excessive expression of omega-3 fatty acid desaturase may be lethal during gestation and detrimental to reproduction (Ji et al. Citation2009).
Imprinted genes are important on embryonic development (Magee et al. Citation2014). In transgenic cloned goats, the irregular expression of imprinted genes probably results in developmental defects (Jia et al. Citation2016). The imprinted gene h19 is transcribed into a long non-coding RNA that involves in the negative regulation of body weight and cell proliferation (Gabory et al. Citation2009). Insulin-like growth factors 2, which is encoded by imprinted gene igf2, is particularly important to foetal growth (Gicquel and Le Bouc Citation2006). Insulin-like growth factor-2 receptor, as the coding protein of imprinted gene igf2r, is essential for foetal growth regulation and normal development (Yang et al. Citation2005; Brown et al. Citation2009). Drosophila-like homologue 1, encoded by imprinted gene dlk1, is a transmembrane signal protein with key roles of controlling cell differentiation all over the embryonic and adult life cycles (Oczkowicz et al. Citation2010). The overexpression of imprinted gene gtl2 which DNA sequence is transcribed into miRNA may be associated with postnatal death in transgenic mice (Kumamoto et al. Citation2017). These imprinted genes all play very important roles on embryonic development.
It is well-known that DNA methylation can be involved in regulation of gene expression and occurs at CpG sites in vertebrates typically (Ehrlich et al. Citation1982; Jabbari and Bernardi Citation2004). The insertion of foreign DNA into an established mammalian genome can lead to alterations in cellular DNA methylation and transcription patterns (Müller et al. Citation2001). Here, we attempt to explain why fat-1 transgenic sheep, also as one kind of researched animals for livestock production, were born dead by analysing DNA methylation of imprinted genes.
Materials and methods
Sample collection
According to Guidelines on the Humane Treatment of Laboratory Animals (HTLA Pub. Chapters 2–6, revised 2006 in China), our animal experiment was approved by Animal Care and Use Committee of the Inner Mongolia Agricultural University and Inner Mongolia Autonomous Region Key Laboratory of Biological Manufacturing, which was also the provider of our test animals. The experimental group included six born dead fat-1 transgenic Mongolian sheep with three ewes and three rams, regarding as fat-1 transgenic sheep-stillbirths of female (TSF) and fat-1 transgenic sheep-stillbirths of male (TSM), respectively. The order of fat-1 transgenic procedure was arranged as culture and passage of donor cells, oocyte collection and enucleation, nuclear transfer, oocyte activation, embryo culture and embryo transfer (Wan et al. Citation2012). The control group contained six newborn normal Mongolian sheep produced via conventional reproduction with three ewes and three rams, identifying as control sheep female-foetuses (CSF) and control sheep male-foetuses (CSM), respectively. We applied mercy-killing to six newborn normal sheep through overdose of pelltobarbitalum natricum. Then we collected heart, lung, liver, kidney and muscle tissues separately from all 12 Mongolian sheep foetuses in experimental and control groups. Each tissue of each individual was cut into small pieces, divided into frozen tubes, and frozen immediately in liquid nitrogen for preservation.
Nucleic acid sequence and CpG islands analyses of imprinted genes
By searching gene sequences on NCBI (https://www.ncbi.nlm.nih.gov/), the related information of the five imprinted genes (h19, igf2, igf2r, dlk1 and gtl2) was recorded, such as gene size, located chromosomes, exon number, intron number and whether to translate (Supplementary file 1: Table S1). The candidate CpG regions of DNA methylation were isolated using online softwares, consisting of CpG Island Searcher (http://cpgislands.usc.edu/, default standard), EMBOSS Cpgplot (http://emboss.bioinformatics.nl/cgi-bin/emboss/cpgplot) and MethPrimer (http://www.urogene.org/methprimer/).
Primer design for candidate CpG regions of imprinted genes
Basing on the candidate CpG regions of DNA methylation in the five imprinted genes (h19, igf2, igf2r, dlk1 and gtl2), online software EpiDesigner (http://www.epidesigner.com) was used to design primers (Supplementary file 1: Table S2). The primers were designed, validated and finally used for the subsequent DNA methylation detection.
DNA methylation detection and analyses of imprinted genes in various tissues
The tissue samples of fat-1 transgenic sheep-stillbirths and wild sheep-live-births were sent to CapitalBio Corporation (Beijing, China) for DNA methylation assay. The DNA methylation levels of different CpG sites in the candidate CpG regions of five imprinted genes (h19, igf2, igf2r, dlk1 and gtl2) were detected by MassArray platform in five tissues (heart, lung, liver, kidney and muscle) of TSF, TSM, CSF and CSM. With statistical data of methylation, we further analysed the differences of DNA methylation.
Results
DNA methylation levels in candidate CpG regions of imprinted genes
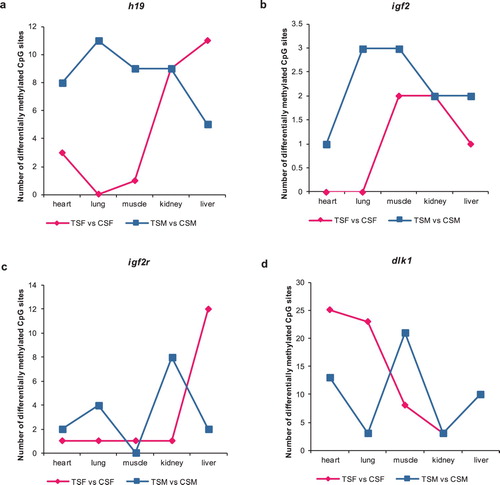
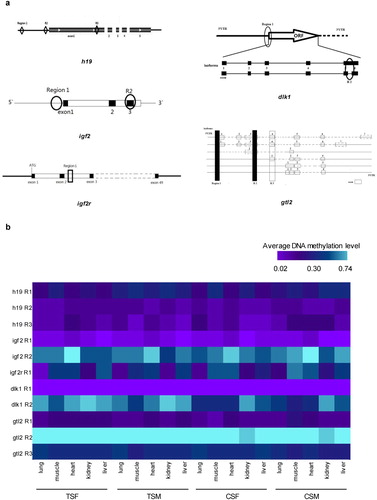
Considering the regulation of gene expression and occurrence of alternative splicing, the candidate CpG regions in which CpG sites can be easily methylated in five imprinted genes (h19, igf2, igf2r, dlk1 and gtl2) were selected by the CpG Island Searcher (default standard), EMBOSS Cpgplot and MethPrimer online softwares (Supplementary file 1: Figures S1–S5). The candidate CpG regions of imprinted genes covered three regions in h19, two regions in igf2, one region in igf2r, two regions in dlk1 and three regions in gtl2 ((a)). By MassArray platform, the DNA methylation levels of different CpG sites were detected in candidate CpG regions of five imprinted genes from five tissues including heart, lung, muscle, kidney and liver (Supplementary file 2: Figures S6–S38). Next, we demonstrated average DNA methylation levels of candidate CpG regions of imprinted genes in tissues sampled from TSF, TSM, CSF and CSM ((b); Supplementary file 1: Tables S3–S17).
Figure 1. Candidate CpG regions and their average DNA methylation levels of imprinted gene h19, igf2, igf2r, dlk1 and gtl2. (a) Schematic diagram of candidate CpG regions in the imprinted gene h19, igf2, igf2r, dlk1 and gtl2. (b) The average DNA methylation levels of different candidate CpG regions of imprinted gene h19, igf2, igf2r, dlk1 and gtl2 in lung, muscle, heart, kidney and liver of TSF, TSM, CSF and CSM. R indicates candidate CpG region.
Differential methylation analysis and gender-neutral methylation pattern
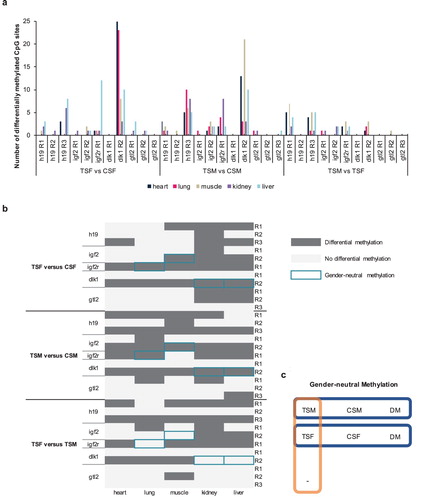
By comparing TSF with CSF, we employed statistical analysis to check methylated CpG sites of significant difference occurring in same candidate CpG regions of same imprinted genes of same tissues, but also comparing TSM with CSM and with TSF (Supplementary file 2: Figures S6–S38). The number of differentially methylated CpG sites were calculated ((a)). When significantly differential methylation of CpG sites occurred, the candidate CpG regions were considered to be differentially methylated. We further designed a cluster graph to illustrate whether candidate CpG regions were differentially methylated or not in the imprinted genes of different tissues ((b)). The cluster analysis demonstrated that candidate CpG regions of gtl2 showed no differential methylation in heart by comparing TSF with CSF, TSM with CSM, and TSM with TSF ((b)), which may be restricted by sample number, so we did not consider imprinted gene gtl2 as target for follow-up analysis and focused on imprinted gene h19, igf2, igf2r and dlk1. We identified a gender-neutral methylation pattern that differential methylation occurred in the candidate CpG regions between TSF and CSF, TSM and CSM, but not TSM and TSF ((c)). We observed that imprinted gene h19 did not exhibit gender-neutral methylation ((b)). However, imprinted gene igf2, igf2r and dlk1 generated gender-neutral methylation ((b)). The findings imply that the deregulated DNA methylation which is influenced by exogenous gene fat-1 in imprinted gene h19 is more likely to differ with gender.
Figure 2. Differential methylation status. (a) Number of differentially methylated CpG sites and (b) whether or not differential methylation occurred in candidate CpG regions of imprinted gene h19, igf2, igf2r, dlk1 and gtl2 in heart, lung, muscle, kidney and liver by comparing TSF with CSF, TSM with CSM, and TSM with TSF. (c) Gender-neutral methylation. R indicates candidate region of CpG. DM and horizontal line denote differential methylation and no differential methylation, respectively.
Regular effect on DNA methylation of imprinted genes in kidney, except for igf2r
According to the statistical data ((a)), we selected and compared the number of differentially methylated CpG sites in imprinted gene h19, igf2, igf2r and dlk1 between TSF and CSF, TSM and CSM marked as ‘TSF vs. CSF’ and ‘TSM vs. CSM’, respectively. The related work has shown that fatty acids are the major fuel source for the kidneys (Kang et al. Citation2015). Combined with n-3 PUFA-related fat-1, in kidney, we discovered that the number of differentially methylated CpG sites were similar in imprinted gene h19, igf2 and dlk1 ((a,b,d)). Conversely, in terms of imprinted gene igf2r, the number of differentially methylated CpG sites exhibited a difference in kidney ((c)). The results indicate that exogenous gene fat-1 may have regular impact on DNA methylation of imprinted gene h19, igf2, dlk1 in kidney both of sheep female- and male-foetuses. Besides, exogenous gene fat-1 could tend to influence DNA methylation of imprinted gene igf2r in kidney of sheep male-foetuses, but not sheep female-foetuses.
Discussion
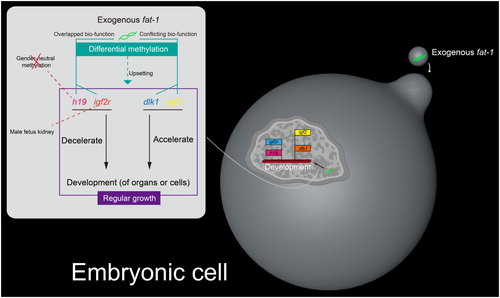
In order to explain why sheep foetuses were born dead under exogenous gene fat-1, we associated exogenous fat-1 with imprinted genes. In kidney and liver, hLF transgene can cause abnormal expression of imprinted gene dlk1 and trigger inflammation of the kidney and liver (Jia et al. Citation2016), but n-3 PUFA which locates downstream of fat-1 can ameliorate nephritis (Zeng et al. Citation2017) and hepatitis (Li et al. Citation2016). Therefore, the compensatory effect on inflammation is perhaps not the cause of neonatal lethality under fat-1 transgene. Subsequently, we noticed that the bio-functions of imprinted gene h19, igf2, igf2r, dlk1 and exogenous gene fat-1 were involved in developmental regulation. Fat-1 and h19 have roles in the negative regulation of cell proliferation (Gabory et al. Citation2009; Liu et al. Citation2016; Liu, Zhou, et al. Citation2016; Liu, Xu, et al. Citation2016). In terms of foetal growth factor insulin-like growth factor 2 and its inhibitor insulin-like growth factor 2 receptor (DeChiara et al. Citation1991; Lau et al. Citation1994; Wang et al. Citation1994), insulin-like growth factor systems are important to embryo development and organogenesis (Li et al. Citation2007). Igf2 can promote embryonic development (Constancia et al. Citation2002). Complete M6P/IGF2R knockout mice presents foetal overgrowth and neonatal lethality (Wylie et al. Citation2003). Dlk1 knockout mice display growth disorders and skeletal malformation (Moon et al. Citation2002). As similar as exogenous fat-1, h19 and igf2r can inhibit development. In contrast to the function of exogenous fat-1, the imprinted gene igf2 and dlk1 can promote growth. In our work, exogenous fat-1 changes the regular DNA methylation in imprinted genes with overlapped or opposite function, which the deregulated DNA methylation in imprinted gene h19 may easily differ with gender. In kidney, exogenous gene fat-1 regularly influences DNA methylation in imprinted gene h19, igf2 and dlk1 both of sheep female- and male-foetuses. But exogenous gene fat-1 affects DNA methylation in imprinted gene igf2r in kidney of sheep male-foetuses more than of sheep female-foetuses (). In brief, we suggest that the differential and deregulated methylation of imprinted genes, which have overlapped or conflicting function with exogenous fat-1, may impede the normal embryo development, eventually raising the neonatal lethality.
Conclusion
Under the detected DNA methylation levels and differential methylation analyses, we discover that the adverse effects of transgene on animal individuals are more likely aroused by conflicting or overlapped bio-function between exogenous gene and imprinted gene, rather than extrinsic source of gene.
Ethical statements
Ethical approval: All procedures performed in studies involving animals were in accordance with the ethical standards of Guidelines on the Humane Treatment of Laboratory Animals (HTLA Pub. Chapters 2–6, revised 2006 in China) and Animal Care and Use Committee of the Inner Mongolia Agricultural University at which the studies were conducted.
Informed consent: Informed consent was obtained from Shenyuan Wang (as animal owner) included in the study.
TAAR_1575224_Suppementary_Tables_Figures.zip
Download Zip (1.1 MB)Acknowledgements
The authors would like to acknowledge to Miss Jing Pan and Mr Feng Wang for sampling assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Algamas-Dimantov A , Yehuda-Shnaidman E , Hertz R , Peri I , Bar-Tana J , Schwartz B. 2014. Prevention of diabetes-promoted colorectal cancer by (n-3) polyunsaturated fatty acids and (n-3) PUFA mimetic. Oncotarget. 5:9851–9863.
- Brown J , Jones E , Forbes BE. 2009. Interactions of IGF II with the IGF-2R/cation independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam Horm. 80:699–719.
- Constancia M , Hemberger M , Hughes J , Dean W , Ferguson-Smith A , Fundele R , Stewart F , Kelsey G , Fowden A , Sibley C , et al. 2002. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 417:945–948.
- DeChiara TM , Robertson EJ , Efstratiadis A. 1991. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 64:849–859.
- Delpech JC , Madore C , Joffre C , Aubert A , Kang JX , Nadjar A , Laye S. 2015. Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharmacology. 40:525–536.
- Ehrlich M , Gama-Sosa MA , Huang L-H , Midgett RM , Kuo KC , McCune RA , Gehrke C. 1982. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 10:2709–2721.
- Gabory A , Ripoche MA , Le DA , Watrin F , Ziyyat A , Forne T , Jammes H , Ainscough JF , Surani MA , Journot L , et al. 2009. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development. 136:3413–3421.
- Gicquel C , Le Bouc Y. 2006. Hormonal regulation of fetal growth. Horm Res. 65:28–33.
- Gravaghi C , Ogrodwski P , Kang JX , Quimby F , Lipkin M , Lamprecht SA. 2011. Cox-2 expression, PGE2 and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 22:360–365.
- Harbige LS. 2003. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 38:323–341.
- Jabbari K , Bernardi G. 2004. Cytosine methylation and CpG, TpG (CpA) and TpA frequencies. Gene. 333:143–149.
- Ji S , Hardy RW , Wood PA. 2009. Transgenic expression of n-3 fatty acid desaturase (fat-1) in C57/BL6 mice: effects on glucose homeostasis and body weight. J Cell Biochem. 107(4):809–817.
- Jia RX , Zhou ZR , Zhang GM , Wang LZ , Fan YX , Wan YJ , Zhang YL , Wang ZY , Wang F. 2016. Analysis of imprinted messenger RNA expression in deceased transgenic cloned goats. Genet Mol Res. 15(1):gmr.15017455.
- Kang HM , Ahn SH , Choi P , Ko YA , Han SH , Chinga F , Park AS , Tao J , Sharma K , Pullman J , et al. 2015. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 21:37–46.
- Kumamoto S , Takahashi N , Nomura K. 2017. Overexpression of microRNAs from the Gtl2-Rian locus contributes to postnatal death in mice. Hum Mol Genet. 26:3653–3662.
- Lai L , Kang JX , Li R , Wang J , Witt WT , Yong HY , Hao Y , Wax DM , Murphy CN , Rieke A , et al. 2006. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat Biotechnol. 24(4):435–436.
- Lau MM , Stewart CE , Liu Z , Bhatt H , Rotwein P , Stewart CL. 1994. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 8:2953–2963.
- Li S , Li Y , Yu S , Du W , Zhang L , Dai Y , Liu Y , Li N. 2007. Expression of insulin-like growth factors systems in cloned cattle dead within hours after birth. Mol Reprod Dev. 74:397–402.
- Li M , Ouyang H , Yuan H , Li J , Xie Z , Wang K , Yu T , Liu M , Chen X , Tang X , et al. 2018. Site-specific fat-1 knock-in enables significant decrease of n-6PUFAs/n-3PUFAs ratio in pigs. G3 (Bethesda). 8(5):1747–1754.
- Li Y , Tang Y , Wang S , Zhou J , Zhou J , Lu X , Bai X , Wang XY , Chen Z , Zuo D. 2016. Endogenous n-3 polyunsaturated fatty acids Attenuate T cell-Mediated hepatitis via Autophagy activation. Front Immunol. 7:350.
- Liu F , Li Z , Lv X , Ma J. 2015. Dietary n-3 polyunsaturated fatty acid intakes modify the effect of genetic variation in fatty acid desaturase 1 on coronary artery disease. PloS One. 10:e0121255.
- Liu X , Pang D , Yuan T , Li Z , Li Z , Zhang M , Ren W , Ouyang H , Tang X. 2016. N-3 polyunsaturated fatty acids attenuates triglyceride and inflammatory factors level in hfat-1 transgenic pigs. Lipids Health Dis. 15:89.
- Liu J , Xu M , Zhao Y , Ao C , Wu Y , Chen Z , Wang B , Bai X , Li M , Hu W. 2016. n-3 polyunsaturated fatty acids abrogate mTORC1/2 signaling and inhibit adrenocortical carcinoma growth in vitro and in vivo. Oncol Rep. 35:3514–3522.
- Liu M , Zhou L , Zhang B , He M , Dong X , Lin X , Jia C , Bai X , Dai Y , Su Y , et al. 2016. Elevation of n-3/n-6 PUFAs ratio suppresses mTORC1 and prevents colorectal carcinogenesis associated with APC mutation. Oncotarget. 7:76944–76954.
- Magee DA , Spillane C , Berkowicz EW , Sikora KM , MacHugh DE. 2014. Imprinted loci in domestic livestock species as epigenomic targets for artificial selection of complex traits. Anim Genet. 45:25–39.
- Moon YS , Smas CM , Lee K , Villena JA , Kim KH , Yun EJ , Sul HS. 2002. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2:5585–5592.
- Müller K , Heller H , Doerfler W. 2001. Foreign DNA integration. Genome-wide perturbations of methylation and transcription in the recipient genomes. J Biol Chem. 276(17):14271–14278.
- Oczkowicz M , Piestrzyska-Kajtoch A , Piorkowska K , Rejduch B , Rozycki M. 2010. Expression of DLK1 and MEG3 genes in porcine tissues during postnatal development. Genet Mol Biol. 33:790–794.
- Pan DK , Zhang L , Zhou YR , Feng C , Long C , Liu X , Wan R , Zhang J , Lin A , Dong E , et al. 2010. Efficient production of omega-3 fatty acid desaturase (sFat-1)-transgenic pigs by somatic cell nuclear transfer. Sci China Life Sci. 53(4):517–523.
- Pohlmeier WE , Hovey RC , Van Eenennaam AL. 2011. Reproductive abnormalities in mice expressing omega-3 fatty acid desaturase in their mammary glands. Transgenic Res. 20(2):283–292.
- Psota TL , Gebauer SK , Kris-Etherton P. 2006. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 98:3i–18i.
- van West D , Maes M (2003) Polyunsaturated fatty acids in depression. Acta Neuropsychiatrica 15:15–21.
- Wan YJ , Zhang YL , Zhou ZR , Jia RX , Li M , Song H , Wang ZY , Wang LZ , Zhang GM , You JH , et al. 2012. Efficiency of donor cell preparation and recipient oocyte source for production of transgenic cloned dairy goats harboring human lactoferrin. Theriogenology. 78:583–592.
- Wang ZQ , Fung MR , Barlow DP , Wagner EF. 1994. Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature. 372:464–467.
- White PJ , Arita M , Taguchi R , Kang JX , Marette A. 2010. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabete. 59:3066–3073.
- Wylie AA , Pulford DJ , McVie-Wylie AJ , Waterland RA , Evans HK , Chen YT , Nolan CM , Orton TC , Jirtle RL. 2003. Tissue-specific inactivation of murine M6P/IGF2R. Am J Pathol. 162:321–328.
- Yang L , Chavatte-Palmer P , Kubota C , O’neill M , Hoagland T , Renard JP , Taneja M , Yang X , Tian XC. 2005. Expression of imprinted genes is aberrant in deceased newborn cloned calves and relatively normal in surviving adult clones. Mol Reprod Dev. 71:431–438.
- Yang C , Shang X , Cheng L , Yang L , Liu X , Bai C , Wei Z , Hua J , Li G. 2017. DNMT 1 maintains hypermethylation of CAG promoter specific region and prevents expression of exogenous gene in fat-1 transgenic sheep. PLoS One. 12:e0171442.
- Zeng Z , Yang H , Wang Y , Ren J , Dai Y , Dai C. 2017. Omega-3 polyunsaturated fatty acids Attenuate Fibroblast activation and kidney Fibrosis involving MTORC2 Signaling Suppression. Sci Rep. 7:46146.
- Zhang P , Zhang Y , Dou H , Yin J , Chen Y , Pang X , Vajta G , Bolund L , Du Y , Ma RZ. 2012. Handmade cloned transgenic piglets expressing the nematode fat-1 gene. Cell Reprogram. 14(3):258–266.