ABSTRACT
An efficient management of the newborn dairy calf around birth results in reduced rearing costs and increased future milk yield. To maintain young calves healthy and maximize performance and profit it is important to create an environment that maintain low levels of stress. Low environment temperature directly affects newborn and young calves’ performance, health and survival due hypothermia. For that reason, the adaptation of the dairy calf to the cold environment is important, with body responses of primary importance. However, some strategies may also be adopted to reduce cold stress induced by heat loss. There are several ways to aid the young calf in low environmental temperature, starting immediately after birth with colostrum feeding. Besides that, factors such as adequate housing, management and nutrition, result in significantly decrease morbidity and mortality, and similar growth rates as calves in the zone of thermoneutrality. The calf physiology adaptation to cope with cold stress and the positive effect of feeding systems, resulting in greater heat production, health, and performance will be approach in this literature review.
Introduction
After birth, among other factors as dystocia, anoxia, and nutrition, the survival of the calves depends on its ability to rapidly adapt to the new environmental conditions (Bellows, Citation1997). When the environmental temperature is low, the calf’s body responds throught heat production (thermogenesis) to cope with the situation (Vermorel et al., Citation1989). However, the body temperature regulation in young calves is not mature as in old animals. Because of that, cold ambient temperature can have some negative effects on calves’ health and survival (Davis and Drackley, Citation1998). Poor calf health can lead to high death losses and have a serious impact on net income for the cattle producer (Bellows, Citation1997). Thus, the efficient growth and performance of young dairy calves are extremely important to decrease replacement heifer raising costs and increase the potential of future milk production (Tozer and Heinrichs, Citation2001; Van De Stroet et al., Citation2016; Chester-Jones et al., Citation2017).
Efficiency and success in dairy calf rearing in cold weather is a function of a high standard feeding management, good hygiene practices and prevention of disease that start soon after birth (Pineda et al., Citation2016). In addition, one of the keys to maximizing profit and keeping young calves healthy is creating an environment that keeps cold stress down, since it contributes to low performance and increased rates of morbidity and mortality in the first few weeks of life (Hulbert and Moisá, Citation2016). For that reason, some strategies were created to reduce cold stress such as dry and deep bedding (Webster, Citation1984; Lago et al., Citation2006), heat lamps (Butler et al., Citation2006; Borderas et al., Citation2009), jackets, blankets or coats (Davis and Drackley, Citation1998) and extra feed supply (Vermorel et al., Citation1983; Drackley, Citation2008; Ghasemi et al., Citation2017). Therefore, this narrative review was prepared with the motivation to increase the knowledge about the body physiology processes and their adaptations to cold stress by the calf presenting some management practices as adequate housing, efficient management and feeding strategies.
Ambient temperature and calves’ thermoneutrality
There is a range of ambient temperatures for all mammals, within which the general metabolism of the organism generates sufficient heat as a byproduct of the metabolism, so that its predetermined body temperature can be maintained. This temperature range is known as the zone of thermoneutrality, and at this temperature, the organism demonstrates its basal metabolic rate (Cannon and Nedergaard, Citation2010). The thermoneutral zone of the young calf varies with age, weight, environmental conditions, and other stressors and ranges from 15°C to 25°C (Davis and Drackley, Citation1998). The point in environmental temperature at the lower end of the thermoneutral zone is termed the lower critical temperature. Value for the lower critical temperature of calves varies with age (). Diesch et al. (Citation2004) studied the physiological status of calves at birth and the perinatal factors that might predispose newborn calves to debility and death. The authors reported that calves born during windy and wet weather, and when temperatures were <10°C, had lower rectal temperatures right after birth (12 min) and took longer to stand, as compared to calves born in dry weather and when temperatures were > 10°C. With increased age, the lower critical temperature declines due an increase in hair thickness and length, skin thickness, and stores of subcutaneous fat.
Table 1. Effect of age of calf on lower critical temperature.
Lower critical temperature is observed in temperate countries mainly during winter. However, some subtropical countries such as Brazil, Argentina and Uruguay, also present temperatures below lower critical temperature during winter in some regions. In part of southwest and south of Brazil, calves may be exposed to cold weather, wind and humidity, requiring body temperature regulation (National Institute of Meteorology – INMET, 2017). Thus, this is an issue for calves born at several regions worldwide, with high importance for milk production. Regardless of the region, at lower critical temperature, the body needs to produce heat to keep the animal alive (Davis and Drackley, Citation1998).
Mechanisms of thermogenesis
Thermogenesis depends on exercise, diet, and climatic conditions, in particular environmental temperature (Vermorel et al., Citation1983; Harper et al., Citation2002). Heat is generated as a by-product of metabolic reactions in biological systems (Girardier and Stock, Citation1983). Animal’s heat production is the result of several phenomena: the metabolic rate of body tissues, the metabolism of brown adipose tissue, shivering, physical activity and the feeding heat increment (Vermorel et al., Citation1983).
Mammalian thermogenesis can be classified as obligatory or facultative. Obligatory reactions include basal metabolic rate and essential reactions such as ingestion and digestion of feed, and metabolism of nutrients. The latter reactions account for a significant proportion of diet-induced thermogenesis, the energy costs of assimilating nutrients and retaining net energy (Harper et al., Citation2002). On the other hand, facultative reactions include everything needed by the animal beyond the basal metabolism. All cells and tissues of the body contribute to obligatory thermogenesis. However, facultative thermogenesis is predominantly the result of metabolic reactions in two types of tissue: skeletal muscle and brown adipose tissue (BAT). In muscle, these processes include exercise-induced thermogenesis and cold-induced shivering thermogenesis; both are mechanisms that require coupled oxidative phosphorylation. In BAT, facultative thermogenic processes include diet-induced thermogenesis and cold-induced non-shivering thermogenesis; both require uncoupled oxidative phosphorylation (Harper et al., Citation2002). Production of heat to maintain homeothermy in the neonate is dependent on shivering thermogenesis in the muscle and non-shivering thermogenesis in BAT (Bellows, Citation1997)
Cold-induced shivering thermogenesis
Shivering is defined as an involuntary rhythmic contraction of skeletal muscle myofibrils involving no voluntary movements or external work (Herpin et al., Citation2005). Shivering involves episodic or sustained vigorous contractions of antagonistic muscle fibres without efficient work output, which causes an increased turnover of the myofibrilar ATP pool and thus heat dissipation (Klingenspor and Fromme, Citation2012). Shivering appears soon after birth in calves housed at 10°C and stops when the hair coat is almost dry. It first affects skin and then skeletal muscles (Vermorel et al., Citation1983). This behaviour has the intensity classified according to the body regions where shivering is observed. Bellows and Lammoglia (Citation2000) classify shivering using a scale from1 to 3, where (1) represents no shivering, (2) moderate shivering of muscles in the back and legs and (3) intense shivering of muscles in back, legs and face of the calf.
According to Vermorel et al. (Citation1983), in 15 h old calves lying in a 37°C water bath, shivering starts when water temperature drops to 32°C (personal results). The lower the water temperature, the more the calf shivers. In addition, inspiration of cold air causes an increase in rhythmic and tonic muscle activity, increasing shivering behaviour (Pozos and Danzl, Citation2001). Shivering is immediately followed by an increase in heat production ranging from 33% to more than 100% (Vermorel et al., Citation1983).
Nutrition of the dam in late gestation could also affect shivering thermogenesis in the neonate. Lammoglia et al. (Citation1999b) evaluating the effects of supplementing cows with fat (1.7% or 4.7% crude dietary fat) during late gestation on cold tolerance in newborn calves. Authors reported that calves born from dams supplemented with high fat presented higher glucose concentrations, which is associated with shivering thermogenesis. Increased concentrations of blood glucose in calves resulted from glycogen mobilization as substrate for the shivering muscle (Lammoglia et al., Citation1999a). Concentrations of lactate are also positively related to shivering, since muscular tremors results in glucose anaerobic oxidation leading to lactate buildup (Pozos and Danzl, Citation2001). As the body start to produce heat by the BAT, shivering is reduced (Cannon and Nedergaard, Citation2004).
Cold-induced non-shivering thermogenesis
Non-shivering thermogenesis has been defined as ‘heat producing mechanisms due to processes that do not involve muscular contractions, such as those involved in ion pumping or mitochondrial loose coupling’ (Herpin et al., Citation2005). Non-shivering thermogenesis contributes to a large proportion of total heat production of newborn calves in environments below the thermoneutral zone. In newborns, cold-induced non-shivering thermogenesis occurs almost exclusively in BAT (Himms-Hagen, Citation1985), which is present in calves and other mammals (Vermorel et al., Citation1983). In smaller mammals at thermoneutrality, nearly half of their energy metabolism goes towards BAT metabolism (Cannon and Nedergaard, Citation2004). However, in environments below thermoneutrality, the predominant energy utilizer is the BAT. The availability of the BAT for the animal’s metabolism and consequent heat production alters based on environmental conditions: it atrophies when is not needed, and it is recruited when a chronic, high demand is encountered (Cannon and Nedergaard, Citation2004).
Brown adipose tissue is located in the perirenal, inguinal and prescapular body regions and accounts to about 2% of body weight () (Vermorel et al., Citation1983). The cells of BAT are usually multilocular, containing several drops of stored triacylglycerol, and are characteristically packed with many large mitochondria (Cannon and Nedergaard, Citation2004). This tissue represents the major portion of the depot fat reserve in a newborn calf, and is about 40% lipid on a wet weight basis (Okamoto et al., Citation1986). In addition, the BAT can also use exogenous triacylglycerol and glucose for thermogenesis (Himms-Hagen, Citation1985). The glucose can serve as a thermogenic substrate when it is abundantly available, i.e. after a meal.
Figure 1. Brown adipose tissue distribution in the animal body (Cannon and Nedergaard, Citation2004).
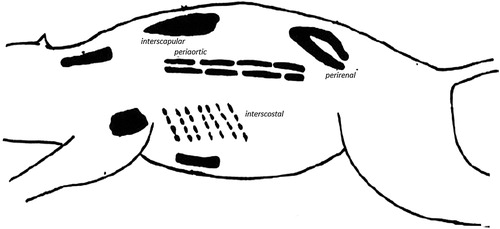
The BAT grows when it is stimulated by prolonged and intense activation of its sympathetic nerve supply (Vermorel et al., Citation1983). Information on body temperature, feeding status, and body energy reserves is coordinated in an area in the brain to the BAT. With a cold stimulation, a signal is transmitted via the sympathetic nervous system to the individual brown adipocytes. The released transmitter, norepinephrine, initiates triglyceride breakdown in the brown adipocytes. The intracellular signal is transmitted via cAMP and protein kinase A, leading to mitochondrial combustion of substrates and heat production (Cannon and Nedergaard, Citation2004). Mitochondria in BAT have a unique proton conductance mechanism that permits them to become reversibly uncoupled and thus to oxidize substrates at an extremely high rate. This mechanism is controlled by the intracellular concentration of fatty acids, mainly generated by the breakdown of endogenous triacylglycerol. The mechanism involves an uncoupling protein (UCP) which has one binding site for purine nucleotides per dimer on the outer surface of the inner mitochondrial membrane (Himms-Hagen, Citation1985).
The activity of BAT lipoprotein lipase increases very rapidly in response to acute sympathetic stimulation. When the cells are thermogenically inactive they become filled with lipids and may superficially resemble white adipose tissue cells (Himms-Hagen, Citation1985). This occurs during the first month of calves’ life, when BAT is rapidly converted to white adipose tissue, which has decreased reactivity to norepinephrine (Vermorel et al., Citation1983).
Cold environment affecting calf survival
The most challenging period for calves is from birth through weaning. During this time, calves experience remarkable physiological, metabolic, and environmental changes (Davis and Drackley, Citation1998). Bellows (Citation1997) reviewed factors affecting calf survival and concluded that at or right after birth, the calf may have the survival compromised for many reasons including anoxia, dystocia, nutrition and suboptimal environmental temperatures. At this moment, the calf moves from the controlled, warm uterine environment, to the often hostile external environment. This transition of ambient demands many physiological actions to maintain normal body temperature especially during cold weather. At low environmental temperature, newborns that have increased heat production to maximum levels in response to cold exposure become hypothermic when the ambient conditions are cold or some failure to produce heat occur that heat loss exceeds heat production (Mellor and Stafford, Citation2004). Failure to produce enough heat can lead to the development of secondary complications including chronic digestive and respiratory disorders, scouring and pneumonia, which affect later performance and increase mortality (Young, Citation1983). Patterson et al. (Citation1987) evaluated the calf loss incidence from birth to weaning and found a calf mortality of approximately 9–13% resultant of chilling/cold exposure, pneumonia and scours, being the severe cold weather conditions the major factor causing variation in mortality rate.
According to Bellows (Citation1997), the ability of the neonate to maintain normal core body temperature is a function of its ability to produce enough heat to balance the evaporative and non-evaporative heat losses. This involves regulating blood flow to the skin, heart rate, respiration rate and hair erection (Davis and Drackley, Citation1998). However, body temperature regulation in calves develops with the age. Newborn are less equipped to deal with cold environment than older calves (Davis and Drackley, Citation1998). Thus, the adaptation of the young dairy calf to a cold environment is mainly dependent upon the availability of adequate housing, management and nutrition, resulting in decrease morbidity and mortality, and similar growth rates as calves in the zone of thermoneutrality (Davis and Drackley, Citation1998; Nonnecke et al., Citation2009; Pineda et al., Citation2016).
Management practices for young calves in cold environment
There are several ways to aid the young calf in reducing its heat loss from the body and increase thermogenesis. An important consideration that helps to reduce heat losses is that the calf has access to a dry, well-bedded shelter that provides protection from wind and extreme environmental conditions. Calf pens should be bedded deeply to provide insulation in cold weather (Davis and Drackley, Citation1998). Bedding is potentially effective to reduce calves’ heat loss. If the bedding is sufficiently deep, the calf can nest and trap a boundary layer of warm air around itself, which reduces the lower critical temperature of the calf (Nordlund, Citation2008).
Lago et al. (Citation2006) evaluating calf respiratory disease and pen microenvironments in naturally ventilated calf barns during the winter assigned a nesting score based on how visible the calf’s legs were when the calf was lying down. Nesting score 1 was assigned when calves lie on top of the bedding with legs exposed. Score 2 was assigned when calves nestle slightly into the bedding, but part of the legs were visible above the bedding. Score 3 was used when the calf appears to nestle deeply into the bedding material and legs were not visible. The authors observed that the prevalence of calf respiratory disease decreased with increasing nesting score. The potential for the calf to nest deeply seems to reduce the risk for chilling and allows for colder and better-ventilated spaces, since the animal will have part of the body protected (Nordlund, Citation2008). Hutches in cold climates are best bedded with long straw, to provide greater isolative effects. A minimum of 15 cm of bedding is recommended and a variety of bedding material can be used. In addition, adequate bedding absorbs moisture, which will help keep the calf’s haircoat dry to maintain its insulating function (Davis and Drackley, Citation1998). Sutherland et al. (Citation2013) rearing calves on river stones, surfaces with low insulation property, at a depth of approximately 20 cm up to 6 weeks in cold environment conditions observed lower skin surface temperature and consequently calves thermal discomfort as compared to calves reared on sawdust at a depth of approximately 20 cm.
According to Butler et al. (Citation2006), heat lamps are also an efficient method for warming newborn calves, being important to consider temperature regulation to avoid possible burns. A study comparing the effectiveness of rewarming newborn calves previously exposed to a cold-water bath until the rectal temperature reached 30°C with added insulation, heat lamp, warm water or warm water plus ethanol showed that the metabolic effort was 50% less for the calves in the warm water than in the in the added insulation and the heat lamp treatments. However, the rewarming by the supplementary heat from infrared heat lamps also may be achieved (Robinson and Young, Citation1988).
Another way to reduce heat losses is using calf jackets, blankets or coats. Rawson et al. (Citation1989) housed calves continuously for two weeks in hutches within environmental chambers in which temperature was cycled on a daily basis either between −30° and −18° C. The authors observed that the insulated coat which extended from the neck to the tail head, covering both sides, provided a 52% increase in whole animal resistance to heat loss (insulation) as compared to calves in the same environmental chambers without coat.
Providing energy by feed is other method to combat hypothermia by heat production. Animals should be fed an extra energy to meet the increase in maintenance energy requirements to produce heat (Davis and Drackley, Citation1998). Okamoto et al. (Citation1986) suggest that in situation where endogenous substrates become depleted due to prolonged cold exposure the provision of colostrum may help to maintain elevated metabolism for a longer time. Noblet and Le Dividich (Citation1981) observed that pigs reared in cold conditions have had rectal temperature positively related to the amount of colostrum intake whereas the rectal temperature from pigs reared in warm conditions was independent of the colostrum intake.
Colostrum as an energy source for newborn calves’ thermogenesis
Bovine colostrum consists of a mixture of lacteal secretions and constituents of blood serum, most notably Ig and other serum proteins, which accumulate in the mammary gland during the prepartum dry period (Barrington and Parish, Citation2001). Colostrum is known to be important for passive immunity transfer (Weaver et al., Citation2000; Uruakpa et al, Citation2002; Baumrucker et al., Citation2010). Newborn calves are born hypogammaglobulinemic or agammaglobulinemic due to the absence of transplacental transfer of antibodies during gestation (McGuirk and Collins, Citation2004). However, the ingestion of colostrum and absorption of antibodies early after birth enable the passive immune transfer (Olson et al., Citation1980). The efficiency of immunoglobulin transfer across the gut epithelium is optimal during the first 4 h of life with a progressive decline 6 h after birth (Godden, Citation2008). Osaka et al. (Citation2014) provided colostrum for the calves within 1 h, between 1 and 6 h, between 6 and 12 h or between 12 and 18 h after birth and observed that the apparent efficiency of absorption of IgG declined by less than 0.3%/h from calving to 12 after birth, and then declined more rapidly at 2.5%/h to at least 18 h after birth. In addition, the authors reported that calves need to consume 120 g of IgG if fed in the first hour after birth or 125 g of IgG if fed between 1 and 6 h, to achieve 10 mg/mL of serum IgG at 24 h. Therefore, the most important management factor in determining health and survival of the neonatal calf is achieving early and adequate intake of high quality colostrum (Godden, Citation2008).
Colostrum intake also aims to stimulate maturation and function of the neonatal gastrointestinal tract (Hammon et al., Citation2014). Colostrum contains several peptide growth factors, which stimulate the growth and differentiation of mammalian cells, such as Insulin-like growth factors (IGF-1 and IGF-2), transforming growth factor beta (TGF-β1 and TGF-β2), growth hormone (GH), epidermal growth factor, and insulin (Pakkanen and Aalto, Citation1997). These hormones promote gastrointestinal tract development, production of digestive enzymes, and absorption capacity of nutrients (Bach, Citation2012). Yang et al. (Citation2015) providing for 28 newborn calves first milking colostrum, transitional milk or bulk tank milk (4 L immediately after birth and then 2 L at 8 h after birth) reported that calves fed high quality colostrum presented higher IgG absorption at 24 and 48 h after feeding as compared to transitional milk or bulk tank milk. Additionally, in the first week calves fed first milking colostrum also presented higher antioxidant activities and serum growth factors, villus length and width, crypt depth, and mucosal thickness as compared to transitional milk or bulk tank milk. On the other hand, calves that received bulk tank milk presented villi severely atrophied, and some histological changes were detected.
In addition to passive immunity and growth factors effects, colostrum feeding is important to neonatal thermogenesis (Hammon et al., Citation2012). According to Herpin et al. (Citation2005), in cold environment body temperature and heat production are positively related to the amount of colostrum intake. In a study where colostrum or water was provided to newborn piglets maintained at thermoneutrality or in cold environment at the first day of life, colostrum provided as much as 75% of energy required for heat production at a low critical temperature (Herpin et al., Citation1994). The authors attempted to circumvent the piglets’ activity during suckling by using tube-feeding and conducting the same feeding routine on sham-fed piglets fed distilled water, to obtain by difference the ‘actual’ thermogenic effect of colostrum, and not the heat associated with suckling and physical activity. The thermogenic effect of colostrum is considered due to metabolic heat production represented by the energy cost associated with digestion, absorption and processing of nutrients (Herpin et al., Citation1994). Vermorel et al. (Citation1983) presenting personal results in their review article reported that 24 Friesian calves held at 10°C increased heat production on average by 18% and 9% during the first and the second hour respectively, following colostrum consumption at 12 h of age.
Beyond heat production by the energy costs of assimilating nutrients and retaining net energy, colostrum may promote thermogenesis as a source of substrate for the BAT. Colostrum supplies lactose, amino acids, and triglycerides (), constituting an excellent energy source (6.7 MJ/kg) to heat production both by diet-induced thermogenesis and by nonshivering thermogenesis (Vermorel et al., Citation1983; Himms-Hagen, Citation1990; Kirovski, Citation2015).
Table 2. Characteristics and composition of Holstein colostrum and milk.
Therefore, efficient management practice feeding calves’ high volume of colostrum during the first hours of life promote passive immunity and thermogenesis, ensuring the best possible start for optimum growth, reduced veterinarian and medical costs, and increased milk yield as a mature animal (Faber et al., Citation2005). The United States Department of Agriculture-National Animal Health Monitoring System (USDA-NAHMS, Citation2014) recommends feeding colostrum at 10% of body weight. Faber et al. (Citation2005) reported that heifer calves fed 4 L of high quality colostrum immediately after birth had veterinary costs from birth until first calving reduced approximately $15.00 per animal (based on health disorders and their respectively treatment costs) compared with cohorts fed 2 L of colostrum. In addition, animals fed the greater volume of colostrum presented greater ADG, with ADG differing by 0.23 kg up to approximately 500 d of age between the two groups of heifers, and produced 1349 kg more milk/d across their second lactation than animals in the 2 L treatment group. The improvements for calves fed a higher volume of colostrum are probably due to greater immunity but also energy supply during the first hours of life. Another study reported that calves fed unlimited amount of colostrum for 3 d after birth and mature milk up to d 28 were able to digest and metabolize high amounts of feed even during the first week of life compared with calves fed restrict amount for 3 d after birth and a commonly recommended amounts of mature milk up to d 28 (Hammon et al., Citation2002). Higher colostrum intake was accompanied by a low plasma concentration of NEFA and cortisol indicating a greater inhibition of fat mobilization and reduced gluconeogenesis stimulation with greater nutrient intake. Moreover, insulin concentrations in calves fed unlimited amount of colostrum and milk were higher than in calves fed commonly recommended amount, since plasma insulin concentrations depend on the amount of ingested colostrum and energy intake.
When calves are fed adequate volumes of colostrum within the first hours of life, environment conditions and the energy obtained from the liquid diet become more important for thermogenesis (Davis and Drackley, Citation1998). With prolonged exposure to even mildly cold conditions, physiological adaptation occurs in animals resulting in increases in thermal insulation, appetite and basal metabolic intensity, as well as alterations in digestive functions. Primary among these changes are an increased resting metabolic rate, and hence an increased energy requirement for maintenance (Young, Citation1981, Citation1983). Therefore, temperature has an important effect on the energy requirement of the young calf.
Feed requirements for calves’ maintenance bellow thermoneutral conditions
In cold weather, the body alters physiologic processes, and hence, requires more nutrients to control temperature through heat production (Drackley, Citation2008). When temperature falls below the lower critical temperature, the energy needed to maintain core body temperature is supplied either by the increased energy intake or from the increased metabolism of tissue reserves (Nonnecke et al., Citation2009).
The National Research Council (NRC, Citation2001) established energy requirements for calves less than 100 kg body weight (BW) in units of metabolizable energy (ME), which in calves is determined by subtracting losses of energy in faeces and urine from total feed (or intake) energy. The ME requirements for maintenance under thermoneutral conditions are approximately 1.75 Mcal/d for a 45-kg calf. Whole milk contains about 5.37 Mcal /kg of solids, whereas milk replacer contains about 4.6–4.7 Mcal/kg, which means that a 45-kg calf requires 325 g of milk solids or 380 g of milk replacer just for maintenance at thermoneutrality.
As environmental temperature decreases, maintenance requirements for ME increases. At −20°C a 45-kg calf requires about 725 g/d of milk replacer powder just to meet maintenance requirements and maintain body temperature. Since energy consumed above maintenance is used for growth, as higher is the maintenance energy requirement, the lower is animal performance, when feeding is not adjusted. shows the effects of BW and environmental temperature on maintenance ME requirements in calves less than 21 days of age (Drackley, Citation2008). In concentrate starters, the main portion of energy is derived from cereal grains such as corn.
Table 3. Maintenance requirements for metabolizable energy as affected by body weight and environmental temperature in calves less than 21 days’ old.
As for energy, protein is required for maintenance and growth as a source of amino acids. However, the protein requirements for maintenance are small (about 30 g/d for a 45-kg calf) and are not believed to be substantially altered by cold stress. Protein requirements are mostly determined by the growth rate. On average, 188 g of protein are deposited for every kilogram of BW gain in calves above or below thermoneutral conditions, which would require 250–280 g of crude protein intake from milk replacer (Drackley, Citation2008).
Cold weather feeding strategies
According to Drackley (Citation2008), practical feeding systems can be made simple, although nutrients requirements for calves are more complex than the industry has recognized. Dairy replacement calves are usually fed limited amounts of whole milk or milk replacer (4 L) and have ad libitum access to a dry grain mixture (calf starter) prior to weaning. According to USDA-NAHMS (Citation2014), from the 1950s to the 1970s the approach was to minimize the cost reducing amount of milk/milk replacer fed. The restricted feeding system is known as a conventional milk-feeding programme. This practice provides milk or milk replacer (MR) at approximately 10% of calf’s BW at birth (Jasper and Weary, Citation2002), volume much lower than ad libitum intakes, which are in the range of 16% to 20% of BW. When MR is fed, it contains 20–22% CP and 15–20% fat and are traditionally reconstituted to approximately 12.5% solids (Cowles et al., Citation2006).
The conventional programme was designed to meet or slightly exceed maintenance requirements of the young calf, allowing about 200–300 g/d of growth under thermoneutral conditions (Drackley, Citation2008). Nutrients for growth are met by voluntary consumption of the starter concentrate rather than solids from milk. Typically, this practice encourages calf starter intake, and stimulates early rumen development, allowing a smoother weaning transition (Bush and Nicholson, Citation1986). A fully functional rumen allows the calf to utilize short chain fatty acids (SCFA) as its primary energy substrate. However, very young calves fed milk replacer at a conventional programme rate during the winter do not consume enough energy to achieve maintenance bellow thermoneutral conditions (Drackley, Citation2008). Therefore, they do not grow at the targeted rates.
Alternatively to conventional milk feeding, some intensive milk-feeding systems allow calves to receive greater or ad libitum volume of milk. Calves may receive higher volumes during the whole milk-feeding period, for example, 8L/d or more from birth to weaning. Intensive programmes allow increased early and future life performance in general by providing more dry matter per day from milk or milk replacer and greater amount of crude protein, with fat content similar or less to the conventional method (Rincker et al., Citation2011). These authors found those results providing a high-protein milk replacer (30.6% CP, 16.1% fat, 15% solids) for intensive diet, whereas the conventional diet was constituted of conventional milk replacer (21.5% CP, 21.5% fat, 12.5% solids). According to Raeth-Knight et al. (Citation2009), solids content of intensive milk replacer ranges from 12.5% to 17.5%. A recent research showed that calves fed milk replacer ad libitum presented greater average weight gain in the first phase of life and milk yield in the first lactation 612 kg above that animals fed approximately 50% less milk replacer (Korst et al., Citation2017). According to Soberon et al. (Citation2012) for every 1 kg of preweaning average weight gain, milk yield increase about 1,113 kg in the first lactation.
However, this practice reduces starter intake, since liquid feed intake is negatively correlated with the solid feed intake (Gelsinger et al., Citation2016), which difficult weaning. A study using medium CP (20.2%) milk replacer reported that Holstein calves fed intensive (8 L/d) presented lower concentrate intake (100.0 g of DM/d) during preweaning than calves in the conventional programme (4 L/d; 362.1 g of DM/d). On the other hand, concentrate intake showed no difference among treatments post-weaning (de Paula et al., Citation2017). Another problem with this programme is that very young animals may not consume greater volumes of the liquid diet. Therefore, an alternative to solve both problems is to alter the volume of feeding with step-up/step-down programme. In this programme, milk feeding is gradually increased to reach a peak in the middle of the milk-feeding period before it is gradually decreased to the original level towards the end of the period (Omidi-Mizaei et al., Citation2015). Omidi-Mirzaei et al. (Citation2015) observed that calves fed a step-down (d 1–29, 6 L/d; d 30–45, 4 L/d; d 46–56, 2 L/d), or step-up/step-down (d 1–5, 6 L/d; d 6–15, 8 L/d; d 16–35, 10 L/d; d 36–42, 8 L/d; d 43–47, 6 L/d; d 48–52, 4 L/d; d 53–56, 2 L/d) system, improved the total DMI (1.21 and 1.43 kg/d, respectively), BW (60.9 and 69.5 kg, respectively) and some body measurements during pre and post-weaning compared to calves in the conventional diet (1.08 kg/d of DMI, and 57.7 kg of BW). According to the authors, the better performance could be explained by the higher nutrient availability due to the greater milk intake, since improvements in growth and feed efficiency occur because of feeding greater amounts of liquid diet. Quigley et al. (Citation2006) also reported that calves fed additional milk replacer in a step-up/step-down programme (28% CP and 16% fat offered at 3.8, 5.6, 7.2, and 3.8 L/d during d 0–7, 8–14, 15–31, and 32–41, respectively) had greater milk intake, BW, BW gain, feed efficiency, than calves fed conventional method (20% CP and 20% fat offered at 3.8 L/d) (Quigley et al., Citation2006). It is important to note that these feeding programmes may be applied to animals raised under normal or bellow thermoneutral temperatures. However, as the energy requirement of calves bellow thermoneutral temperatures is higher, this programmes will not result in increased performance when compared to calves under thermoneutral temperatures (Davis and Drackley, Citation1998).
From birth to the first 2–3 weeks of age, the calf consumes negligible amounts of dry feed and relies almost entirely on milk or milk replacer to meet nutrient requirements. During this phase, the solids in milk and milk replacer are digested by enzymes in the abomasum and small intestine and the rumen is undeveloped (Xu, Citation1996). The digestive enzymes allow highly efficient digestion of milk proteins, lactose, and triacylglycerides, and smaller digestion of non-milk proteins or polysaccharides such as starch (Drackley, Citation2008). As the calf begins to consume starter, its fermentation leads to a fast differentiation of the rumen epithelium so that the SCFA produced from microbial fermentation can be absorbed and metabolized. The animal will depends exclusively on fermentation of the solid diet only after weaning (Drackley, Citation2008).
Because of their lack of rumen functionality, young calves should be fed extra energy by increasing the amount of a good quality liquid diet to meet the increase in maintenance energy requirements when housed below the thermoneutral zone. According to the NRC (Citation2001), dietary requirements during the preruminant phase are best met with high-quality liquid diets formulated with a rich source of carbohydrates, proteins and fats that are efficiently digested, with the addition of starter during the transition phase. Allowing calves greater intake of liquid feed during early life is closer to natural conditions in which calves would have ad libitum access to milk (Drackley, Citation2008).
Drackley (Citation2008) suggests that at low environmental temperature some producers could use feeding strategies to improve calf growth and health. Producers may increase the volume of milk or milk replacer fed at each feeding or increase solids content to each feeding, increasing energy intake. For those that are already feeding larger amounts of milk or replacer, a third meal may be added to the feeding routine. Another way to increase the energy intake is by feeding a higher energy content milk replacer or supplement the milk replacer with added fat or additional solids. On the other hand, feeding starter diet with supplementary fat has been discouraged mainly due to the reduced voluntary starter intake, which diminishes the energy intake and rumen development in calves. However, starter supplementation with fat may be beneficial when feeding under the effects of cold stress (Ghasemi et al., Citation2017). The authors indicate that young calves increase their energy intake during cold stress, and feed intake is not limited by metabolic factors as fat supplements (3%) are fed.
Final considerations
Decreased heat losses in the newborn dairy calf depends on the availability of adequate nutrition, housing and management, resulting in significant reduction in morbidity and mortality, and similar growth rates as calves in the thermoneutrality zone. Besides the role in the passive immune transfer process, colostrum feeding is an important tool to increase newborn calves’ thermoregulatory responses during cold environment, increasing future productivity. In addition, for calves submitted to low environmental temperature within first months of life, an elevated plane of nutrition increases heat production, allowing maintained or even increased calves’ performance.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Fernanda Lavínia Moura Silva http://orcid.org/0000-0001-5430-8412
Carla Maris Machado Bittar http://orcid.org/0000-0001-9836-7203
Additional information
Funding
References
- Bach A. 2012. Ruminant nutrition symposium: optimizing performance of the offspring: nourishing and managing the dam and postnatal calf for optimal lactation, reproduction, and immunity. J Anim Sci. 90:1835–1845. doi: 10.2527/jas.2011-4516
- Barrington GM , Parish SM. 2001. Bovine neonatal immunology. Vet Clin North Am Food Anim Pract. 17:463–476. doi: 10.1016/S0749-0720(15)30001-3
- Baumrucker CR , Burkett AM , Magliaro-Macrina AL , Dechow DC. 2010. Colostrogenesis: mass transfer of immunoglobulin g1 into colostrum. J Dairy Sci. 93:3031–3038. doi: 10.3168/jds.2009-2963
- Bellows RA. 1997. Factors affecting calf survival. Range Beef Cow Symposium XV Proceedings. 141–150.
- Bellows RA , Lammoglia MA. 2000. Effects of severity of dystocia on cold tolerance and serum concentrations of glucose and cortisol in neonatal beef calves. Theriogenology. 53:803–813. doi: 10.1016/S0093-691X(99)00275-7
- Borderas FT . de Passillé AMB and Rushen J 2009. Temperature preferences and feed level of the newborn dairy calf. Appl Anim Behav Sci 120, 56–61. doi: 10.1016/j.applanim.2009.04.010
- Bush RS , Nicholson JWG. 1986. The effects of weaning schedule, duration of milk feeding and fishmeal on calf performance. J Anim Sci. 66:691–698.
- Butler L , Daly R , Wright C. 2006. Cold stress and newborn calves. Extension Extra. 73:1–3.
- Cannon B , Nedergaard J. 2004. Brown adipose tissue, function and physiological significance. Physiol Rev. 84:277–359. doi: 10.1152/physrev.00015.2003
- Cannon B , Nedergaard J. 2010. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 214:242–253. doi: 10.1242/jeb.050989
- Chester-Jones H , Heins B J , Ziegler D , Schimek D , Schuling S , Ziegler B , de Ondarza MB , Sniffen CJ , Broadwater N. 2017. Relationships between early-life growth, intake, and birth season with first-lactation performance of Holstein dairy cows. J Dairy Sci. 100:3697–3704. doi: 10.3168/jds.2016-12229
- Cowles KE , White RA , Whitehouse NL , Erickson PS. 2006. Growth Characteristics of Calves Fed an Intensified Milk Replacer Regimen with Additional Lactoferrin. J Dairy Sci. 89:4835–4845. doi: 10.3168/jds.S0022-0302(06)72532-2
- Davis CL and Drackley JK 1998. The development, nutrition, and management of the young calf. Ames , Iowa State University Press. 79–89.
- de Paula MR , Oltramari CE , Silva JT , Gallo MPC , Mourão GB , Bittar CMM. 2017. Intensive liquid feeding of dairy calves with a medium crude protein milk replacer, effects on performance, rumen, and blood parameters. J Dairy Sci. 98:458–477.
- Diesch TJ , Mellor DJ , Stafford KJ , Ward RN. 2004. The physiological and physical status of single calves at birth in a dairy herd in New Zealand. N Z Vet J. 52:250–255. doi: 10.1080/00480169.2004.36436
- Drackley JK. 2008. Calf nutrition from birth to breeding. Vet Clin North Am Food Anim Pract. 24:55–86. doi: 10.1016/j.cvfa.2008.01.001
- Faber SN , Faber NE , McCauley TC , Ax RL. 2005. Case study. Effects of colostrum ingestion on lactational performance . Prof Anim Sci. 21:420–425. doi: 10.15232/S1080-7446(15)31240-7
- Gelsinger SL , Heinrichs AJ , Jones CM. 2016. A meta-analysis of the effects of preweaned calf nutrition and growth on first-lactation performance. J Dairy Sci. 99:6206–6214. doi: 10.3168/jds.2015-10744
- Ghasemi E , Azad-Shahraki M , Khorvash M. 2017. Effect of different fat supplements on performance of dairy calves during cold season. J Dairy Sci. 100:5319–5328. doi: 10.3168/jds.2016-11827
- Girardier L and Stock MJ. 1983. Heat production in mammals. Mammalian Thermogenesis Eds. Chapman and Hall 225, 709–710.
- Godden S. 2008. Colostrum management for dairy calves. Vet. Clin Food Anim Pract. 24:19–38. doi: 10.1016/j.cvfa.2007.10.005
- Hammon HM , Schiessler G , Nussbaum A , Blum JW. 2002. Feed intake patterns, growth performance, and metabolic and endocrine traits in calves fed unlimited amounts of colostrum and milk by automate, starting in the neonatal period. J Dairy Sci. 85:3352–3362. doi: 10.3168/jds.S0022-0302(02)74423-8
- Hammon HM , Steinhoff-Wagner J , Schönhusen U and Metges CC 2014. Role of colostrum and colostrum components on glucose metabolism in neonatal calves. J Anim Sci 2013.91, 685–695
- Hammon HM , Steinhoff-Wagner J , Schönhusen U , Metges CC , Blum JW. 2012. Energy metabolism in the newborn farm animal with emphasis on the calf, endocrine changes and responses to milk-born and systemic hormones. Domest Anim Endocrinol. 43:171–185. doi: 10.1016/j.domaniend.2012.02.005
- Harper ME , Antoniou A , Bevilacqua L , Bezaire V , Monemdjou S. 2002. Cellular energy expenditure and the importance of uncoupling. J Anim Sci. 80:E90–E97. doi: 10.2527/animalsci2002.80E-Suppl_2E90x
- Herpin P , Dividich J , Berthon D , Hulin J. 1994. Assessment of thermoregulatory and postprandial thermogenesis over the first 24 h after birth in pigs. Exp Physiol. 79:1011–1019. doi: 10.1113/expphysiol.1994.sp003815
- Herpin P , Louveau I , Damon M and Le Dividich J. 2005. Environmental and hormonal regulation of energy metabolism in early development of the pig. In: Burrin DG and Mersmann H, editor. Biology of metabolism in growing animals. New York (NY): Elsevier Press; p. 345–374.
- Himms-Hagen J. 1985. Brown adipose tissue metabolism and thermogenesis. Annu Rev Nutr. 5:69–94. doi: 10.1146/annurev.nu.05.070185.000441
- Himms-Hagen J. 1990. Brown adipose tissue thermogenesis: Interdisciplinary studies. FASEB. 4:2890–2898. doi: 10.1096/fasebj.4.11.2199286
- Hulbert LE , Moisá SJ. 2016. Stress, immunity, and the management of calves. J Dairy Sci. 99:3199–3216. doi: 10.3168/jds.2015-10198
- Jasper L , Weary DM. 2002. Effects of ad libitum milk intake on dairy calves. J Dairy Sci. 85:3054–3058. doi: 10.3168/jds.S0022-0302(02)74391-9
- Kirovski D. 2015. Endocrine and metabolic adaptations of calves to extra-uterine life. Acta Vet Belgrade. 65:297–318. doi: 10.1515/acve-2015-0025
- Klingenspor M , Fromme T. 2012. Brown adipose tissue. Mol Nutr Med. 1–46.
- Korst M , Koch C , Kesser J , Müller U , Romberg FJ , Rehage J , Eder K and Sauerwein H 2017. Different milk feeding intensities during the first 4 weeks of rearing in dairy calves, Part 1, Effects on performance and production from birth over the first lactation. J Dairy Sci. 100:3096–3108.
- Lago A , McGuirk SM , Bennett TB , Cook NB , Nordlund KV. 2006. Calf respiratory disease and pen microenvironments in naturally ventilated calf barns in winter. J Dairy Sci. 89:4014–4025. doi: 10.3168/jds.S0022-0302(06)72445-6
- Lammoglia MA , Bellows RA , Grings EE , Bergman JW. 1999a. Effects of prepartum supplementary fat and muscle hypertrophy genotype on cold tolerance in newborn calves. J Anim Sci. 77:2227–2233. doi: 10.2527/1999.7782227x
- Lammoglia MA , Bellows RA , Grings EE , Bergman JW , Short RE , MacNeil MD. 1999b. Effects of feeding beef females supplemental fat during gestation on cold tolerance in newborn calves. J Anim Sci. 77:824–834. doi: 10.2527/1999.774824x
- McGuirk SM , Collins M. 2004. Managing the production, storage and delivery of colostrum. Vet Clin North Am Food Anim Pract. 20:593–603. doi: 10.1016/j.cvfa.2004.06.005
- Mellor DJ , Stafford KJ. 2004. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet J. 168:118–133. doi: 10.1016/j.tvjl.2003.08.004
- Noblet J , Le Dividich J. 1981. Energy metabolism of the newborn pig during the first 24 h after birth. Biol Neonate. 40:175–182. doi: 10.1159/000241487
- Nonnecke BJ , Foote MR , Miller BL , Fowler M , Johnson TE , Horst RL. 2009. Effects of chronic environmental cold on growth, health, and select metabolic and immunologic responses of preruminant calves. J Dairy Sci. 92:6134–6143. doi: 10.3168/jds.2009-2517
- Nordlund KV. 2008. Practical considerations for ventilating calf barns in winter. Vet Clin Food Anim Pract. 24:41–54. doi: 10.1016/j.cvfa.2007.10.006
- NRC . 2001. Nutrient requirements of dairy cattle. 7th rev. ed. Washington (DC): National Academy of Sciences.
- Okamoto M , Robinson JB , Christopherson RJ , Young BA. 1986. Summit metabolism of newborn calves with and without colostrum feeding. Can. J Anim Sci. 66:937–944. doi: 10.4141/cjas86-103
- Olson DP , Papasian CJ , Ritter RC. 1980. The effects of cold stress on neonatal calves. II. Absorption of colostral immunoglobulins. Can J Comp Med. 44:19–23.
- Omidi-Mirzaei H , Khorvash M , Ghorbani GR , Moshiri B , Mirzaei M , Pezeshki A , Ghaffari MH. 2015. Effects of the step-up/step-down and step-down milk feeding procedures on the performance, structural growth, and blood metabolites of Holstein dairy calves. J Dairy Sci. 98:1–7. doi: 10.3168/jds.2014-9260
- Osaka I , Matsui Y , Terada F. 2014. Effect of the mass of immunoglobulin (Ig)G intake and age at first colostrum feeding on serum IgG concentration in Holstein calves. J Dairy Sci. 97:6608–6612. doi: 10.3168/jds.2013-7571
- Pakkanen R , Aalto J. 1997. Growth factors and antimicrobial factors of bovine colostrum. Int Dairy J. 7:285–297. doi: 10.1016/S0958-6946(97)00022-8
- Patterson DJ , Bellow RA , Burgening PJ , Carr JB. 1987. Occurrence of neonatal and postnatal mortality in range beef cattle. I. Calf loss incidence from birth to weaning, backward and breech presentations and effects of calf loss on subsequent pregnancy rate of dams . Theriogenology. 28:557–569. doi: 10.1016/0093-691X(87)90273-1
- Pineda A , Ballou MA , Campbell JM , Cardoso FC , Drackley JK. 2016. Evaluation of serum protein-based arrival formula and serum protein supplement (Gammulin) on growth, morbidity, and mortality of stressed (transport and cold) male dairy calves. J Dairy Sci. 99:9027–9039. doi: 10.3168/jds.2016-11237
- Pozos RS , Danzl D. 2011. Human physiological responses to cold stress and hypothermia. Pages 351–382 in Medical Aspectsof Harsh Environments. Vol. 1. Pandolf KB and Burr RE, Textbooks of Military Medicine, Washington, USA.
- Quigley JD , Wolfe TA , Elsasser TH. 2006. Effects of additional milk replacer feeding on calf health, growth, and selected blood metabolites in calves. J Dairy Sci. 89:207–216. doi: 10.3168/jds.S0022-0302(06)72085-9
- Raeth-Knight M , Chester-Jones H , Hayes S , Linn J , Larson R , Ziegler D , Ziegler B , Broadwater N. 2009. Impact of conventional or intensive milk replacer programs on Holstein heifer performance through six months of age and during first lactation. J Dairy Sci. 2:799–809. doi: 10.3168/jds.2008-1470
- Rawson RE , Dziuk HE , Good AL , Anderson JF , Bates DW , Ruth GR. 1989. Thermal insulation of young calves exposed to cold. Can J Vet Res. 53:275–278.
- Rincker LE D , VandeHaar MJ , Wolf CA , Liesman JS , Chapin LT , Weber Nielsen MS. 2011. Effect of intensified feeding of heifer calves on growth, pubertal age, calving age, milk yield, and economics. J Dairy Sci. 94:3554–3567. doi: 10.3168/jds.2010-3923
- Robinson JB , Young BA. 1988. Metabolic heat production of neonatal calves during hypothermia and recovery. J Anim Sci. 66:2538–2544. doi: 10.2527/jas1988.66102538x
- Soberon F , Raffrenato E , Everett RW , Van Amburgh ME. 2012. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J Dairy Sci. 95:783–793. doi: 10.3168/jds.2011-4391
- Sutherland MA , Stewart M , Schütz KE 2013. Effects of two substrate types on the behaviour, cleanliness and thermoregulation of dairy calves. Appl Anim Behav Sci 147, 19- 27. doi: 10.1016/j.applanim.2013.04.018
- Tozer PR , Heinrichs AJ. 2001. What affects the costs of raising replacement dairy heifers: A multiple-component analysis. J Dairy Sci. 84:1836–1844. doi: 10.3168/jds.S0022-0302(01)74623-1
- Uruakpa FO , Ismond MAH , Akobundu ENT. 2002. Colostrum and its benefits, a review. Nutr Res. 22:755–767. doi: 10.1016/S0271-5317(02)00373-1
- USDA . 2014. Dairy cattle management practices in the United States. Fort Collins (CO): APHIS.
- Van De Stroet DL , Calderón Díaz JA , Stalder KJ , Heinrichs AJ , Dechow CD. 2016. Association of calf growth traits with production characteristics in dairy cattle. J Dairy Sci. 99:8347–8355. doi: 10.3168/jds.2015-10738
- Vermorel M , Dardillat C , Vernet J , Saido DC , Demigne C 1983. Energy metabolism and thermoregulation in the newborn calf. Annales de Recherches Vétérinaires 4, 382–389.
- Vermorel M , Vernet J , Dardillat C , Saido DC , Demigne C. 1989. Energy metabolism and thermoregulation in the newborn calf; Variations during the first day of life and differences between breeds. Can J Anim Sci. 69:103–111. doi: 10.4141/cjas89-013
- Weaver DM , Tyler JW , VanMetre DC , Hostetler DE and Barrington GM 2000. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med 14, 569–577. doi: 10.1111/j.1939-1676.2000.tb02278.x
- Webster J 1984. Calf husbandry, health and welfare. Collins, London, UK . 71–97.
- Xu R. 1996. Development of the newborn gi tract and its relation to colostrum/milk intake, a review. Reproduction. Fertil Dev. 8:35–48. doi: 10.1071/RD9960035
- Yang M , Zou Y , Wu ZH , Li SL and Cao ZJ. 2015. Colostrum quality affects immune system establishment and intestinal development of neonatal calves. J Dairy Sci. 98:1–11.
- Young BA. 1981. Cold stress as it affects animal production. J Anim Sci. 52:154–163. doi: 10.2527/jas1981.521154x
- Young BA. 1983. Ruminant cold stress, effect on production. J Anim Sci. 57:1601–1607. doi: 10.2527/jas1983.5761601x
