ABSTRACT
This study aimed to determine the effectiveness of guanidinoacetic acid (GAA) with or without methionine (Met) compared to creatine (CREA) at enhancing duck’s performance, restoring tissue CREA and improving meat quality. Mulard ducklings (n = 250) were randomly assigned to control (without additives), or control plus CREA, GAA, GAA + 0.2%Met (GAAMet0.2) or GAA + 0.4%Met (GAAMet0.4) groups in a completely random experimental design. Dietary supplementation of CREA or GAA significantly increased (P < 0.05) overall weight gain and improved feed:gain ratio. Supplementation of GAA (especially GAAMet0.4 group) significantly increased (P < 0.05) carcass and breast yield. Meat pH values were higher (P < 0.05) with dietary GAA + Met or CREA. Providing of dietary GAA + Met led to higher levels of plasma CREA than dietary CREA itself. The molecular investigation indicated that dietary CREA or GAA with Met enhanced the gene expression of hepatic insulin-like growth factor-1, growth hormone and muscle myogenin. Finally, dietary GAA + Met was superior to CREA in improving duck’s performance based on molecular markers related to growth (IGF-1 and growth hormone) and myogenesis (upregulating myogenin and downregulating myostatin). Although, dietary GAA + Met enhanced muscle’s CREA loading than CREA, the long-term GAA supplementation in ducks may induce methyl-groups shortage for protein synthesis, this was resolved with Met addition in our study.
1. Introduction
The world production of meat-type ducks has strongly increased with increased demand for animal protein. Selection of energy feed ingredients are major cost during the ducks force-feeding period and optimal energy level is essential for lowering feed cost. Increasing dietary energy level may enable faster gains or enhance meat production in a given period (Saleh Citation2004).
Creatine and creatine phosphate system is, heavily, involved in energy metabolism and storage by maintaining ATP concentrations and buffer muscle lactic acid accumulation (Bessman Citation1987). The animal demand for creatine either from exogenous protein-rich dietary sources of animal origin (e.g. fish meal) or by endogenous synthesis, in the liver where creatine can be synthesized from guanidinoacetic acid (GAA). Usually farm animals fed on diets containing reduced quantities of animal protein or with no animal protein, may be lacking creatine. On the other hand, GAA, creatine, and creatinine represent a one-way metabolic process, meaning that creatine cannot be re-converted to GAA, and creatinine cannot be re-converted to creatine. Hence, in the prospect of diminishing the amounts of animal-proteins included in animal feeds without affecting their growth rate, dietary incorporation of creatine or its precursor GAA is essential particularly for growing animals demanding continuous high energy supply and might restore the tissue’s creatine load.
Dietary supply of creatine promotes muscle hypertrophy due to higher total creatine and phosphocreatine storage, which stimulate the production of muscular IGF-I and protein synthesis in muscle (Burke et al. Citation2008). GAA is an immediate precursor of CREA that requires only a methyl-group transfer from S-adenosylmethionine (SAM); a universal methyl donor synthesized from methionine (Walker Citation1979).
Creatine as a feed additive displays some problems, such as high cost and instability, compared with GAA, which is more stable and less expensive (Baker Citation2009). Thus, supplemental GAA, can be used as an alternative to creatine in chick’s feed and efficiently increased levels of muscle creatine and breast meat yield (Michiels et al. Citation2012).
From another point, the hepatic synthesis of creatine from GAA utilizes a substantial ratio of methyl groups from SAM (Stead et al. Citation2001). In contrast, supplemental creatine has the potential to spare methyl groups via negative feedback on Arginine:glycine amidinotransferase activity (McGuire et al. Citation1984), lowering GAA production and the need for methyl groups used in creatine synthesis, which has been demonstrated in creatine supplemented rats (Deminice et al. Citation2009). Therefore, it comes out that dietary GAA supplementation considerably increases the methylation demand and lead to accumulation of homocysteine (risk factor for cardiovascular disease) in the blood; resulted from methylation reaction (Stead et al. Citation2001; Setoue et al. Citation2008). Thus, direct methyl donors are good candidates to avoid the harmful accumulation of homocysteine.
Methionine (Met) is the first limiting amino acid for poultry, required for muscle and feather metabolism and its supplementation is critical to maximizing duck’s performance. Moreover, Met is a source of methyl group needed for creatine synthesis from GAA and consequently their addition with methionine should spare Met availability for protein synthesis (McBreairty et al. Citation2015).
Furthermore, the influence of nutrients on gene expression related to animal growth contributes to understanding different variations in bird’s performance. Growth hormone (GH) is an important regulator of growth rate and body composition, through the GH receptor pathway (Kita et al. Citation2005). Also, myogenin is one of the most important genes responsible for the formation of muscle fibres, and its expression level is associated with muscle growth (Koishi et al. Citation1995). Contrarily, myostatin suppress muscle growth and/or induce muscle atrophy and appears to act as a negative regulator of muscle development (Sandri Citation2008). Since limited data in the literature was found on the effects of feeding creatine or GAA to ducks, the purpose of this study was to investigate the effects of dietary creatine and GAA with methionine supplementation on performance, meat quality, muscle-tissue creatine storesand growth-related genes in meat producing ducks.
2. Material and methods
The present study was approved by the Animal Care and Welfare Committee of the Faculty of Veterinary Medicine, Zagazig University.
2.1. Diets and experimental design
The present study was carried out on 250 one-day-old Mulard ducklings that were obtained from a commercial duck hatchery. On arrival, the birds were weighed and randomly assigned into five experimental groups; each group contained 50 ducklings that were equally subdivided into five replicates and raised for 42 days. The temperature was kept at 33°C up to 3 days of age, and then it was reduced gradually to room temperature.
Ducklings feeding programme consisted of starter diet (up to 21 days) and finisher diet (22–42 days). The ducks had free access to water and experimental diets in wet mash form. The composition of the experimental control diet is shown in . Five dietary groups were considered: (1) control diet (C); (2) control diet supplemented with 0.05% creatine (CREA); (3) control diet supplemented with 0.05% of guanidinoacetic acid (GAA); (4) control diet supplemented with 0.05% GAA plus 0.2% Methionine (GAAMet0.2); (5) control diet supplemented with 0.05% GAA plus 0.4% of Methionine (GAAMet0.4). GAA was obtained from, Evonik Industries (Hanau, Germany) and CREA from Germany and packed in USA (California Gold Nutrition).
Table 1. Ingredient composition and calculated nutrient content of basal diets.
2.2. Growth performance measurements
Average live body weight of ducks was measured at the end of starter and finisher periods and feed intake was recoded at the end of the two rearing periods. And, accordingly, growth performance parameters, as body weight gain, feed:gain ratio and protein efficiency ratio were calculated as we previously reported (Ibrahim et al. Citation2018).
2.3. Sampling
At the end of the feeding period, 5 ducks per group were randomly selected, fasted overnight, and weighed then slaughtered in the next morning to determine carcass characteristics, and nutritional composition and quality of breast meat. Breast samples were collected for analysis of CREA, GAA contents. For gene expression analyses of Myogenin, Myostatin, IGF-1, and β-actin the samples were taken immediately after slaughter, snap frozen in liquid nitrogen, and stored at −80°C till analyzed. Blood samples were taken in EDTA tubes and plasma was separated and the samples were stored at −20°C until analysis of creatine, GAA and homocysteine contents.
2.4. Carcass characteristics and meat quality
After carcasses chilling for 24 h at 2°C, 5 ducks per group were weighed to determine carcass weight, carcass yield including head and toe (g), breast weight (g), and breast yield (g/kg, without bones). The chemical composition of breast meat for moisture, crude protein and intramuscular fat was done according to AOAC Citation2002). For meat quality measurements, right side of breast meat was used to determine postmortem pH (at 0.5, 12 and 24 h), and conductivity at 24 h (μS; PQM metre). Drip loss (%; proportionate weight loss of a sample hanging in a plastic bag for 48 h at 2°C). The same sample was used for cooking and thaw loss, after storage at −20°C, thaw loss (%; proportional weight loss of a meat sample before frozen storage at −20°C and after overnight defrosting at 4°C) and cooking loss (%; proportionate weight loss of a sample after cooking in an open plastic bag in a water bath at 70°C for 40 min followed by cooling).
2.5. Analysis of plasma homocysteine and creatine and GAA in breast tissues and plasma
HPLC determination of plasma and tissue creatine and GAA concentrations was done according to the method described by Buchberger and Ferdig (Citation2004) and plasma homocysteine concentrations was following the method of Vester and Rasmussen (Citation1991). Briefly, 10 g of muscle tissue was chopped, mixed with 120 ml of deionized water and homogenized with a rotor mixer. The suspension was mixed with deionized water and centrifuged, and the supernatant was then filtrated. The plasma samples were thoroughly mixed with 500 ml of protein precipitation solution consisting of 0.1 M ZnSO4 in methanol/water (30:70, v/v). After centrifugation (4000 rpm, 10 min), 500 ml of the liquid fraction was subjected to ultra-filtration (10 kDa filters). The filtrate from blood and tissues was mixed with 300 ml of 1.3 M KOH and 150 ml of 0.9% aqueous ninhydrin solution. After incubation for 15 min at room temperature, 100 ml of 5% ascorbic acid solution and 100 ml of 5 M phosphoric acid were added and the mix was heated at 90°C for 30 min. After cooling at room temperature for 5 min the samples were run on the HPLC.
2.6. Analysis of gene expression by real-time qPCR
Firstly, total RNA was isolated from muscles samples using the E.Z.N.A.® spin column total RNA extraction kit (Omega Bio-Tech, Cat NO R6834-01, Canada) following manufacturer’s instructions. Total RNA purity was measured by Nano-Drop 1000 Spectrophotometer (Thermo-Scientific, USA). Then, total RNA was reverse transcribed into cDNA, using QIAGEN® LongRange 2-Step RT-PCR Kit, following manufacturer’s instructions. One microliter of total cDNA was mixed with 12.5 μL of 2× SYBR Green qPCR mix with ROX from Bio-Rad (USA), with addition of 5.5 μL of RNase-free water and 0.5 μL of each forward and reverse primers for the target genes. The values for the specific targets were normalized using those of β-actin to express the relative expression. The primer sequences of studied genes are the following: growth hormone (GH), F: 5′ ACAGGGAAGGATGATGCC 3′ R: 5′ AGCCAGGACTGGATGAGAAC 3′, Insulin-like growth factor 1 (IGF-1), F: 5′ GCCATCTGCAGGATACTTTGC 3′ R: 5′ CTGGGAGAATGCCCATTGGT 3′, Myogenin, F: 5′ CGGATCACCTCCTGCCTGA 3′; R: 5′ CGTCCTCTACGG CGATGCT3′, Myostatin, F: 5′ GCAAGTCCAAAAACCTACAA 3′ R: 5′ CTTCACATCAATACTCTGCCA 3′; and β-actin, F: 5′ GTGGATCAGCAAGCAGGAGT 3′; R: 5′ TTTGTCACAAGGGTGTGGGT 3′.
2.7. Statistical analyses
The experimental design is a completely randomized design and the data was evaluated using linear mixed model’s procedure. post hoc comparisons were applied, whenever appropriate, using Duncan’s test. All statistical procedures were performed using PASW statistics 18 (SPSS Inc., USA). Statistical significance was considered at P ≤ 0.05. Fold change was considered using the (2−ΔΔCt) method to quantify mRNA levels before performing the statistical analysis of gene expression.
4. Results
4.1. Growth performance
Performance of ducks in starter and finisher periods and allover performance results are shown in . In starter period, ducks fed on GAAMet0.4 recorded the highest final body weight and body weight gain, followed by ducks supplemented with GAAMet0.2, then CREA supplemented group, and, least, GAA supplemented group when compared with the control group. The average daily feed intake of ducklings fed on GAAMet0.2 and CREA was significantly increased (P < 0.05) followed by groups fed on diets supplemented with GAAMet0.4 then GAA supplemented group in comparison with the control group which recorded the lowest feed intake in starter period. On the other hand, the gain:feed ratio of ducklings in starter period was higher in all GAA groups supplemented with Met, followed by CREA supplemented group then group supplemented with GAA, compared to control ducklings which recorded the lowest gain:feed ratio. Whilst, in the finisher period, the final body weight and body gain of ducks was significantly increased (P < 0.05) when supplemented with GAAMet0.4 followed by GAAMet0.2 and CREA when compared with GAA and control groups. Ducks groups fed on GAAMet0.4 and CREA recorded lower gain:feed ratio followed by groups fed on GAAMet0.2 and GAA when compared with control group. The allover performance results estimated that the final body weight and weight gain were higher in ducks supplemented with GAAMet0.4 than those fed on diets supplemented with GAAMet0.2 or CREA, where the lowest final body weight and weight gain were recorded in GAA then control groups. Total feed intake significantly decreased (P < 0.05) in ducks supplemented with GAAMet0.4 compared with CREA or GAAMet0.2. Duck groups fed on GAAMet0.4 achieved most favourable final gain: feed ratio followed by CREA then GAAMet0.2 groups when compared with GAA group. Finally, the addition of GAAMet0.4 significantly increased the protein efficiency ratio than the addition of CREA or GAAMet0.2 in duck’s diets.
Table 2. Effect of CREA or (GAA) with or without methionine supplementation on Moulard duck’s performance (starter, grower and allover period).
4.2. Carcass characteristics and meat quality
Results related to carcass measurement and meat quality are shown in . Addition of Met0.4 to GAA recorded a significant increased carcass and breast meat yield (P < 0.05), followed by groups supplemented with GAAMet0.2 and CREA when compared with GAA and control groups. The moisture content was not affected by the treatments, while crude protein content was significantly increased (P < 0.05) in groups supplemented with GAAMet0.4 and CREA when compared with other groups. While, fat content was only reduced in the GAA group (without added methionine). After 24 h, the pH values were significantly decreased (P < 0.05) in CREA and GAAMet groups when compared with GAA (without Met), whereas higher pH value was recorded in the control group. None of the drip loss, thaw loss, or cooking loss % were affected by the studied dietary treatments.
Table 3. Effect of CREA or (GAA) with or without methionine supplementation on carcass characteristics and meat quality in Moulard ducks at slaughter (d 42).1
4.3. Creatine loading effect
Inclusion of creatine and GAA either alone or with methionine in duck’s diets significantly decreased the breast meat GAA content and elevated the breast meat content of creatine (). The groups supplemented with GAA plus Met exhibited decreased breast meat GAA content than the group supplemented with GAA alone. In addition, the breast meat content of creatine was inversely correlated with GAA content.
Table 4. Plasma concentrations of CREA, GAA and homocyteine and Breast muscle concentrations of CREA and GAA of Moulard ducks at slaughter (d 42).
4.4. Gene expression
Data of mRNA expression for growth hormone (GH), insulin-like growth factor-1 (IGF-I), myogenin and myostatin genes are shown in . Duck groups supplemented with GAAMet0.4 recorded the highest mRNA level of growth hormone gene expression followed by CREA and GAAMet0.2 when compared with GAA and control groups. The mRNA expression level of GH significantly increased (P < 0.05) in CREA and GAAMet groups than in GAA or control groups. In addition, there was no significant difference between groups supplemented with GAA plus 0.2% or 0.4% Met.
Figure 1. Effect of CREA or GAA on the relative mRNA expression of (A) growth hormone, GH; (B) insulin-like growth factor-1, IGF-1; (C) mygenin and myostatin genes of Mulard ducks at slaughter (d 42).
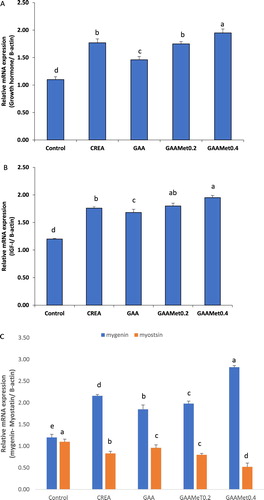
Myogenin mRNA expression in duck’s muscle was markedly increased and myostatin gene expression was significantly decreased (P < 0.05) in CREA and GAAMet groups compared to GAA and control groups.
5. Discussion
The control diet in this study was formulated according to NRC of poultry (Citation1994), also the methionine level in the control diet was calculated and meet the minimum requirement reported for ducks in NRC of poultry (1994). Our aim in this study was not to question control diet´s level of methionine (reported in NRC) but the aim from our study was to describe whether the addition of creatine or its precursor guanidinoacetic acid will show better results in meat-producing ducks. In addition, we assumed that methionine level reported in NRC for ducks is sufficient in the control diet. In fact, the addition of more methionine (alone) in the diet was not beneficial in broilers (Sigolo et al. Citation2019). However, the addition of guanidinoacetic acid required more methionine; as a source of methyl groups, than stated in NRC, which resulted in favourable/beneficial results in the present study.
5.1. Effects on duck’s performance and feed utilization
Our experimental design was built on the use of creatine (CREA) and its precursor guanidinoacetic Acid (GAA) in energy metabolism and improving the performance of ducks fed on whole-plant diets. In addition, comparing between dietary CREA and GAA on tissue CREA loading effect has not been done in ducks before and whether chronic use of the GAA will limit methionine (Met) availability and affects duck’s performance and protein synthesis or not. Thus, we expected that supplementation of the GAA with Met as a methyl-group donor will spare Met for protein synthesis and growth.
The role of CREA or GAA (especially with 0.4% Met; GAAMet0.4 group) on duck’s performance were prominent in both starter and finisher periods. The outcomes of the entire rearing period concluded that using of CREA or GAA positively affected duck’s growth rates, mainly due to better feed utilization. Moreover, using of dietary GAAMet0.4 was more effective at enhancing duck’s performance than GAAMet0.2 or GAA alone, while there is no difference between CREA and GAA with addition of 0.2% Met (GAAMet0.2 group). This was an expected response had not been previously demonstrated in ducks; as using GAA with addition of Met (2 or 4 gm/kg feed) enhanced duck’s growth by compensating Met required for growth and consumed from conversion of the GAA to CREA. As If the dietary Met is strongly restricted, the process of transmethylation of other nutrients will be reduced. Recently, a study described the protecting role of methionine (normal level or excess) in heat stressed broilers by decreasing the gene expression of genes related to muscle breakdown and increasing gene expression of genes related to protein synthesis (Del Vesco et al. Citation2015). Also, the results from Ringel et al. (Citation2008) report significant performance improvements with dietary GAA supplementation. GAA supplementation by 0.6 and 1.2 g/kg to broilers restored the availability of creatine and resulted in efficient feed conversion and gain (Michiels et al. Citation2012). In addition, increasing muscle creatine content with high-energy diets may lead to a better utilization of energy and dietary nutrients, consequently, improving growth and gain:feed ratio. The mechanisms of creatine in enhancing muscle performance and protein synthesis, may be related to increased muscle anaerobic capacity by restoring phosphocreatine (PCr), thus, preserve the normal physiological role and delay the start of muscle exhaustion (Casey et al. Citation1996). Or, could be due to increased intramuscular absorption of PCr which result in osmotic draw of fluid into the muscle cell, superhydration process, and increase the cell volume (Hultman et al. Citation1996). Dietary creatine supplementation is expected to reduce the feed intake as it can restore the energy requirements of the body with the CREA and PCr shuttle. Additionally, GAA is required for high creatine supply in fast-growing chicken to support muscle growth (Brosnan et al. Citation2009). Recently, positive effects on broiler growth performance were noted when GAA was provided (Abudabos et al. Citation2014). Mousavi et al. (Citation2013) also described that broiler’s FCR was improved by 0.06% GAA supplementation in a complete-fortified diet.
5.2. Effects on ducks’ carcass measurements and meat quality
The results from the current study indicated that, dietary GAA and CREA improved carcass and breast meat yield of ducks. Also, increasing dietary Met supplementation to 4 g/kg of feed, enhanced the effect of the GAA and increased breast meat and carcass yield than CREA supplementation and tended to increase muscle hydration, reduce the intramuscular fat and increase breast muscle protein content. In addition, the effect of CREA supplementation is the same of GAAMet2. These effects were in accordance with Ringel et al. (Citation2008) who reported that an increased breast meat yield with dietary GAA inclusion. Cellular hydration is an important anabolic signal controlling and triggering protein synthesis (Persky and Brazeau Citation2001). Juan et al. (Citation2011) found that dietary inclusion of 3% creatine to broiler increased the synthesis of muscle protein and lowered abdominal fat than in the control group and the same authors indicated that creatine increase liver Carnitine palmitoyltransferase and Peroxisome proliferator-activated receptor alpha expression resulted in a higher fatty acid β-oxidation. Thus, decreasing the fat deposition in broilers Feeding of CREA or GAA didn’t affect meat quality except for the decline in muscle pH and muscle tenderness as GAA especially when supplemented with Met was more efficient than CREA in muscle pH decline thereby impacting meat quality development. Poultry meat quality is greatly affected by postmortem glycolysis, the pH of meat is highly dependent upon postmortem glycolysis (Fletcher et al. Citation1997) and on the amount of glycogen present in muscle before slaughter. The breast muscle of broilers contains type II fast-twitch muscle fibres (Von Lengerken et al. Citation2002) which utilize energy by anaerobic glycolysis (Zhang et al. Citation2014). It is well recognized that aerobic glycolysis yields more ATP than anaerobic glycolysis (38 mol vs. 2 mol of ATP/1 mol of glucose) David and Michael (Citation2008). In normal postmortem condition, the muscle could swiftly utilize its creatine stores to produce more PCr for yielding ATP. Thus, in these muscle fibres, great amount of lactic acid is accumulated (acid meat), therefore increased protein degradation, drop in muscle pH, diminished water-holding capacity, and finally reducing the marketing value of poultry meat.
So, our study expected that CREA supplementation can improve the meat quality by two mechanisms, initially, it improves the absorption of glucose into muscle thus, decreasing the conversion of glucose to glycogen in the liver and, consequently, increase muscle store of glycogen. On the other way, CREA can limit the drop in pH through Phosphagen system which is the most instant means of buffering ATP through creatine kinase, which catalyzes the transfer of phosphate from phosphocreatine (PCr) to ADP, yielding ATP and creatine (Meyer and Foley, Citation1996). Thus, creatine kinase helps prevent rises in free ADP and utilize H+ which lead to a transitory alkaline shift at the start of the contraction, besides restricting postmortem glycolysis (Meyer and Foley, Citation1996). These results were also supported by (Zhang et al. Citation2014), who reported that dietary inclusion of 1200 mg/kg creatine monohydrate (CMH) for 14 days before slaughter significantly reduced drip loss, muscle lactate, and glycolytic capacity in broilers pectoralis major thus keeping good meat quality.
On other hand, dietary intake of guanidinopropionic acid is more useful in delaying drop in pH affecting meat retail value. As many researches documented that cellular creatine uptake in skeletal muscle is competitively inhibited by beta-guanidinopropionic acid (β-GPA), this effect encouraged a modification from glycolytic to oxidative metabolism and improved cellular uptake of glucose (Oudman et al. Citation2013). In contrast, normal muscle required additional ATP as continued muscle contraction lead to greater ATP hydrolysis and in this time the second way for ATP production is via myokinase response (2ADP → AMP + ATP). Subsequently, AMP is deaminated to IMP by AMP deaminase. Both effects of myokinase and AMP deaminase preserve normal ATP/ADP ratio during high energy demands (Meyer and Foley Citation1996). As rises in AMP and ADP stimulate anaerobic degradation of glycogen producing lactic acid and H+. From this point, dietary GAA intake can aid an additional mean for improving muscle quality through increases in AMP deaminase activity in order to blunt the increases in AMP and ADP that occur with greater ATP hydrolysis.
5.3. Creatine loading effects
Supplemental GAA increased plasma CREA concentrations, demonstrating that ducks can convert GAA to CREA. However, when supplemental GAA was provided with 4 mg Met/kg feed, the plasma GAA increases were less and the plasma creatine was more increased. As Met is used in the process of converting GAA to CREA, the lower blood concentrations of GAA in the presence of supplemental Met may be due to the Met-caused increased rate of conversion of GAA to CREA. Furthermore, muscle concentration of CREA is more in GAA supplemented muscle than in CREA supplemented muscle.
Our finding indicated that, Plasma homocysteine, a marker that is inversely related to methyl group supplementation, was decreased in CREA and GAA plus Met; as homocysteine can be recycled into Met in presence of methyl group. These results agreed with Michiels et al. (Citation2012) who found that dietary GAA increased the breast meat CREA levels of broilers. We had expected that a methyl group deficiency could be induced by the GAA because methyl groups are required for the conversion of GAA to creatine. On the contrary, creatine supplementation can spare methyl groups by negative feedback on arginine:glycine amidinotransferase enzyme activity (McGuire et al. Citation1984), decreasing GAA synthesis and methyl groups demand required for creatine production, besides lowering homocysteine yield in rats supplemented with dietary creatine (Deminice et al. Citation2009). The mechanism responsible for higher levels of breast muscle creatine could be a result of hepatic creatine conveyance, endogenous muscle synthesis or a combination of both. Increased concentrations of extracellular creatine have been found to reduce Cr transport (Loike et al. Citation1988). Thus, it is suggested that the higher plasma CREA levels achieved by dietary CREA supplementation for ducks downregulates the CREA transport from the liver and the reduced amount of CREA stored in muscle compared to GAA supplementation. Thus, GAA supplementation was more effective at enhancing levels of tissue CREA, even though both CREA and GAA supplementation resulted in high plasma CREA concentrations. Nevertheless, GAA supplementation requires higher methyl group supply for CREA synthesis and this can limit the methyl group needed for other transmethylation reactions. Similarly, McBreairty et al. (Citation2015) found higher hepatic creatine concentration in pigs fed on dietary creatine or GAA, although, only dietary GAA supplementation led to higher muscle creatine concentration and lower hepatic S-adenosylmethionine levels compared to the control. The results support the hypothesis that dietary GAA enhances the CREA load in duck’s tissues fed on plant diets and consequently improves cell energy organization and performance.
5.4. Effects on gene expression
In prospect of the relationship between growth hormone (GH) and insulin-like growth factor 1 (IGF-I) synthesis and release, the role of IGF-I in the proliferation and differentiation of muscle cells and protein synthesis (Oksbjerg et al. Citation2006) and the phycological function of myogenin was associated with muscle growth. The present results confirmed that dietary CREA or GAA with Met supplementation positively affected these abovementioned genes controlling duck’s growth, in addition using of Met up to 4 gm/kg of diet triggered GAA role, this was accompanied by increasing of breast weight. In addition, development of the skeletal muscle is regulated by myogenic controlling factors (including MyoD, Myf5, MyoG, and MRF4), myocyte enhancer factor 2 (including MEF2A, B, C, and D), myostatin and IGF-I signalling pathways (Zanoz and Gailly Citation2013). Also, the activation of IGF-1 genes promotes protein synthesis and muscle development (Glass Citation2003). Michiels et al. (Citation2012) reported that inclusion of GAA 1.2 g/kg of feed for broiler raised IGF-I level, which could be related to muscle growth. Similarly, Burke et al. (Citation2008) showed anabolic effects of CREA supplementation in humans might also be intermediated by upregulation of muscle IGF-I expression. Indeed, previous work indicated that creatine may increase the differentiation of myogenic satellite cells and protein synthesis in ovine (Vierck et al. Citation2003). Wen et al. (Citation2017) specified that the high methionine diets augmented mRNA levels of myogenic monitoring factors (MRF4, Myf5) and myocyte enhancer factor 2 (MEF2A and MEF2B) in the fast-growing broilers, and reduced myostatin mRNA level in the slow growing broilers.
6. Conclusion
Supplementation of GAA plus Met, and less with CREA, positively influenced duck’s performance. It is advisable to add Met in combination with GAA up to 4 g/kg feed when used for the long term to support its anabolic effect on muscle growth potential and protein synthesis. Moreover, GAA is more effective than CREA in supporting the muscle CREA load and enhanced growth hormone, insulin-like growth factor 1, myogenic genes expression associated with superior growth performance and breast muscle yield of ducks. These results reach to its desired maximal beneficial effects with Met addition.
Acknowledgements
The present authors financially supported this research. All study data will be available from the corresponding author upon request.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ahmed Abdelfattah-Hassan http://orcid.org/0000-0003-3478-1098
References
- Abudabos AM , Saleh F , Lemme A , Zakaria AH. 2014. The relationship between guanidine acetic acid and metabolisable energy level of diets on performance of broiler chickens. Ital J Anim Sci. 13(3):3269. doi: 10.4081/ijas.2014.3269
- AOAC . 2002. Official Methods of Analysis. 17th Ed. Arlington, VA : Association of Official Analytical Chemists.
- Baker DH. 2009. Advances in protein-amino acid nutrition of poultry. Amino Acids. 37(1):29–41. doi: 10.1007/s00726-008-0198-3
- Bessman SP. 1987. The creatine phosphate energy shuttle-the molecular asymmetry of a ‘pool’. Anal Biochem. 161:519–523. doi: 10.1016/0003-2697(87)90483-0
- Brosnan JT , Wijekoon EP , Warford-Woolgar L , Trottier NL , Brosnan ME , Brunton JA , Bertolo RFP. 2009. Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J Nutr. 139:1292–1297. doi: 10.3945/jn.109.105411
- Buchberger W , Ferdig M. 2004. Improved high-performance liquid chromatographic determination of guanidine compounds by precolumn derivatization with ninhydrin and fluorescence detection. J Sep Sci. 27:1309–1312. doi: 10.1002/jssc.200401866
- Burke DG , Candow DG , Chilibeck PD , MacNeil LG , Roy BD , Tarnopolsky MA , Ziegenfuss T. 2008. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exe. 18:389–398. doi: 10.1123/ijsnem.18.4.389
- Casey A , Constantin-Teodosiu D , Howell S , Hultman E , Greenhaff PL. 1996. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol-Endoc M. 271:E31–E37.
- David LN , Michael MC. 2008. Glycolysis, gluconeogenesis, and the pentose phosphate pathway. In: Arh K , Rossignol R , editor. Lehninger principles of biochemistry. 5th ed. New York, NY : W.H. Freeman and Company; p. 527–563.
- Del Vesco AP , Gasparino E , Grieser DO , Zancanela V , Voltolini DM , Khatlab AS , Guimaraes SEF , Soares MAM , Neto ARO. 2015. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stress-exposed broilers. Plos One. 10(2):0115821. doi: 10.1371/journal.pone.0115821
- Deminice R , Portari GV , Vannucchi H , Jordao AA. 2009. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr. 102:110–116. doi: 10.1017/S0007114508162985
- Fletcher DL , Qiao M , Smith D. 1997. The relationship of broiler breast meat color and pH to shelf-life and odor development. Poult. Sci. 76:1042–1046. doi: 10.1093/ps/76.7.1042
- Glass DJ. 2003. Signalling pathways that mediate skeletal 478 muscle hypertrophy and atrophy. Nat Cell Biol. 5:87–90. doi: 10.1038/ncb0203-87
- Hultman E , Soderlund K , Timmons JA , Cederblad G , Greenhaff PL. 1996. Muscle creatine loading in men. Journal of Applied Physiology. 81(1):232–237. doi: 10.1152/jappl.1996.81.1.232
- Ibrahim D , El-Sayed R , Khater SI , Said EN , El-Mandrawy SA. 2018. Changing dietary n-6: n-3 ratio using different oil sources affects performance, behavior, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Animal Nutrition. 4(1):44–51. doi: 10.1016/j.aninu.2017.08.003
- Juan C , Man W , Kong YL , Zou SX. 2011. Creatine pyruvate enhances lipolysis and protein synthesis in broiler chicken. Agr Sci China. 10:1977–1985. doi: 10.1016/S1671-2927(11)60199-5
- Kita K , Nagao K , Okumura J. 2005. Nutritional and tissue specificity of IGF- I and IGFBP-2 gene expression in growing chickens - a review. Asian Australas J Anim Sci. 18:747–754. doi: 10.5713/ajas.2005.747
- Koishi K , Zhang M , McLennan IS , Harris AJ. 1995. Myod protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn. 202:244–254. doi: 10.1002/aja.1002020304
- Loike JD , Zalutsky DL , Kaback E , Miranda AF , Silverstein SC. 1988. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci USA. 85:807–811. doi: 10.1073/pnas.85.3.807
- McBreairty LE , Robinson JL , Furlong KR , Brunton JA , Bertolo RF. 2015. Guanidinoacetate is more effective than creatine at enhancing tissue creatine stores while consequently limiting methionine availability in Yucatan miniature pigs. Plos One. 10(6):0131563. doi: 10.1371/journal.pone.0131563
- McGuire DM , Gross MD , Van Pilsum JF , Towle HC. 1984. Repression of rat kidney L-arginine:glycine amidinotransferase synthesis by creatine at a pre-translational level. J Biol Chem. 502(259):12034–12038.
- Meyer RA , Foley JM. 1996. Cellular processes integrating the metabolic response to exercise. Handbook of Physiology. Exercise: Regul Integr Mult Syst. 12:841–869.
- Michiels J , Maertens L , Buyse J , Lemme A , Rademacher M , Dierick NA , De Smet S. 2012. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult Sci. 91:402–412. doi: 10.3382/ps.2011-01585
- Mousavi S , Afsar A , Lotfollahian H. 2013. Effects of guanidinoacetic acid supplementation to broiler diets with varying energy contents. J Appl Poult Res. 22(1):47–54. doi: 10.3382/japr.2012-00575
- National Research Council (NRC) . 1994. Nutrition Requirements of poultry. 9th Ed. Washington, DC : National Academy Press.
- Oksbjerg N , Nissen PM , Vestergaard M. 2006. Serum from heifer calves treated with bovine growth hormone affects the rate of proliferation of C2C12 myogenic cells dependent on the plane of nutrition: The role of insulin-like growth factor- I and IGF-binding proteins-2 and -3. J Anim Physiol Anim Nutr. 90:177–184. doi: 10.1111/j.1439-0396.2005.00587.x
- Oudman I , Clark J F , Brewster LM. 2013. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: a systematic review. Plos One. 8(1):52879. doi: 10.1371/journal.pone.0052879
- Persky AM , Brazeau GA. 2001. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 53:161–176.
- Ringel J , Lemme A , Araujo LF. 2008. The effect of supplemental guanidino acetic acid in Brazilian-type broiler diets at summer conditions. Poult Sci. 87(Suppl. 1):154.
- Saleh EA. 2004. Effects of dietary nutrient density on performance 526 and carcass quality of male broilers grown for further processing. Int J Poult Sci. 3:1–10. doi: 10.3923/ijps.2004.1.10
- Sandri M. 2008. Signaling in muscle atrophy and hypertrophy. Physiology. 23:160–170. doi: 10.1152/physiol.00041.2007
- Setoue MO , Morita TS , Sugiyama K. 2008. Hyperhomocysteinemia induced by guanidinoacetic acid is effectively suppressed by choline and betaine in rats. Biosci Biotechnol Biochem. 72(7):1696–1703. doi: 10.1271/bbb.70791
- Sigolo S , Deldar E , Seidavi A , Bouyeh M , Gallo A , Prandini A. 2019. Effects of dietary surpluses of methionine and lysine on growth performance, blood serum parameters, immune responses, and carcass traits of broilers. J Appl Anim Res. 47(1):146–153. doi:10.1080/09712119.2019.1583571.
- Stead LM , Au KP , Jacobs RL , Brosnan ME , Brosnan JT. 2001. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol-Endoc M. 281:E1095–E1100.
- Vester B , Rasmussen K. 1991. High performance liquid chromatography method for rapid and accurate determination of homocysteine in plasma and serum. Eur J Clin Chem Clin Biochem. 29:549–554. 35.
- Vierck JL , Icenoggle DL , Bucci L , Dodson MV. 2003. The effects of ergogenic compounds on myogenic satellite cells. Med Sci Sport Exerc. 35:769–776. doi: 10.1249/01.MSS.0000065005.96298.01
- Von Lengerken G , Maak S , Wicke M. 2002. Muscle metabolism and meat quality of pigs and poultry. Vet Zootech-Lith. 20(42):82–86.
- Walker JB. 1979. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 50:177–242.
- Wen C , Jiang X , Ding L , Wang T , Zhou Y. 2017. Effects of dietary methionine on breast muscle growth, myogenic gene expression and IGF-I signaling in fast-and slow-growing broilers. Sci Rep-UK. 7(1):1924. doi: 10.1038/s41598-017-02142-z
- Zanoz N , Gailly P. 2013. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 70(21):4117–4130. doi: 10.1007/s00018-013-1330-4
- Zhang L , Li JL , Gao T , Lin M , Wang XF , Zhu XD , Gao F , Zhou GH. 2014. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animal. 8:1955–1962. doi: 10.1017/S1751731114001906
