 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Three graded levels of MHA (methionine hydroxyl analogue) added into the broilers’ diets were based on the assumption that the relative bioavailability of MHA sources to DL-methionine (DLM) on a molar basis was 100%, 90% or 80% to meet the requirement of total sulphur amino acids. DLM was used as the reference control group. Live body weight at 21 and 35 days was retarded (p < 0.05) in chicks fed a diet containing 100% equivalent MHA-FA (methionine hydroxyl analogue free acid) across dietary treatments. Chicks fed the diet containing MHA-Ca vs. MHA-FA grew faster at 35 days (p < 0.05). Chicks consumed least (p < 0.05) when 100% equivalent MHA-FA was added during 1–21 days. FCR was enhanced in MHA-Ca (methionine hydroxyl analogue calcium salt) vs. MHA-FA at all days measured. MHA effect on mortality, being higher (p < 0.05) in chicks on MHA-FA vs. MHA-Ca, was noted. Serum concentration of total cholesterol was lowest (p < 0.05) in chicks fed on DLM-added diet, but highest (p < 0.05) in those fed 100% equivalent MHA-Ca. Serum concentration of immunoglobulin A was low (p < 0.05) in chicks fed on 100% or 80% equivalent MHA-Ca compared with the rest of treatments. Collectively, MHA-Ca performed better than MHA-FA as to performance traits.
1. Introduction
Methionine is considered the first limiting amino acid in poultry due to its high demand on protein synthesis and feather development. Methionine requirements, on an as-fed basis, during the production stage of broiler chickens varied 0.50–0.52% for starter phase, 0.38–0.45% for grower phase and 0.32–0.44% for finisher phase (NRC, Citation1994; Cobb Citation2012; KFSF Citation2012; Ross Citation2014). Thus, it is common practice to supplement synthetic methionine in poultry diets to balance the dietary amino acids. The commonly used synthetic methionine products include DL-methionine (DLM) and methionine-hydroxy analogue (MHA) and the latter is present either a free acid (MHA-FA, containing 88% of active substance) or a calcium salt (MHA-Ca, containing 84% of active substance). Although MHA is not considered as true amino acid as it lacks amino group in their structure, it is known that it converts to biologically active L-methionine. Thus, there has been an on-going controversial debate concerning the biological availability of MHA compared to DLM in poultry.
Although it is not conclusive, it is generally accepted that MHA contains a low biological efficiency compared with DLM. For example, Sauer et al. (Citation2008) pointed out that the relative efficiencies of MHA-FA were estimated at 81% and 79% of the values for DLM, on an equimolar basis, in broiler chickens. Similarly, a low efficiency of MHA-FA (73% on equimolar basis) relative to DLM was reported (Hoehler et al. Citation2005). In addition, it was shown that the relative efficiency of MHA-Ca compared with DLM was 75% (on an equimolar basis) for feed conversion ratio and breast meat yield (Elwert et al. Citation2008). In comparative study between MHA-Ca and MHA-FA in broiler chicks, the former exhibited higher methionine efficacy than the latter (Boebel and Baker Citation1982), although both forms were inferior to DLM. In contrast, equal bio-efficacy of MHA-FA (Elkin and Hester Citation1983; Liu et al. Citation2006; Xi et al. Citation2007; Swennen et al. Citation2011; Conde-Aguilera et al. Citation2016) or MHA-Ca (Bishop and Halloran Citation1977; Baker and Boebel Citation1980; Dilger and Baker Citation2008) compared with DLM has been also reported in broiler chicks. Nonetheless, it is common practice in feed industry to add MHA sources in the diets of chickens albeit that the bioavailability relative to DLM is still under controversy. Recently, MHA-Ca has been re-introduced into the market (Elwert et al. Citation2008). To our surprise, there are hardly any reports on comparing the MHA sources, which thus prompted us to study the effect of the graded levels of MHA sources (MHA-FA and MHA-Ca) on performance in broiler chickens. In this study, we also included DLM as the reference to meet the methionine requirement.
2. Materials and methods
2.1. Animals, diets and experimental design
A total of one thousand fifty day-old feather-sexed male chicks (Ross 308) were purchased from local hatchery. Upon arrival, they were randomly allotted into one of 35 pens (1.8 m × 1.8 m) with rice husks as a bedding material. Each pen was equipped with nipples and pan feeder. The experiment was arranged in a completely randomized design (CRD). Each treatment had 5 replicates, 30 chicks per replicate (n = 5 replicates/treatment). All experimental diets ( and ) were formulated to contain equal crude protein, total sulphur amino acids (TSAA) and energy. The DLM-added diet was used as the control diet. Three graded levels of MHA sources, based on the bioavailability, were added to meet the TSAA requirement. The relative bioavailability of MHA sources to DLM, on a molar basis, was 100%, 90% or 80%, which were used to meet the TSAA requirement in diet formulation (). As MHA-FA and MHA-Ca contain 88% and 84% of active substance, three levels (2.94, 3.26, and 3.68 g/kg diet for the starter phase and 1.92, 2.12, and 2.40 g/kg diet for the grower phase) of MHA-FA, and three levels (3.08, 3.42, and 3.84 g/kg diet for the starter phase and 2.00, 2.22, and 2.50 g/kg diet for the grower phase) of MHA-Ca were supplemented into the starter and grower diets to meet the TSAA requirement (, , and ). The calculated amino acid levels were confirmed by analysis, being the recovery rate ranged from 98% to 102%. Feed and water were provided ad libitum. Continuous lighting programme was used, and the temperature of facility was maintained at 32°C during the first week and gradually decreased to reach 23°C at 3 weeks and kept thereafter. All experimental protocols were approved by the Animal Care Committee of KonKuk University.
Table 1. Ingredient and chemical composition of the starter diet.
Table 2. Ingredient and chemical composition of the finisher diet.
Table 3. Treatment arrangement.
2.2. Sampling
Feed intake and body weight per pen were measured on a weekly basis and used to calculate feed conversion ratio (FCR). At 5 weeks, 3 chicks per pen were randomly selected and humanely killed by cervical dislocation. Immediately after euthanasia, blood was collected by heart puncture. Serum samples were obtained by gentle centrifugation for 1300 rpm x 15 min and stored at −20°C until use. Immediately after blood sampling, left breast and thigh meats, liver and abdominal fat were sampled. And left breast meats, left leg meats, liver, and abdominal fat were weighed and expressed as the relative weight to live body weight. Production index (PI) was calculated by the following formula:
PI = ((livability × average daily gain))/((feed conversion ratio × 10)) (Fernandes et al. Citation2014).
2.3. Serum characteristics
At 5 weeks, serum samples were used to measure serum parameters such as potassium, sodium, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), glucose, total cholesterol, and triglycerides. All analysis was done at the commercial laboratory (Samkwang Medical Laboratories, Secho, Seoul).
Serum nitric oxide (NO) levels were measured as described (Lee et al. Citation2013). In brief, serum samples (100 µl) were mixed with an equal volume of freshly prepared Griess reagent (Sigma) containing 1% (w/v) sulphanilamine in 5% phosphoric acid and 0.1% (w/v) N-naphthyl ethylenediamine, the mixture was incubated for 10 min at room temperature, and the optical density at 540 nm was measured using a microtiter plate reader (Synergy 2, BioTek, Winooski, VT, USA). Nitrite concentrations were calculated from a standard curve using NaNO2.
Serum IgA level was determined using quantitative ELISA kit (Bethyl Laboratories, Montgomery, TX, USA), following the manufacturer’s instruction. The serum samples were diluted at 1:1,000 and tested in triplicate (Kang and Kim Citation2016).
2.4. Statistical analysis
Pen was considered an experimental unit. All data obtained were subjected to one-way analysis of variance (ANOVA) across treatment groups, and two-way ANOVA if the effect of MHA sources and added doses was tested (SPSS Ver. 21, IBM Corp., USA). Mortality data were transformed to prior to analysis. Data on mortality and production index were graphed with the use of GraphPad Prism (GraphPad Software Inc.). If a significant effect was noted, the significance between treatments was determined by Tukey’s test. The level of statistical significance was preset at p < 0.05.
3. Results and discussion
It is clear from this study that growth performance of chicks fed a diet containing 100% equivalent MHA-FA were least, those fed MHA-Ca vs. MHA-FA performed better, and excess MHA (e.g. 80% equivalent) in the diets compared with DLM did not improve growth performance (). Consequently, our study agrees with the earlier studies (Boebel and Baker Citation1982; van Weerden et al. Citation1982) showing that MHA-FA was inferior to MHA-Ca for chicks as methionine source. Boebel and Baker (Citation1982) postulated that non-monomeric forms present in MHA-FA was mainly attributed to lower methionine activity due to its less efficient utilization by the chicks. However, Martin-Venegas et al. (Citation2006) reported no difference in intestinal absorption in vitro and in vivo whether MHA-FA existed in either monomers or the mixture of monomer, dimer, and polymeric compounds. The reason for the reduced body weight gain of chicks (1–21 d) fed 100% equivalent MHA-FA containing diet is in part due to the lower feed intake and lower MHA-FA availability ().
Table 4. Effect of different methionine sources on growth performance in broiler chickens.
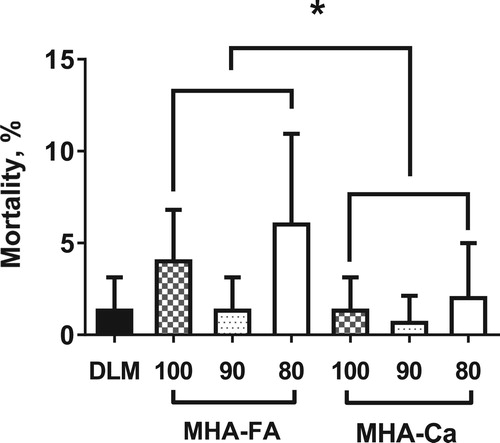
Mortality ranged from 0.7% to 6.0% and was not influenced by the treatments (). However, high mortality rate occurred in chicks fed on diets containing 100% or 80% equivalent MHA-FA, leading to the MHA effect. The clear explanation on why MHA-FA vs. MHA-Ca increased mortality is not readily available at this stage. In most studies, mortalities were not affected by MHA-FA compared with DLM (Daenner and Bessei Citation2003; Mandal et al. Citation2004) although Abdel-Maksoud et al. (Citation2010) noted a marginal increase in mortalities in MHA-FA vs. DLM. It cannot be explained by low methionine activity or MHA-induced toxicity. Lemme et al. (Citation2002) reported that the graded levels of DLM or MHA-FA that was beyond our addition levels did not affect mortality and total mortalities were 1.7% and 4.0% in two trials. In contrast to our finding, Willemsen et al. (Citation2011) reported that addition of MH-FA vs. DLM lowered mortalities in chickens raised on ambient environment or exposed to high temperature.
Figure 1. Effect of different methionine sources on mortality in broiler chickens data are expressed as means ± SD, n = 5. Asterisk (*) denotes significantly increased mortality in the MHA-FA compared with MHA-Ca group (p < 0.05). DLM = DL-methionine, MHA-FA = methionine hydroxyl analogue free acid, MHA-Ca = methionine hydroxyl analogue calcium salt.
The altered performance by methionine sources was clearly reflected in production index which was lowest (p < 0.05) in 100% equivalent MHA-FA compared with the rest of treatments. And the production index affected by MHA sources, being MHA-FA lower compared with MHA-Ca (). Recently, however, Zou et al. (Citation2015) reported that production index increased with increasing level of MHA-FA in diets. Tentatively, according to our finding, MHA-Ca vs. MHA-FA is more efficient to meet the requirement of methionine in broiler chickens. As determined by the production index, adding MHA-FA with 90% methionine efficacy and MHA-Ca with 100% methionine efficacy in practical diets seems appropriate. No economic incentives were found beyond this level (i.e. 80% methionine efficacy in MHA-FA; 90% and 80% methionine efficacy in MHA-Ca) as manifested by unaltered or slight decrease in performance, mortality and production index. In contrast to performance, meat yields were not affected by any of treatments although there was a slight decrease (p > 0.05) in relative percentage of breast meat in 100% equivalent MHA sources ().
Figure 2. Effect of different methionine sources on production index in broiler chickens data are expressed as means ± SD, n = 5. Letters (a,b) indicate a significant different between treatments (p < 0.05). Asterisk (*) denotes significantly elevated production index in the MHA-Ca compared with MHA-FA group (p < 0.05). DLM = DL-methionine, MHA-FA = methionine hydroxyl analogue free acid, MHA-Ca = methionine hydroxyl analogue calcium salt.
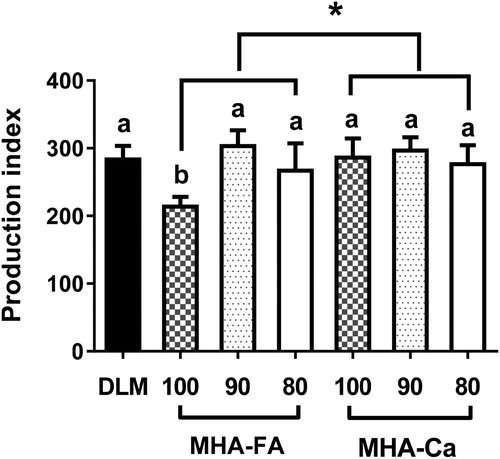
Table 5. Effect of different methionine sources on meats, abdominal fat, and organ weights in broiler chickens at 5 weeks of age.
As to blood parameters, interesting results have been emerged from this study. In general, MHA-fed chickens tended to have higher concentrations of sodium, glucose, GPT and total cholesterol in serum samples compared with DLM-fed chickens (). In essence, the hypercholesterolemic effect by MHA vs. DLM was clearly detected. Serum concentration of GOT, an indicator of liver function (Diaz et al. Citation2003), was lowered in DLM-treated group compared with MHA-treated groups. It is well documented that MHA upon absorption is efficiently converted to L-methionine in the liver of chickens (Dibner and Knight Citation1984; Dibner and Ivey Citation1992). Whether the observed difference in blood parameters is the consequence of altered liver function by MHA needs to be clarified. It should be however remembered that the observed values in serum samples are not overwhelmingly deviating from the reference values (Bowes et al. Citation1989) for GPT (184–273 IU/L), sodium (145–154 mmol/L) and cholesterol (115–163 mg/dL) except for glucose (268–282 mg/dL). The low glucose level (168–221 mg/dL) seen in this study can be explained by the experimental design which no-fasting was practised. It is noted that serum IgA concentration was highest in DLM-fed chicks, and lower in MHA-Ca vs. MHA-FA (, panel A). Although we did not measure IgY and IgM which are two most dominant immunoglobulins, IgA is also considered important as it is the third dominant immunoglobulin in blood and first dominant immunoglobulin in the intestine. In contrast, nitric oxide, an important mediator of immunity, was not altered by dietary treatments (, panel B). Further in-depth studies are needed to provide the insight into whether host immune responses can be altered depending on MHA sources.
Figure 3. Effect of different methionine sources on serum immunoglobulin A (panel A) and nitric oxide (panel B) in broiler chickens data are expressed as means ± SD, n = 5. Letters (a,b) indicate a significant different between treatments (p < 0.05). Asterisk (*) denotes significantly elevated production index in the MHA-Ca compared with MHA-FA group (p < 0.05). DLM = DL-methionine, MHA-FA = methionine hydroxyl analogue free acid, MHA-Ca = methionine hydroxyl analogue calcium salt.
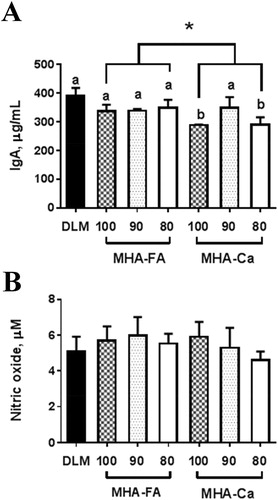
Table 6. Effect of different methionine sources on blood characteristics in broiler chickens at 5 weeks of age.
4. Conclusions
The present study showed that MHA-Ca vs. MHA-FA is considered an effective methionine source for broiler chickens as manifested by the former being higher in production index and lower in mortality compared with the latter. In addition, the cholesterol-lowering action by DLM compared with MHA sources and difference in immune parameter between MHA sources were noted. From a practical perspective, it is recommended that adding MHA sources with 90% equivalent to DLM, on an equimolar ratio, into a diet of broiler chicks is considered relevant to feed industry.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Kyung-Woo Lee http://orcid.org/0000-0002-3533-7979
Additional information
Funding
References
- Abdel-Maksoud A , Yan F , Cerrate S , Coto C , Wang Z , Waldrop PW. 2010. Effect of arginine level and source and level of methionine on performance of broilers 0 to 18 days of age. Int J Poult Sci. 9:14–20. doi: 10.3923/ijps.2010.14.20
- Baker DH , Boebel KP. 1980. Utilization of the D- and L-Isomers of methionine and methionine hydroxy analogue as determined by chick bioassay. J Nutr. 110:959–964. doi: 10.1093/jn/110.5.959
- Bishop RB , Halloran HR. 1977. The effect of methionine or methionine hydroxy analogue Supplementation on chick response to total sulfur amino acid intake. Poult Sci. 56:383–385. doi: 10.3382/ps.0560383
- Boebel KP , Baker DH. 1982. Efficacy of the calcium salt and free acid forms of methionine hydroxy analog for chicks. Poult Sci. 61:1167–1175. doi: 10.3382/ps.0611167
- Bowes VA , Julian RJ , Stirtzinger T. 1989. Comparison of serum biochemical profiles of male broilers with female broilers and white Leghorn chickens. Can J Vet Res. 53:7–11.
- Cobb 700 . 2012. Broiler performance and nutrition supplement. Cobb-Vantress. [accessed 2018 Jan]. http://www.cobb-vantress.com/docs/default-source/cobb-700-guides/cobb700_broiler_performance_nutrition_supplement_english9294AABB12037B70EE475E39.pdf .
- Conde-Aguilera JA , Cholet JCG , Lessire M , Mercier Y , Tesseraud S , van Milgen J. 2016. The level and source of free-methionine affect body composition and breast muscle traits in growing broilers. Poult Sci. 95:2322–2331. doi: 10.3382/ps/pew105
- Daenner E , Bessei W. 2003. Influence of supplementation with liquid DL-methionine hydroxy analogue-free acid (Alimet) or DL-methionine on performance of broilers. J Appl Poult Res. 12:101–105. doi: 10.1093/japr/12.2.101
- Diaz GJ , Roldan LP , Cortes A. 2003. Intoxication of crotalaria pallida seeds to growing broiler chicks. Vet Hum Toxicol. 45:187–189.
- Dibner JJ , Ivey FJ. 1992. Capacity in the liver of the broiler chick for conversion of supplemental methionine activity to L-methionine. Poult Sci. 71:700–708. doi: 10.3382/ps.0710700
- Dibner JJ , Knight CD. 1984. Conversion of 2-hydroxy-4-(methylthio)butanoic acid to L-methionine in the chick: A stereospecific pathway. J Nutr. 114:1716–1723. doi: 10.1093/jn/114.9.1716
- Dilger RN , Baker DH. 2008. Cyst(e)ine imbalance and its effect on methionine precursor utilization in chicks. J Anim Sci. 86:1832–1840. doi: 10.2527/jas.2007-0712
- Elkin RG , Hester PY. 1983. A Comparison of methionine sources for broiler chickens fed corn-soybean meal diets under simulated commercial grow-out conditions. Poult Sci. 62:2030–2043. doi: 10.3382/ps.0622030
- Elwert C , de Abreu Fernandes E , Lemme A. 2008. Biological effectiveness of methionine hydroxy-analogue calcium salt in relation to DL-methionine in broiler chickens. Asian-Australas J Anim Sci. 21:1506–1515. doi: 10.5713/ajas.2008.80201
- Fernandes BCS , Martins MRFB , Mendes AA , Milbradt EL , Sanfelice C , Martins BB , Aguiar EF , Bresne C. 2014. Intestinal integrity and performance of broiler chickens fed a probiotic, a prebiotic, or an organic acid. Rev Bras Cienc Avic. 16:417–424. doi: 10.1590/1516-635X1604417-424
- Hoehler D , Lemme A , Jensen SK , Vieira SL. 2005. Relative effectiveness of methionine sources in diets for broiler chickens. J Appl Poult Res. 14:679–693. doi: 10.1093/japr/14.4.679
- Kang HK , Kim CH. 2016. Effects of dietary supplementation with rice bran oil on the growth performance, blood parameters, and immune response of broiler chickens. J Anim Sci Technol. 58:12. doi: 10.1186/s40781-016-0092-6
- KFSP . 2012. Korean feeding standard for poultry. 2nd ed. Gyeonggi-do : National Institute of Animal Science.
- Lee KW , Lillehoj HS , Jang SI , Lee SH , Bautista DA , Ritter GD , Lillehoj EP , Siragusa GR. 2013. Comparison of live Eimeria vaccination with in-feed salinomycin on growth and immune status in broiler chickens. Res Vet Sci. 95:110–114. doi: 10.1016/j.rvsc.2013.02.005
- Lemme A , Hoehler D , Brennan JJ , Mannion PF. 2002. Relative effectiveness of methionine hydroxy analog compared to DL-methionine in broiler chickens. Poult Sci. 81:838–845. doi: 10.1093/ps/81.6.838
- Liu YL , Song GL , Yi GF , Hou YQ , Huang JW , Vazquez-Anon M , Knight CD. 2006. Effect of supplementing 2-hydroxy-4-(methylthio) butanoic acid and DL-methionine in corn-soybean-cottonseed meal diets on growth performance and carcass quality of broilers. Asian-Australas J Anim Sci. 19:1197–1205. doi: 10.5713/ajas.2006.1197
- Mandal AB , Elangovan AV , Johri TS. 2004. Comparison bio-efficacy of liquid DL-methionine hydroxyl analogue free acid with DL-methionine in broiler chickens. Asian-Australas J Anim Sci. 17:102–108. doi: 10.5713/ajas.2004.102
- Martin-Venegas R , Soriano-Garcia JF , Vinardell MP , Geraert PA , Ferrer R. 2006. Oligomers are not the limiting factor in the absorption of DL-2-hydroxy-4-(methylthio)butanoic acid in the chicken small intestine. Poult Sci. 85:56–63. doi: 10.1093/ps/85.1.56
- NRC . 1994. Nutrient requirements of poultry. 9th rev. ed. Washington, DC : Natl. Acad. Press.
- Ross 308 . 2014. Ross 308 broiler: nutrition specifications. Aviagen. [accessed 2018 Jan]. http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross308BroilerNutritionSpecs2014-EN.pdf .
- Sauer N , Emrich K , Piepho HP , Lemme A , Redshaw MS , Mosenthin R. 2008. Meta-analysis of the relative efficiency of methionine-hydroxy-analogue-free-acid compared with DL-methionine in broilers using nonlinear mixed model. Poult Sci. 87:2023–2031.
- Swennen Q , Geraert PA , Mercier Y , Everaert N , Stinckens A , Willemsen H , Li Y , Decuypere D , Buyse J. 2011. Effects of dietary protein content and 2-hydroxy-4-methylthibutanoic acid or DL-methionine supplementation on performance and oxidative status of broiler chickens. Br J Nutr. 106:1845–1854. doi: 10.1017/S0007114511002558
- van Weerden EJ , Bertram HL , Schutte JB. 1982. Comparison of DL-methionine, DL-methionine-Na, DL-methionine hydroxyl analogue-Ca, and DL-methionine hydroxyl analogue free acid in broilers by using a crystalline amino acid diet. Poult Sci. 61:1125–1130. doi: 10.3382/ps.0611125
- Willemsen H , Swennen Q , Everaert N , Geraert PA , Mercier Y , Stinckens A , Decuypere D , Buyse J. 2011. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult Sci. 90:2311–2320. doi: 10.3382/ps.2011-01353
- Xi PB , Yi GF , Lin YC , Zheng CT , Jiang ZY , Vazquez-Anon M , Song GL , Knight CD. 2007. Effect of methionine source and dietary crude protein level on growth performance, carcass traits and nutrient retention in Chinese color-feathered chicks. Asian-Australas J Anim Sci. 20:962–970. doi: 10.5713/ajas.2007.962
- Zou L , Wang D , Liu J , Bai Y , Liang Z , Zhang T. 2015. Effects of DL-2-hydroxy-4-(methylthio) butanoic acid on broilers at different dietary inclusion rates. Brit Poult Sci. 56:337–344. doi: 10.1080/00071668.2015.1021296
