?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Gossypol, a secondary metabolite found in cotton (Gossypium spp.), is known to be toxic to a variety of animals, particularly monogastric mammals and commercial poultry (Gallus domesticus). Gossypol toxicosis in poultry include reduced weight, decreased egg production, and egg yolk discolouration. However, there is limited published data regarding gossypol toxicity in northern bobwhites (Colinus virginianus), which may encounter cottonseed products in the environment and subsequently ingest gossypol. We determined the oral LD50 of gossypol in northern bobwhites following the Environmental Protection Agency’s OCSPP 850.2100 Guideline: Avian Acute Oral Toxicity Test. Through a range-finding test, we estimated that the LD50 was between 200 and 1,000 mg/kg body weight (BW). Following the range-finding test, we administered a single oral dose of refined gossypol to quail at 262, 342, 447, 585, and 765 mg/kg BW. We observed quail daily for mortality and any signs of intoxication throughout a 14-day observation period. We performed gross necropsies and had histopathology done on select organ tissues from experimental animals. Using the probit analysis, we determined that the oral LD50 of gossypol in northern bobwhites is 651 mg/kg BW (95% CI 579–731). Hepatocellular pigment accumulation and pancreatic necrosis were important lesions interpreted as evidence of gossypol toxicity.
1. Introduction
Cottonseed (Gossypium spp.) is a high-protein feed source that is used as a feed supplement and primary ration for a variety of livestock (Campbell et al. Citation2010). Although cottonseed-based feeds are generally well-tolerated by ruminant species, toxicity has been documented in swine (Sus scrofa), other monogastric mammals, and commercial poultry (Gallus domesticus). This toxicity has been linked to gossypol, a secondary plant metabolite found in cotton plant seeds, roots, and foliage (Gadelha et al. Citation2014).
The gossypol LD50 has been determined for several mammalian species, including rats (925–1,350 mg/kg), mice (500–950 mg/kg), rabbits (350–600 mg/kg), guinea pigs (280–300 mg/kg) (Eagle et al. Citation1948), and pigs (550 mg/kg) (Lyman et al. Citation1963). Although Abou-Donia (Citation1976) asserted ‘chickens are slightly more sensitive than rats, but [are less] sensitive [than pigs],’ there are no published LD50 data for birds. We chose to study gossypol toxicity in northern bobwhites (Colinus virginianus), a species of significant economic and cultural value.
Gossypol occurs in both a free and bound form (Zeng et al. Citation2015). Free gossypol is the active form that readily causes toxic effects while bound gossypol is the inactive, non-toxic form. Free gossypol readily binds to proteins and minerals, usually lysine and iron (Nagalakshmi et al. Citation2007; Gadelha et al. Citation2014). When this occurs, it inhibits lysine and iron absorption. When gossypol is ingested, the gastrointestinal tracts absorb it, and it passes into the blood stream (Lindsey et al. Citation1980; Abou-Donia et al. Citation1989; Kim et al. Citation1996). From there, the liver and kidneys metabolize and accumulate gossypol. Gossypol is excreted in the bile and feces, with a minimal amount being excreted in the urine.
High concentrations of free gossypol cause toxicosis, particularly in monogastric mammals and birds (Singla and Meenu Citation2008; Campbell et al. Citation2010). Gossypol toxicity in avian species has a different presentation than in monogastrics mammals. In swine, gossypol is cardiotoxic, and death results from cardiac failure (Burlatschenko Citation2002). Common signs of gossypol toxicity include pulmonary edema with excessive abdominal fluid, hypoprothrombinemia, and severe dyspnea (Abou-Donia Citation1976; Burlatschenko Citation2002). The no-observed-adverse-effect-level (NOAEL) for feeding free gossypol to swine is below 0.01% or 100 mg/kg feed (Hale and Lyman Citation1957). In commercial poultry, clinical signs of gossypol toxicity include: decreased egg production and egg size, egg yolk discolouration, and decreased feed efficiency and growth rate, all resulting in weight loss and cachexia (Abou-Donia Citation1976). The NOAEL for broilers (G. domesticus) is 200 mg/kg feed, which is equivalent to 20–30 mg/kg body weight (BW)/day; at this amount, gossypol does not affect growth or egg production and size (EFSA Citation2008).
The objective of this study is to evaluate the effects of experimental gossypol administration to northern bobwhites, with emphasis on mortality and the determination of the LD50 of gossypol, and identification of clinical, gross, and microscopic correlates of gossypol toxicosis.
2. Materials and methods
2.1. Bird management
Research was conducted at the Tarleton State University Northern Bobwhite Research Center, a biosafety level 1 laboratory, in Stephenville, TX during February–April 2016 under the auspices of the Tarleton State University Animal Care and Use Committee (06-016-2015). These experiments were conducted in accordance with the guidelines specified in the Animal Welfare Act and associated Animal Welfare Regulations and Public Health Service Policy (HHS Citation2015; USDA Citation2017).
We kept the birds in a facility that was a concrete building with constructed pens of varying sizes made from wood, chicken wire, and mesh netting. We housed the general breeding population in a 4.88 × 5.18 × 3.05 m pen; all control and treatment birds came from this population. During the study, the temperature was between 15.1°C and 21.3°C and relative humidity varied from 44.6% to 77.2%. We fed birds a standard commercial game bird feed (Purina® Game Bird + Turkey Startena SM, Gray Summit, MO, USA). Prior to feeding, Colorado State University Veterinary Diagnostic Laboratory in Fort Collins, CO tested feed for the presence of insecticides, aflatoxin B1, fumonisin, and arsenic and all tests came back negative. We provided feed and water ad libitum and maintained birds in non-reproductive condition by restricting the photoperiod to 0700–1700 h daily. All experimental and control birds were euthanized by cervical dislocation at the end of this study (AVMA Citation2013).
We randomly selected 100 birds for this study from our general population. Birds were 36 or 42 weeks old at the time of dosing. All birds were in apparent good health and did not have any physical deformities. We determined the mean BW from a sample of the breeding population ( = 199.66 g, n = 124). Birds used in the study weighed ± 10% of the mean population BW (range = 179.69–219.63 g).
2.2. Experimental design
We determined the acute toxicity of gossypol using a two-stage oral toxicity test, composed of a range-finding test and a definitive test (EPA Citation2012). For the range-finding test, we randomly assigned 40 birds to 3 treatment groups and one control group (5 male and 6 female). Treatment doses were 20 (5 male and 5 female), 200 (5 male and 5 female) and 1,000 (5 male and 4 female) mg/kg BW (due to laboratory error). Because the range-finding test showed the LD50 to be between 200 and 1,000 mg/kg BW, we geometrically spaced doses between 200 and 1,000 mg/kg BW for the definitive test. We assigned 60 birds to 5 treatment groups and one control group. Treatment doses were 262, 342, 447, 585, and 765 mg/kg BW. Each treatment group contained 10 birds (5 male and 5 female).
Sixteen days prior to dosing, we moved the test birds from the breeding population pen and randomly assigned them to individual galvanized 25.40 × 50.80 × 50.80 cm3 metal breeding cages. Cages were in rows stacked 5 × 12 and lights were hung parallel to the rows, so that all cages received roughly the same amount of illumination. Birds acclimated to the test facility, photoperiod, and basal diet for 16 days. We withheld feed from all birds 15 h prior to dosing to prevent regurgitation. In preparation for dose day, we filled capsules with the appropriate dose of free gossypol, which had an average purity of 99.31% (AdooQ BioScience, Irvine, CA, USA). We calculated gossypol doses using the mean BW of the 100 test birds rather than the BW of each individual bird due to time constraints and personnel limitations. Control groups received a placebo dose containing no gossypol. To minimize observer bias, a third-party person randomized the capsules and assigned doses. On dose day, we administered a single oral dose of gossypol in capsules early in the morning. Once birds ingested the capsule, we continuously monitored the birds for the first 2 h after dosing. Afterwards, we checked on the birds every hour for 6 h. We documented behaviour, gross appearance, mortality, and any signs of intoxication once a day before dosing and twice a day after dosing, once in the morning and once in the afternoon, for 14 days, the remainder of the study.
We performed gross necropsies on all birds that died during each experiment. Necropsies were performed within 24 h of a bird’s death, and cadavers were refrigerated at 2.8°C if the postmortem interval was greater than one hour. Necropsies of birds in the initial range test included photo documentation of the coelomic viscera in situ and individual photographs of the brain, heart, lungs, liver, kidneys, spleen, ventriculus, pancreas, and gonads. Individual organs were then weighed and preserved in 10% neutral buffered formalin. Preserved tissues were routinely processed for histological evaluation by paraffin embedding, 5-μm-thick sections were prepared for each tissue, and sections were stained with standard haematoxylin and eosin. Tissue sections were evaluated by board-certified veterinary and atomic pathologists. Because the evaluation of the range-finding test birds revealed differences in the weight of the heart, spleen, and ventriculus, only these organs were evaluated for the definitive test. We did not assess histopathology for the definitive test.
2.3. Statistical analysis
We used IMB® SPSS® Statistics Version 23 (Armonk, NY, USA) to conduct a probit analysis to determine the dose–response curve (Bliss Citation1934). To analyse organ weights from the range-finding test, we used a Welch’s t-test (Welch Citation1947); if the data did not have a normal distribution, we ran a Wilcoxon–Mann–Whitney rank sum test with Bonferroni correction (Mann and Whitney Citation1947). For the definitive test, we used a one-way analysis of variance (ANOVA) test and a Tukey’s honest significant difference (HSD) post-hoc test (Welch Citation1947; Tukey Citation1949); if the data did not have a normal distribution, we used a Kruskal–Wallis H-test and Dunn’s post-hoc test for multiple comparisons of independent samples with Bonferroni correction (Kruskal and Wallis Citation1952; Dunn Citation1964). We set α = 0.05 for all tests and performed all analyses using R version 3.3.2 (R Core Team Citation2017).
3. Results
3.1. Gossypol toxicity – clinical signs, mortality rate, and LD50
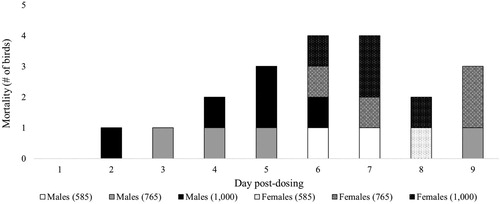
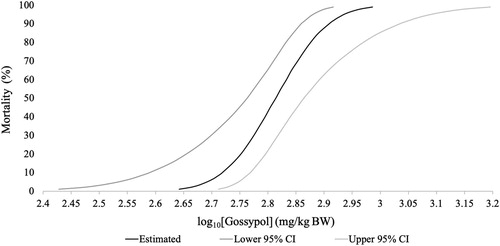
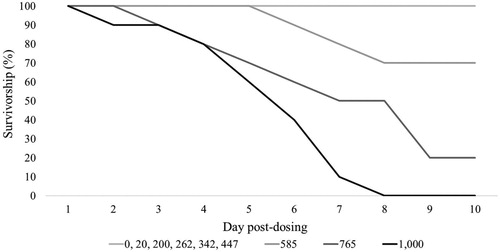
Gossypol toxicity signs included: weakness, lethargy, puffed-up and ruffled feathers, diarrhea, and decreased feed consumption and weight loss. The severity and length of symptoms varied with the gossypol dose; these factors increased in severity in birds receiving higher doses. All birds receiving the 1,000 mg/kg BW dose died (n = 9) while all birds receiving the 20 and 200 mg/kg BW doses survived (n = 20); 3 birds from the 585 mg/kg BW dose and 8 birds from the 765 mg/kg BW dose died while all other birds survived (n = 39) ( and ). Analysis resulted in an oral LD50 of gossypol of 651 mg/kg BW (95% CI 579–731) ().
Figure 1. Survivorship (%) of birds from the range-finding and definitive tests from 1 to 10 days post-dosing.
3.2. Necropsy, histopathology, and organ weights
Gross lesions observed at necropsy included: ectopic tissue in males dosed with 585 (n = 2) and 765 (n = 1) mg/kg BW; gallbladder distention in males and females dosed with 585 (n = 1), 765 (n = 3), and 1,000 (n = 2) mg/kg BW; and mineralization deposits in males dosed with 765 (n = 1) and 1,000 (n = 2) mg/kg BW. Post-mortem artefacts included gas distended intestinal tract in most of the birds and bile staining on the right rib cage of birds dosed with 585 (n = 1), 765 (n = 3), and 1,000 (n = 4) mg/kg BW.
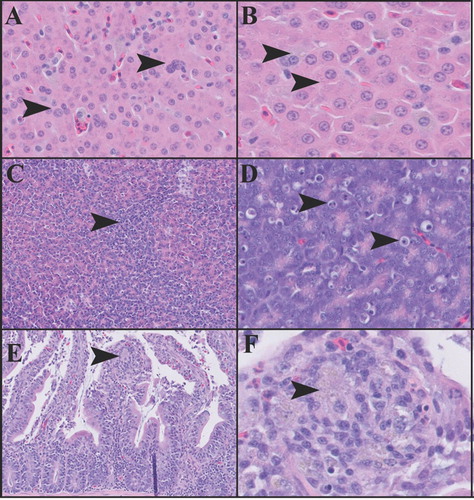
Histological examination was performed on select organs from the 1,000 mg/kg BW experimental group and included brain, heart, lung, and liver (n = 9); kidney and ventriculus (n = 8); pancreas (n = 5); duodenum (n = 3); ovary (n = 2); and testis and spleen (n = 1). Tissues from untreated control animals were not available for histological examination. The most prominent microscopic lesions were consistently observed in the liver ((A,B)) and pancreas ((C,D)). In all liver sections, there was mild to moderate Kupffer cell and hepatocellular pigment accumulation was accompanied by increased variability in hepatocellular size and increased frequency of karyomegalic and multinucleated hepatocytes. Finely granular grey-green and coarsely granular golden-brown pigment were present in both hepatocytes and Kupffer cells. The proportion of golden-brown pigment was increased in the Kupffer cells. Infrequent periportal and random nodular aggregates of heterophils and Kupffer cells, and foci of extramedullary haematopoiesis were also present in some liver sections. In all pancreatic sections, the exocrine pancreatic tissue had an enhanced nodular pattern, with round lobules of normal exocrine acini surrounded by rims of smaller, more densely packed and mildly disorganized acinar cells. The smaller acinar cells had enlarged nuclei, increased cytoplasmic basophilia with depleted or absent zymogen granules. A subset of affected cells exhibited cytoplasmic vacuolation, and individual acinar cell necrosis, characterized by cleared spaces containing shrunken cells with pyknotic or karyorrhectic nuclei, was commonly observed in these affected areas. Occasional perivascular clusters of lymphocytes were also present in some pancreatic sections.
Figure 4. Tissue sections of the liver (A and B), pancreas (C and D), and duodenum (E and F) from birds dosed with 1,000 mg/kg BW of gossypol. Liver (A), 200× magnification. Hepatocytes and Kupffer cells contain pigment, and there is marked variability in the size and number of hepatocytes nuclei (arrowheads). Liver (B), 400× magnification. Arrowheads indicate pigment accumulation in hepatocytes. Pancreas (C), 100× magnification. Arrowhead indicates the zonal region in which there is a collapse of exocrine acini, and remaining exocrine acinar cells exhibit increased cytoplasmic basophilia. Pancreas (D), 200× magnification. Arrowheads indicate individual exocrine acinar cell necrosis (apoptosis). Duodenum (E), 200× magnification. Arrowhead indicates cluster of pigment-laden macrophages within a blunted villus tip. Duodenum (F), 400× magnification. Arrowhead indicates cluster of pigment-laden macrophages. A small number of heterophils are present at the base of the arrowhead.
In six of the nine examined brains, there were multiple regions in which cerebral glial cells, particularly astrocytes, exhibited mild to moderate cytoplasmic vacuolation (cytotoxic edema). Affected astrocytes had enlarged and occasionally vesicular nuclei, however, no inflammatory infiltrates or degenerative neuronal changes were observed. In all examined sections of heart, there was scant pericardial adipose tissue, and what tissue remained exhibited a moderate degree of atrophy, with small regions of serous atrophy present in the most severely affected birds. Some sections of heart contained small regions exhibiting a mild degree of cardiomyocyte karyomegaly, with affected nuclei often having an elongated cigar-like shape. In all examined sections of lung, there was mild diffuse congestion, and acute haemorrhage into some parabronchi, the latter of which was interpreted as collection artefacts. There were infrequent small foci of collecting tubule mineralization in all the examined kidneys. In all three sections of duodenum, the villus tips contained aggregates of macrophages with pale grey-green intracytoplasmic pigment similar to that observed in the hepatocytes ((E,F)). In some of the sections, the pigment laden macrophages were accompanied by small numbers of heterophils. Mild hemosiderosis was the only microscopic finding in the single examined section of the spleen. No significant microscopic changes were observed in the ventriculus, or in the two sections of the ovary, and one section of the testis.
In addition to the microscopic lesions described above, gout was present to a varying degree in three of the birds. In the most severely affected bird, the renal parenchyma was severely disrupted by numerous and gouty tophi, and small uric acid deposits widely disseminated over the coelomic surfaces of the viscera, and small gouty tophi were also present within the lungs and liver. In the other two birds, the gout was limited to very small gouty tophi within the kidneys.
There were no differences between the control and 1,000 mg/kg BW dose for the liver, ventriculus, lungs, kidneys, ovary, testis, pancreas, and brain weights (P ≥ 0.05) (). There was a difference for the heart and spleen. The heart (n = 20, W = 80, P ≤ 0.05) and spleen (n = 19, W = 73, P ≤ 0.05) weighed less for the 1,000 mg/kg BW dosed birds than for the control birds (). There was no difference in the ventriculus weights between the control and the 585 and 765 mg/kg BW doses ( = 0.281, P ≥ 0.05, n = 21); however, there was a difference in the spleen weights (
= 9.113, P ≤ 0.01, n = 21) (). Dunn’s post-hoc test revealed differences between the control and both treatment groups, but not between the two treatment groups (). The results from the ANOVA test showed a difference in the heart weight (F
2,18 = 6.683, P ≤ 0.01, n = 21). There was no difference between the 585 (0.345 ± 0.046 g, n = 3) and 765 mg/kg BW (0.629 ± 0.228 g, n = 8) dose groups (P ≥ 0.05); however, there was a difference between the control (0.866 ± 0.251 g, n = 10) and both treatment groups (P ≤ 0.01). Both the spleen and heart weighed less for the 585 mg/kg BW dosed birds than for birds receiving the control and 765 mg/kg BW dose.
Table 1. Organ weights (g) for control and 1,000 mg/kg BW dosed birds from the range-finding test.
Table 2. Ventriculus and spleen weights (g) for control and 585 and 765 mg/kg BW dosed birds from the definitive test.
4. Discussion
Signs of gossypol toxicity in northern bobwhites are consistent with the literature and what previous studies found in poultry, specifically weakness, lethargy, and decreased feed consumption and weight loss (Lindsey et al. Citation1980). Puffed, ruffled feathers and a change in the consistency and colour of droppings are common non-specific signs of illness in avian species. Birds receiving doses from 200 to 1,000 mg/kg BW had diarrhea within 2–3 h after being dosed. Previous studies on poultry did not reference diarrhea as an observed side effect of gossypol ingestion (Lindsey et al. Citation1980). This sign has been seen in rabbits, dogs, and swine (Schwartz and Alsberg Citation1924; Eagle Citation1950; Smith Citation1957); however, this study is the first to note its appearance in gallinaceous birds. The toxicity symptoms varied with the gossypol dose ingested. Clinical signs lasted longer and were more severe in birds receiving higher doses than in birds receiving lower doses of gossypol.
The mortality rate in our study was high. This is most likely because we used free gossypol with an average purity of 99.31%. Additionally, the mortality rate increased with increasing levels of gossypol. Previous studies done by Lillie and Bird (Citation1950) and Couch et al. (Citation1955) support this, however, other studies done by Gamboa et al. (Citation2001) and Eagle and Davies (Citation1957) have found otherwise. Upon further analysing our mortality data, we observed that males died before females (Figure ). This phenomenon has not been seen in other gossypol LD50 studies. The mechanism is unknown and further investigation is warranted. However, northern bobwhites are not as sensitive to gossypol toxicity as other species. The 651 mg/kg BW LD50 for bobwhites is in between that of rats, 925–1,350 mg/kg (Eagle et al. Citation1948), and swine, 550 mg/kg (Lyman et al. Citation1963), confirming what Abou-Donia stated (Citation1976).
Gross necropsy findings of experimental and control animals were limited to ectopic tissue, gallbladder distention, and gout in a subset of the experimental group. The ectopic tissue was not evaluated histologically and was interpreted as a coincidental occurrence and not an effect of the gossypol ingestion. Birds receiving high doses (585–1,000 mg/kg BW) consumed less total feed post-dosing (Farthing et al. Citation2018) and subsequently there was no stimulus for bile excretion, which explains why a few birds had distended gallbladders. Mild renal gout was present in two birds, and severe disseminated gout was present in one bird. The gout was interpreted as a nonspecific change secondary to dehydration and/or alterations in hepatic and renal function, and not a primary effect of gossypol intoxication.
Histological evaluation of the high-dose (1,000 mg/kg BW) experimental animals revealed clinically significant lesions in numerous tissues, with the pancreas and liver most consistently and severely affected. In the liver, pigment accumulation was prominent within hepatocytes and Kupffer cells, and was associated with increased variability in hepatocytes size and the number and size of nuclei within affected hepatocytes. The nature of the grey-green could not be definitively determined with routine stains. In previous studies the pigment was interpreted as bile, however, it is possible that it may represent some combination of bile, iron, and gossypol pigment. It is notable that the fine grey-green pigment granules were distinct from the small number of coarse golden-brown hemosiderin pigment granules also present within some hepatocytes and most Kupffer cells. It is also notable that the grey-green pigment was also present within clusters of macrophages within villus tips of the intestine, and affected macrophages were associated with a small amount of heterophilic inflammation. Previous studies have described accumulation of the protein-bound (+) enantiomer of gossypol in intestine and liver, and it is strongly suspected that gossypol is a component of the intracellular grey-green pigment observed in the liver and intestine in this study (Farthing et al. Citation2018). Unlike previous studies (Henry et al. Citation2001; Lordelo et al. Citation2005; Lordelo et al. Citation2007; Blevins et al. Citation2010), lymphoplasmacytic portal hepatitis and biliary hyperplasia were not observed in this study. It is possible that the duration of gossypol exposure was adequate to have resulted in the development of these chronic changes prior to completion of the experimental trial.
The prominent degenerative and regenerative lesions in the pancreas exhibited a zonal pattern with lobules of exocrine acini encircled by thin zones of acinar cell degeneration and regeneration. Moderate to large numbers of individual necrotic exocrine acinar cells within these zones, and most affected cells exhibited features of apoptosis including marked cell shrinkage with nuclear pyknosis and karyorrhexis. Notably, the individual necrotic cells did not recruit acute inflammatory cells into the affected areas. Collectively these changes are suspected to represent the direct effects of gossypol toxicity on pancreatic exocrine cells. The islets were not affected, and only small amount of perivascular lymphoplasmacytic inflammation was present. Previous studies of gossypol intoxication in poultry have evaluated alterations in pancreatic weight, however, this is the first study to describe histologic changes in the pancreas associated with gossypol toxicosis. It is not clear whether the proportion of affected exocrine pancreatic tissue was adequate to have impaired digestion, however, it is possible that decreased digestive capacity could have contribute to the marked weight loss observed clinically.
Histological examination of the heart revealed that there was scant pericardial adipose tissue in nearly all the birds, and in many cases the small amount of remaining fat exhibited serous atrophy. These are interpreted as sequelae to the hyporexia or anorexia and weight loss described in the clinical and gross findings. The serous atrophy is significant as it indicates a sudden shift to a negative energy balance, presumably secondary to the onset of gossypol toxicosis.
In the brain, multiple birds exhibited varying degrees of glial cell cytoplasmic vacuolation suggestive of cytotoxic edema. The etiopathogenesis and general clinical significance of these changes was not clear, however, in most severely affected birds it was of adequate severity to have been clinically significant. It is possible that metabolic disturbances associated with hepatic or renal disease, or possibly hypoxaemia secondary to anaemia could have been contributory.
Both the spleen and heart weighed less for birds receiving the 585, 765, and 1,000 mg/kg BW gossypol doses compared to the control birds. This finding contrasts with what Henry et al. (Citation2001) found. They found that the heart and spleen weighed more for male broiler chicks receiving 800 and 1,600 mg/kg of feed containing purified gossypol than for the control chicks (Henry et al. Citation2001). In our study, there were no clear histological correlates for these weight changes. Because it was unlikely that these weight changes contributed to morbidity and mortality, this left us to question if there was any clinical significance to this finding. The reason why the heart weighed less was due to atrophy of pericardial fat because of systemic weight loss. The change in spleen weight was unclear, considering there was only one spleen available for histopathology and there were no significant lesions. Spleen weight can change depending on whether it is congested with blood, has increased or decreased lymphoid tissue, haematopoietic tissue, or has inflammation (Elmore Citation2007). Future studies examining the effects of gossypol on gallinaceous birds should have histopathology done on spleens from both treatment and control birds.
Overall, this study is the first to determine the LD50 of gossypol in an avian species. It is also the first study to note diarrhea as a clinical sign in gallinaceous birds and to describe histologic changes in the pancreas associated with gossypol toxicosis.
Acknowledgements
We thank Colorado State University Veterinary Diagnostic Laboratory for analysing the feed sample and J. Swenson, DVM for providing her knowledge and assistance with this study. A special thank you to the research technicians, especially J. D. Hall, C. T. Hall, J. A. Lowe, T. S. Farthing, J. M. Roberts, S. G. Aguallo, and A. M. McAnally, and fellow undergraduate and graduate students in the Department of Wildlife, Sustainability, and Ecosystem Sciences at Tarleton State University, without whom this research would not have been possible. We gratefully acknowledge reviewers for their time, feedback, and contributions to this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abou-Donia MB. 1976. Physiological effects and metabolism of gossypol. Residue Rev. 61:125–160.
- Abou-Donia MB , Othman MA , Obih P. 1989. Interspecies comparison of pharmacokinetic profile and bioavailability of (±)–gossypol in male Fischer–344 rats and male B6C3F mice. Toxicology. 55:37–51. doi: 10.1016/0300-483X(89)90173-X
- American Veterinary Medical Association (AVMA) . 2013. AVMA guidelines for the euthanasia of animals. IL : AVMA.
- Blevins S , Siegel PB , Blodgett DJ , Ehrich M , Saunders GK , Lewis RM. 2010. Effects of silymarin on gossypol toxicosis in divergent lines of chickens. Poult Sci. 89:1878–1886. doi: 10.3382/ps.2010-00768
- Bliss CI. 1934. The method of probits. Science. 79:38–39. doi: 10.1126/science.79.2037.38
- Burlatschenko S. 2002. Suspected gossypol toxicosis in a sow herd. J Swine Health Prod. 11:137–139.
- Campbell TA , Bullock SL , Long DB , Hewitt DG , Dowd MK. 2010. Visitation to cottonseed storage sites by feral swine and evidence of gossypol exposure. Hum–Wildl Interact. 4:145–151.
- Couch JR , Chang WY , Lyman CM. 1955. The effect of free gossypol on chick growth. Poult Sci. 34:178–183. doi: 10.3382/ps.0340178
- Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics. 6:241–252. doi: 10.1080/00401706.1964.10490181
- Eagle E. 1950. Effect of repeated doses of gossypol on the dog. Arch Biochem. 26:68–71.
- Eagle E , Castillon LE , Hall CM , Boatner CH. 1948. Acute oral toxicity of gossypol and cottonseed pigment glands for rats, mice, rabbits and Guinea pigs. Arch Biochem. 18:271–277.
- Eagle E , Davies DL. 1957. Feed value and protein-quality determinations on cottonseed meals. J Am Oil Chem Soc. 34:454–459. doi: 10.1007/BF02638004
- Elmore SA. 2007. Enhanced histopathology of the spleen. Toxicol Pathol. 34:648–655. doi: 10.1080/01926230600865523
- European Food Safety Authority (EFSA) . 2008. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on gossypol as undesirable substance in animal feed. EFSA J. 908:1–55.
- Farthing AL , Mathewson HA , Roberts JM , Schwertner TW , Guay KA. 2018. Response of northern bobwhites fed single doses of gossypol. Wildl Soc Bull. 42:460–466. doi: 10.1002/wsb.907
- Gadelha IC , Fonseca NB , Oloris SC , Melo MM , Soto-Blanco B. 2014. Gossypol toxicity from cottonseed products. Scientific World J. 2014:1–11.
- Gamboa DA , Calhoun MC , Kuhlmann SW , Haq AU , Bailey CA. 2001. Tissue distribution of gossypol enantiomers in broilers fed various cottonseed meals. Poult Sci. 80:920–925. doi: 10.1093/ps/80.7.920
- Hale F , Lyman CM. 1957. Effect of protein level in the ration on gossypol tolerance in growing-fattening pigs. J Animal Sci. 16:364–367. doi: 10.2527/jas1957.162364x
- Henry MH , Pesti GM , Brown TP. 2001. Pathology and histopathology of gossypol toxicity in broiler chicks. Avian Dis. 45:598–604. doi: 10.2307/1592900
- Kim HL , Stipanovic RD , Calhoun MC. 1996. Accumulation of gossypol enantiomers in ovine tissues. Comp Biochem Physiol B Biochem Mol Biol. 113:417–420. doi: 10.1016/0305-0491(95)02061-6
- Kruskal WH , Wallis WA. 1952. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 47:583–621. doi: 10.1080/01621459.1952.10483441
- Lillie RJ , Bird HR. 1950. Effect of oral administration of pure gossypol and of pigment glands of cottonseed on mortality and growth of chicks. Poult Sci. 29:390–393. doi: 10.3382/ps.0290390
- Lindsey TO , Guthrie LD , Hawkins GE. 1980. Physiological responses of lactating cows to gossypol from cottonseed meal rations. Dairy Sci. 63:562–573. doi: 10.3168/jds.S0022-0302(80)82972-9
- Lordelo MM , Calhoun MC , Dale NM , Dowd MK , Davis AJ. 2007. Relative toxicity of gossypol enantiomers in laying and broiler breeder hens. Poult Sci. 86:582–590. doi: 10.1093/ps/86.3.582
- Lordelo MM , Davis AJ , Calhoun MC , Dowd MK , Dale NM. 2005. Relative toxicity of gossypol enantiomers in broilers. Poult Sci. 84:1376–1382. doi: 10.1093/ps/84.9.1376
- Lyman CM , El-Nockrashy AS , Dollahite JW. 1963. Gossyverdurin: A newly isolated pigment from cottonseed pigment glands. J Am Oil Chem Soc. 40:571–575. doi: 10.1007/BF02822469
- Mann HB , Whitney DR. 1947. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 18:50–60. doi: 10.1214/aoms/1177730491
- Nagalakshmi D , Rama Rao SV , Panda AK , Sastry VR. 2007. Cottenseed meal in poultry diets: A review. Poult Sci. 44:119–134. doi: 10.2141/jpsa.44.119
- R Core Team . 2017. R: a language and environment for statistical computing. Vienna : R Foundation for Statistical Computing. https://www.R-project.org .
- Schwartz EW , Alsberg CL. 1924. Pharmacology of gossypol. J Arg Res. 28:191–198.
- Singla N , Meenu . 2008. Toxic and antifertility effects of single oral dose of gossypol in lesser bandicoot rat. Bandicota bengalensis Gray and Hardwicke. Pest Mgmt Econ Zool. 16:215–222.
- Smith HA. 1957. The pathology of gossypol poisoning. Am J Pathol. 33:353–365.
- Tukey JW. 1949. Comparing individual means in the analysis of variance. Biometrics. 5:99–114. doi: 10.2307/3001913
- United States Department of Agriculture (USDA) . 2017. Animal welfare act and animal welfare regulations. Animal and Plant Health Inspection Service 41–35–076.
- United States Department of Health and Human Service (HHS) . 2015. Public health service policy on human care and use of laboratory animals. Bethesda, MD : National Institutes of Health Office of Laboratory Animal Welfare.
- United States Environmental Protection Agency (EPA) . 2012. Ecological effects test guidelines: OCSPP 850.2100: Avian acute oral toxicity test. Office of Chemical Safety and Pollution Prevention 712–C–025.
- Welch BL. 1947. The generalization of ‘student's’ problem when several different population variances are involved. Biometrika. 34:28–35.
- Zeng QF , Bai P , Wang JP , et al. 2015. The response of meat ducks from 15 to 35 d of age to gossypol from cottonseed meal. Poult Sci. 94:1277–1286. doi: 10.3382/ps/pev070