ABSTRACT
The spleen combines the innate and adaptive immune systems, and the early conceptus must regulate the maternal intrauterine immune and systemic immune response during early pregnancy in sheep. However, it is unclear whether early pregnancy exerts its effects on the characterization of the T helper (Th) cytokines profile in the spleen during early pregnancy in ewes. In this study, spleens were obtained at day 16 of the oestrous cycle and at days 13, 16 and 25 of pregnancy (n = 6 for each group) from ewes, and qRT-PCR, western blot and immunohistochemistry analysis were used to analyze the Th cytokines profile of the spleens. Our results showed that there was down-regulation of IFN-γ at days 13 and 16 of pregnancy, but there was upregulation of IL-2, IL-5 and IL-6 at day 25 of pregnancy, TNF-β at days 16 and 25 of pregnancy, and IL-4 and IL-10 in all pregnant groups. Immunohistochemistry results showed that the IL-6 protein was limited to the capsule, trabeculae and splenic cords. This paper reported, for the first time, that characterization of the Th cytokines profile varied in the maternal spleen during early pregnancy, which may be essential for successful pregnancy in sheep.
KEYWORDS:
Introduction
During pregnancy, there is an absence of any maternal immune response against the foetus and placenta, which is also known as maternal immune tolerance (Williams Citation2012). The chemokines and cytokines produced by the conceptus result in a variety of maternal immunological adjustments against the conceptus in sheep and cattle (Hansen Citation2011; Yang et al. Citation2014), and there is a shift of T helper 1 (Th1) cytokines to Th2 cytokines in the maternal endometrium induced by the conceptus and progesterone (P4) in ruminants (Hansen Citation2011; Zhang et al. Citation2015). Th1 cytokines include interleukin (IL)-2, interferon-gamma (IFN-γ) and tumour necrosis factor beta (TNF-β), which are involved in cell-mediated cytotoxicity and inflammatory responses, whereas Th2 cytokines are anti-inflammatory cytokines, including IL-4, IL-5, IL-6 and IL-10 (Ott and Gifford Citation2010). Th2 cytokines promote a humoral response and are generally beneficial to a normal pregnancy, whereas Th1 cytokines normally suppressed in pregnancy, and significantly higher concentrations of Th1 cytokines are produced in spontaneous abortion groups (Raghupathy et al. Citation2000; Sykes et al. Citation2012a). There are trends towards changes in the Th2 cytokine profile and suppression of the Th1 cytokine profile in the peripheral blood and the maternal-fetal interface (Sykes et al. Citation2012b). We also revealed that IFN-γ is down-regulated, and IL-4, IL-5, IL-6, IL-10 and IL-13 are upregulated in peripheral blood mononuclear cells (PBMCs) during early pregnancy in cattle (Yang et al. Citation2016). There were a downregulation of TNF-β and IL-2 and an upregulation of IL-5 and IL-10 in the maternal lymph nodes during early pregnancy in sheep (Yang et al. Citation2019).
Lymphatic organs are the major parts of the immune system in the mammalian body, and the spleen is the largest lymphatic organ (de Porto et al. Citation2010). The spleen is one of the secondary lymphatic organs and has been described as a central organ of the immune system that combines the innate and adaptive immune system in a uniquely organized way (Mebius and Kraal Citation2005). The spleen is compartmentalized into red and white pulp. The red pulp participates in filtration of red blood cells and reserve of monocytes, and white pulp is implicated in active immune response (Swirski et al. Citation2009). The circulating immune cells actively contribute to the establishment of embryo implantation, and the first signal of pregnancy recognition works on the maternal immune system before implantation (Fujiwara et al. Citation2009). We recently reported that there are changes in the expression of the P4 receptor and P4-induced blocking factor in the ovine spleen (Yang et al. Citation2018a), which indicates that the early conceptus exerts its effects on the maternal spleen. There is a strong down-regulation of the expression of cofilin-1, F-actin capping protein subunit alpha and malate dehydrogenase proteins in splenic CD4+ lymphocytes, which indicates that preimplantation pregnancy can alter the activation state of peripheral CD4+ lymphocytes in mice (Chelmonska-Soyta et al. Citation2014). The Th cytokines play an important role in the immune system. However, it has been unclear whether early pregnancy exerts its effects on the characterization of the Th cytokines profile in the ovine spleen. In this study, the spleens from nonpregnant and early pregnant ewes were sampled to study the expression of IL-2, IFN-γ, TNF-β, IL-4, IL-5, IL-6 and IL-10, which may be beneficial in understanding the effects of early pregnancy on maternal splenic immunomodulation in sheep.
Materials and methods
Animals and experimental design
All procedures were approved by the Hebei University of Engineering Animal Care and Use Committee, and all experiments were conducted following the guidelines of the National Standards for Laboratory Animals of China (GB 14925-2010). Small-tail Han ewes approximately 18 months of age were housed at the farm of Handan Boyuan Animal Husbandry Co., Ltd. in China, and ovine oestrus was detected daily by a vasectomized ram. Twenty-four ewes were randomly divided into a nonpregnancy group (day 16 of the oestrous cycle) and three pregnant groups (days 13, 16 and 25 of pregnancy; n = 6 for each group), and the first day of coition was counted as day 0 of pregnancy or nonpregnancy. The ewes in the pregnant groups were mated twice with fertile rams in a 12-h interval after the detection of sexual receptivity, but the ewes assigned to the nonpregnant group were not mated with an intact ram. The effects of early pregnancy on the expression of Th cytokines in the ovine spleens are mainly due to P4 and interferon-tau (IFNT). There were significantly higher concentrations of P4 on days 12–13 in plasma, and lower concentrations of P4 on days 15–16 during the ovine oestrous cycle (Mcnatty et al. Citation1973). IFNT (Protein X) and additional proteins were detected between days 14 and 21 in sheep (Godkin et al. Citation1982). The day 16 of non-pregnant ewe is not under the effects of both P4 and IFNT. Therefore, splenic samples were collected at days 13, 16, and 25 of pregnancy, and day 16 of the oestrous cycle at the time of slaughter. The spleens were obtained from the ewes on days 13, 16 and 25 of pregnancy, as well as day 16 of the oestrous cycle at the time of slaughter, and pregnancy was determined by observation of the conceptus in the uterus after dissection. The transverse pieces of the spleens (0.3 cm3) were fixed in fresh 4% (w/v) paraformaldehyde in PBS buffer (pH 7.4), and the remaining mixture of the splenic red pulp and white pulp were frozen in liquid nitrogen for subsequent quantitative real-time PCR (qRT-PCR) and western blot analysis.
RNA extraction and qRT-PCR assay
Samples were crushed into fine powders in liquid nitrogen, and the powders were dissolved in TRIzol (Invitrogen, California, USA), and then the total RNA was extracted according to the manufacturer's instructions. Genomic DNA removal and cDNA synthesis were performed using a FastQuant RT kit (With gDNase, Tiangen Biotech Co., Ltd., Beijing). The primer sequences of IFN-γ, TNF-β, IL-2, IL-4, IL-5, IL-6, IL-10 and GAPDH were designed and synthesized by Shanghai Sangon Biotech Co., Ltd. (), and assessed by BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) at NCBI. PCR amplification efficiency of each pair of primers was assessed before quantification, and was found to be in an acceptable range (between 0.9 and 1.1). The primer product was sequenced to check for specificity, and the expression of the targeted genes was determined by a Bio-rad CFX96 real-time PCR system with 20 μl. The qPCR was performed using a SuperReal PreMix Plus kit (Tiangen Biotech), and the melting curve was analyzed to guarantee the specificity of the amplification after PCR reactions. The PCR amplifications were carried out at 95°C for 10 sec, 55–58°C (55°C for IL-5 and IL-6, 58°C for IFN-γ, TNF-β, IL-4, IL-2 and IL-10) for 20 sec, and 72°C for 25 sec, and the number of PCR cycles was 40. The GAPDH PCR reaction was the same as IFN-γ, TNF-β, IL-2, IL-4, IL-5, IL-6, and IL-10, respectively (Yang et al. Citation2019). The relative expression values for the qRT-PCR assay were calculated by the 2−ΔΔCt analysis method, with GAPDH used for normalization of the PCR data (Wong and Medrano Citation2005). The relative expression value was set as 1 for the group on day 16 of the oestrous cycle.
Table 1. Primers used for qRT-PCR.
Western blot
The total proteins of the splenic samples were extracted by RIPA Lysis Buffer (Biosharp, BL504A), and a BCA Protein Assay kit (Tiangen Biotech) was used to measure the protein concentration. Equal amounts of total proteins (10 μg/lane) were separated using 12% SDS-PAGE and the proteins were transferred to 0.22 μm polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). In addition, the control proteins of TNF-β (Sigma-Aldrich Co. LLC., T7799), IL-5 (Sigma-Aldrich Co. LLC., I5273) and IL-10 (Sigma-Aldrich Co. LLC., I9276) were used for validity of antibodies. IFN-γ, TNF-β, IL-2, IL-4, IL-5, IL-6 and IL-10 were detected by western blot using a mouse anti-IFN-γ monoclonal antibody (Abcam, ab27919, 1:1000), mouse anti-TNF-β monoclonal antibody (Santa Cruz Biotechnology, Inc., SC-28345, 1:1000), rabbit anti-IL-2 polyclonal antibody (Abcam, ab193807, 1:1000), mouse anti-IL-4 monoclonal antibody (Bio-Techne, MAB2469, 1:1000), mouse anti-IL-5 monoclonal antibody (Santa Cruz Biotechnology, Inc., SC-8433, 1:1000), rabbit anti-IL-6 polyclonal antibody (Abcam, ab193853, 1:1000) and mouse anti-IL-10 monoclonal antibody (Santa Cruz Biotechnology, Inc., SC-32815, 1:1000), respectively. Secondary goat anti-mouse IgG-HRP (Biosharp, BL001A) and goat anti-rabbit IgG-HRP (Biosharp, BL003A) were diluted to 1:10000. A Pro-light HRP chemiluminescence detection reagent (Tiangen Biotech) was used to detect the immunoreactive bands. These procedures were validated using positive tissue (endometrium) and negative control tissue (fat), and the target bands were present in the endometrium without a band in the fat. Sample loading was monitored with the anti-GAPDH antibody (Santa Cruz Biotechnology, Inc., sc-20357) at a dilution of 1:1000. Quantity One V452 software (Bio-Rad Laboratories) was used to semi-quantify the intensity of the blots, and the relative levels were calculated using GAPDH. The expression of GAPDH protein was measured by western blot, and there was no difference among the four groups.
Immunohistochemistry analysis
The fixed splenic samples were embedded in paraffin, and the paraffin-embedded sections were deparaffinized in xylene and rehydrated in ethanol. The sections were stained by hematoxylin and eosin (HE). The endogenous peroxidase activity of the rehydrated sections was quenched using 3% H2O2, and 5% normal goat serum in PBS was used to reduce nonspecific binding. Immunohistochemical localization of IL-6 in the splenic tissue was performed using the rabbit anti-IL-6 polyclonal antibody (Abcam, ab193853, 1:100). For a negative control, nonimmune goat serum was used in place of the primary antibody. A DAB kit (Tiangen Biotech) was used to visualize the antibody binding sites in the tissue sections. Finally, the images were captured using a light microscope (Nikon Eclipse E800, Japan) with a digital camera (AxioCam ERc 5s), and the intensity of staining and density of the stained cells were analyzed through the images. The immunostaining intensity of the different splenic tissue samples from different ewes (n = 6 for each group) was rated by 2 different investigators in a blinded fashion, and histological subtypes were analyzed by assigning an immunoreactive intensity of a scale of 0–3, as described previously (Kandil et al. Citation2007). An intensity of 3+ was given to the cells with the highest staining intensity, and an intensity of 0 was assigned to the cells with no immunoreactivity.
Statistical analyses
The data for the relative expression levels of IFN-γ, TNF-β, IL-2, IL-4, IL-5, IL-6 and IL-10 mRNA and proteins were analyzed using a completely randomized design with six animals per group via the Proc Mixed model of SAS (Version 9.1; SAS Institute, Cary, NC). For spleens from different stages of gestation or pregnancy status, the model contained the random effect of the ewe and the fixed effects of the stage of gestation, pregnancy status and the interaction between the stage of gestation and pregnancy status. The comparisons among the relative expression levels of the different groups were performed using the Duncan method and controlling the experimentwise type ± error equal to 0.05. Data are presented as least squares means. Groups were considered significantly different at P < 0.05.
Results
Relative expression levels of Th1 cytokines mRNA and proteins in the spleens
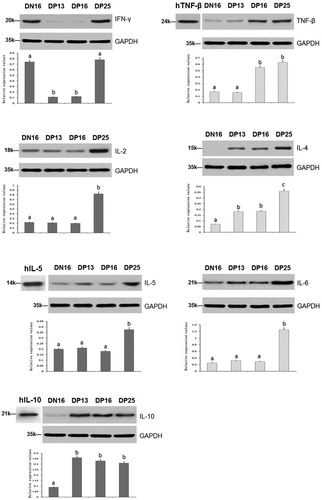
The qRT-PCR assay and western blot revealed ( and ) that the relative expression levels of IFN-γ mRNA and protein were higher in the spleens at day 16 of the oestrous cycle and day 25 of pregnancy than that days 13 and 16 of pregnancy (P < 0.05), and the relative expression levels of TNF-β mRNA and protein were higher at days 16 and 25 of pregnancy than that at day 16 of the oestrous cycle and day 13 of pregnancy (P < 0.05). Furthermore, the relative expression levels of IL-2 mRNA and protein were the highest in the spleens at day 25 of pregnancy among nonpregnant and early pregnancy ewes (P < 0.05), but there was no significant difference among nonpregnant or days 13 and 16 the pregnant ewes (P > 0.05) ( and ).
Figure 1. Relative expression values of Th1 cytokines (IL-2, IFN-γ and TNF-β) and Th2 cytokines (IL-4, IL-5, IL-6 and IL-10) mRNA in spleens of nonpregnant and pregnant ewes measured by quantitative real-time PCR.
Note: DN16 = Day 16 of the oestrous cycle; DP13 = Day 13 of pregnancy; DP16 = Day 16 of pregnancy; DP25 = Day 25 of pregnancy. Significant differences (P < 0.05) are indicated by different letters within same colour column.
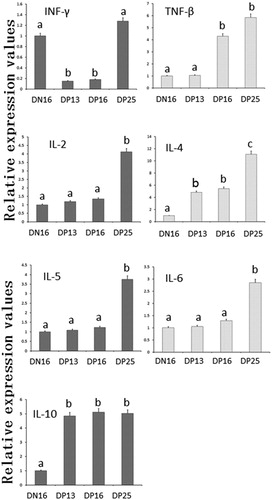
Figure 2. Expression of Th1 cytokines (IL-2, IFN-γ and TNF-β) and Th2 cytokines (IL-4, IL-5, IL-6 and IL-10) proteins in spleens of nonpregnant and pregnant ewes analyzed by western blot.
Note: hTNF-β = Recombinant human TNF-β protein; hIL-5 = Recombinant human IL-5 protein; hIL-10 = Recombinant human IL-10 protein; DN16 = Day 16 of the oestrous cycle; DP13 = Day 13 of pregnancy; DP16 = Day 16 of pregnancy; DP25 = Day 25 of pregnancy. Significant differences (P < 0.05) are indicated by different superscript letters within the same colour column.
Relative expression levels of Th2 cytokines mRNA and proteins in the spleens
There was an upregulation of the relative expression levels of IL-4 mRNA and protein at day 25 of pregnancy (P < 0.05), and the relative expression levels were the lowest in the nonpregnant group (P < 0.05), but there was no significant difference between days 13 and 16 of pregnancy (P > 0.05) ( and ). The relative expression levels of IL-5 and IL-6 mRNA and proteins were the highest in the spleens at day 25 of pregnancy among nonpregnant and early pregnancy ewes (P < 0.05), but there was no significant difference among nonpregnant or days 13 and 16 of the pregnant groups (P > 0.05) ( and ). Furthermore, the relative expression levels of IL-10 mRNA and protein were the lowest in the spleens at day 16 of the oestrous cycle among nonpregnant and early pregnancy ewes (P < 0.05), but there was no significant difference among the pregnant ewes (P > 0.05) ( and ).
The immunohistochemistry for IFN-γ and IL-6 proteins in the spleens
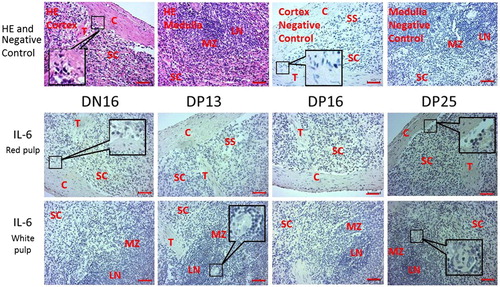
The immunohistochemistry for the IL-6 protein was limited to the capsule, trabeculae and splenic cords (). Furthermore, the staining intensity for IL-6 was 1+, 1+, 1+, and 3+ for the spleens from day 16 of the oestrous cycle, and the spleens from days 13, 16 and 25 of pregnancy, respectively (). The staining intensity was as follows: 0 = negative; 1+ = weak; 2+ = strong; 3+ = stronger.
Figure 3. Representative immunohistochemical localization of IL-6 protein in spleens of nonpregnant and pregnant ewes. The spleen is divided into red pulp and white pulp, and surrounded by a thickened capsule. The red pulp and white pulp are not separated completely in this figure. Capsule (C) with several trabeculae (T) projects into the substance of the spleen.
Note: HE = stained by hematoxylin and eosin; SS = splenic sinuses; SC = splenic cords; MZ = marginal zone; LN = lymphoid nodule; DN16 = day 16 of the oestrous cycle; DP13 = day 13 of pregnancy; DP16 = day 16 of pregnancy; DP25 = day 25 of pregnancy. Bar = 50 µm.
Discussion
The spleen is part of the circulatory system and has an unusual structure of lymphoid compartments to combine the innate and adaptive immune system (Mebius and Kraal Citation2005). The size of the maternal spleen increases an average of 50% in late pregnancy in mice (Davis et al. Citation1961), and the relative content of splenic macrophages increases two-fold during mid-pregnancy in mice (Mattsson et al. Citation1984). In this study, there was a decrease in the levels of IFN-γ mRNA and protein at days 13 and 16 of pregnancy ( and ). IFN-γ is the only member of the type II class of interferon and it has antiviral, immunoregulatory, and antitumor properties (Schroder et al. Citation2004). IFN-γ plays an essential role in Th0 cells differentiating into Th1 cells, which induces a Th1-adapted immune response (Maley et al. Citation2006). IFN-γ is also an inducer of the Class II major histocompatibility complex (MHC) molecule that activates macrophages (Schoenborn and Wilson Citation2007), which is harmful to a normal pregnancy. It has been reported that there is a down-regulation of IFN-γ at the feto-maternal interface (Saito Citation2000), in the endometria (Beltman et al. Citation2013) and PBMCs (Yang et al. Citation2016). Therefore, a decrease in the level of IFN-γ in the maternal spleen at days 13 and 16 of pregnancy may be needed to maintain early pregnancy in sheep.
TNF-β, also known as lymphotoxin-alpha (LT-α), has a significant impact on the maintenance of the immune system, including the development of secondary lymphoid organs (Ruddle Citation2014). As a signalling molecule, TNF-β can regulate cell survival, proliferation, differentiation and apoptosis (Bauer et al. Citation2012), prevent tumour growth and destroy cancerous cell lines, and it plays an essential role in innate immune regulation (Fernandes et al. Citation2016). It has been reported that TNF-α, another member of the tumour necrosis factor superfamily, is implicated in decidualization, placentation, and prevention of abortion (Ozbilgin et al. Citation2015). Therefore, upregulation of TNF-β at days 16 and 25 in pregnant spleens may be involved in the regulation of maternal splenic immunity during pregnancy in sheep.
IL-2 is a pleiotropic cytokine and has direct effects on T-cell differentiation, which plays key roles in regulating functions of the immune system (Liao et al. Citation2011). IL-2 regulates the immune response by promoting naive CD4+ T-cell differentiation into Th1 and Th2 cells and is involved in mediating tolerance and limiting inappropriate immune reactions by regulating the development and maintenance of T regulatory cells and activation-induced cell death (Liao et al. Citation2013). The blood serum level of IL-2 increases during the second week of gestation compared with that in anoestrus and dioestrus, which is essential for the development and maintenance of pregnancy in bitches (Maciel et al. Citation2014). It has also been reported that recombinant bovine IL-2 can enhance immunity and protect against abortion induced by Brucella abortus vaccines in cattle (Wyckoff et al. Citation2005), and there is upregulated expression of IL-2 mRNA in pregnant PBMCs compared with that in nonpregnant PBMCs in cattle (Yang et al. Citation2016). Our results indicated that there was upregulated expression of IL-2 mRNA and protein in the spleens at day 25 of pregnancy ( and ), which may be helpful for establishing a successful pregnancy in sheep.
IL-4 induces the differentiation of naive Th cells (Th0 cells) into Th2 cells that subsequently produce additional IL-4 in a positive feedback loop, and IL-4 also decreases the production of Th1 cells and IFN-γ (Sokol et al. Citation2008). The serum concentration of IL-4 is elevated during early gestation in bitches, which depends on P4, and P4 can act as a potent inhibitor of Th1 cytokines (Pantaleo et al. Citation2013). Trophoblast IFNT enhances the expression of IL-4 mRNA by effector T cells in cattle (Tuo et al. Citation1999), and P4 also significantly upregulates the expression of IL-4 in PBMCs in pregnant cows (Maeda et al. Citation2013). IFNT induces upregulation of the conjugated proteins of interferon-stimulated gene 15 kDa protein in maternal spleen during early pregnancy in sheep (Yang et al. Citation2018b). We found that the relative levels of IL-4 mRNA and protein were higher in the pregnant groups ( and ), which may be due to the high serum concentrations of P4 and IFNT during early pregnancy.
Th2 cells and mast cells produce IL-5 that stimulates B cell growth and increases immunoglobulin secretion through binding to its receptor. The dendritic cells treated with placental growth factor results in the suppression of naive CD4+ T-cell proliferation, but the CD4+ T-cells increase IL-5 secretion, which is beneficial for normal pregnancies in humans (Lin et al. Citation2007). The spontaneous in vitro secretion of IL-5 is lower in cases of preeclampsia compared with normal pregnancy (Jonsson et al. Citation2005). Pregnancy-specific glycoprotein increases the proliferation of IL-5-secreting cells in vivo, which has been implicated in successful pregnancy (Martínez et al. Citation2012). Our results indicated that the expression levels of IL-5 mRNA and protein in the spleens were significantly higher at day 25 of pregnancy ( and ), which may be necessary for pregnancy maintenance in sheep.
IL-6 plays pivotal roles in the inflammatory response, T-cell differentiation and adaptive immunity, and it has regulatory functions in embryo implantation, placental development and immune adaptations to allow tolerance in pregnancy (Prins et al. Citation2012). There is an increase in endometrial IL-6 during early pregnancy in pigs, which is implicated in embryo-uterine interactions and helpful for successful implantation of the conceptus (Blitek et al. Citation2012). IL-6 is involved in the regulating invasiveness of embryonic ectoderm cells through activation of matrix metalloproteinase-2, which is essential for normal placental development and successful pregnancy (Jiang et al. Citation2016). The expression of IL-6 and its receptor in the endometrial tissues increases dramatically during mid-to late-pregnancy and decreases at term, which indicates that IL-6 and its receptor signalling system are involved in the maintenance of pregnancy in pigs (Yoo et al. Citation2017). Our data showed that the expression levels of IL-6 mRNA and protein were significantly higher in the spleens at day 25 of pregnancy ( and ), which suggests that upregulation of IL-6 in the spleen may be involved in embryo implantation in sheep.
IL-10 can down-regulate the expression of Th1 cytokines and MHC class II antigens with subsequent multiple and pleiotropic effects in immunoregulation. IL-10 is secreted by immune and nonimmune cells and plays a vital role in maintaining maternal immune tolerance in an autocrine and paracrine manner during pregnancy (Cheng and Sharma Citation2015), and exogenous IL-10 administration can normalize immune cell subsets and prevent the development of pregnancy preeclampsia in mice (Chatterjee et al. Citation2015). IL-10 is derived from natural killer (NK) cells and plays a unique role in facilitating the dialogue between dendritic cells (DC) and NK cells at the maternal-fetal interface during the establishment of a healthy gestation (Blois et al. Citation2017). This study showed that the levels of IL-10 mRNA and protein in the pregnant groups were higher than those in the nonpregnant group ( and ), which suggested that the upregulation of expression of IL-10 in the maternal spleen may be involved in maternal immune tolerance in sheep.
Our immunohistochemistry results showed that the immunostaining of the IL-6 protein was limited to the capsule, trabeculae and splenic cords (). The staining intensity for IL-6 at day 25 of pregnancy was stronger than that at day 16 of the oestrous cycle, and at days 13 and 16 of pregnancy (). The blood flowing in the splenic artery drains directly into the splenic sinuses of the red pulp (Mebius and Kraal Citation2005), and the monocytes in red pulp differentiate irreversibly into DCs or macrophages upon tissue entry to regulate systemic immunity through blood circulation (Swirski et al. Citation2009). The splenic monocytes increase their motility, in response to tissue injury, exit the spleen en masse to participate in wound healing (Swirski et al. Citation2009). Therefore, we suggest that the change in the expression of Th cytokines in maternal spleen may affect the function of the splenic monocytes. The monocytes exit the spleen, in response to the conceptus in maternal uterus, to regulate the uterine immune function, which is beneficial for embryonic development during early pregnancy in sheep.
In conclusion, there was upregulation of TNF-β, IL-2, IL-4, IL-5, IL-6 and IL-10 in the spleens during early pregnancy, but IFN-γ was down-regulated at days 13 and 16 of pregnancy. Furthermore, the IL-6 protein was limited to the capsule, trabeculae and splenic cords. Therefore, we suggest that early pregnancy exerts its effects on the spleen to regulate the Th cytokines profile, which may be important for maintaining a normal pregnancy during early pregnancy in sheep.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ling Yang http://orcid.org/0000-0003-4385-0024
Additional information
Funding
References
- Bauer J , Namineni S , Reisinger F , Zöller J , Yuan D , Heikenwälder M. 2012. Lymphotoxin, NF-ĸB, and cancer: the dark side of cytokines. Dig Dis. 30:453–468. doi: 10.1159/000341690
- Beltman ME , Forde N , Lonergan P , Crowe MA. 2013. Altered endometrial immune gene expression in beef heifers with retarded embryos. Reprod Fertil Dev. 25:966–970. doi: 10.1071/RD12232
- Blitek A , Morawska E , Ziecik AJ. 2012. Regulation of expression and role of leukemia inhibitory factor and interleukin-6 in the uterus of early pregnant pigs. Theriogenology. 78:951–964. doi: 10.1016/j.theriogenology.2012.05.016
- Blois SM , Freitag N , Tirado-González I , Cheng SB , Heimesaat MM , Bereswill S , Rose M , Conrad ML , Barrientos G , Sharma S. 2017. NK cell-derived IL-10 is critical for DC-NK cell dialogue at the maternal-fetal interface. Sci Rep. 7:2189. doi: 10.1038/s41598-017-02333-8
- Chatterjee P , Chiasson VL , Seerangan G , Tobin RP , Kopriva SE , Newell-Rogers MK , Mitchell BM. 2015. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. 28:135–142. doi: 10.1093/ajh/hpu100
- Chelmonska-Soyta A , Ozgo M , Lepczynski A , Herosimczyk A , Buska-Pisarek K , Kedzierska A , Nowak D , Mazur AJ. 2014. Proteome of spleen CD4 lymphocytes in mouse preimplantation pregnancy. J Physiol Pharmacol. 65:719–731.
- Cheng SB , Sharma S. 2015. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol. 73:487–500. doi: 10.1111/aji.12329
- Davis WH , Beer JR , Cook EF. 1961. Effects of pregnancy on the spleen in mice. J Mammal. 42:53–56. doi: 10.2307/1377240
- de Porto AP , Lammers AJ , Bennink RJ , ten Berge IJ , Speelman P , Hoekstra JB. 2010. Assessment of splenic function. Eur J Clin Microbiol Infect Dis. 29:1465–1473. doi: 10.1007/s10096-010-1049-1
- Fernandes MT , Dejardin E , dos Santos NR. 2016. Context-dependent roles for lymphotoxin-β receptor signaling in cancer development. Biochim Biophys Acta. 1865:204–219.
- Fujiwara H , Ideta A , Araki Y , Takao Y , Sato Y , Tsunoda N , Aoyagi Y , Konishi I. 2009. Immune system cooperatively supports endocrine system-primed embryo implantation. J Mamm Ova Res. 26:122–128. doi: 10.1274/jmor.26.122
- Godkin JD , Bazer FW , Moffatt J , Sessions F , Roberts RM. 1982. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13-21. J Reprod Fertil. 65:141–150. doi: 10.1530/jrf.0.0650141
- Hansen PJ. 2011. The immunology of early pregnancy in farm animals. Reprod Domest Anim. 46:18–30. doi: 10.1111/j.1439-0531.2011.01850.x
- Jiang XY , Lu TM , Shu WH , Zhou HY. 2016. Correlation between IL-6 and invasiveness of ectoderm cells of embryo in early pregnancy. J Biol Regul Homeost Agents. 30:559–563.
- Jonsson Y , Matthiesen L , Berg G , Ernerudh J , Nieminen K , Ekerfelt C. 2005. Indications of an altered immune balance in preeclampsia: a decrease in in vitro secretion of IL-5 and IL-10 from blood mononuclear cells and in blood basophil counts compared with normal pregnancy. J Reprod Immunol. 66:69–84. doi: 10.1016/j.jri.2005.02.002
- Kandil D , Leiman G , Allegretta M , Trotman W , Pantanowitz L , Goulart R , Evans M. 2007. Glypican-3 immunocytochemistry in liver fine-needle aspirates: a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer. 111:316–322. doi: 10.1002/cncr.22954
- Liao W , Lin JX , Leonard WJ. 2011. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 23:598–604. doi: 10.1016/j.coi.2011.08.003
- Liao W , Lin JX , Leonard WJ. 2013. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 38:13–25. doi: 10.1016/j.immuni.2013.01.004
- Lin YL , Liang YC , Chiang BL. 2007. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J Leukoc Biol. 82:1473–1480. doi: 10.1189/jlb.0307164
- Maciel GS , Uscategui RR , de Almeida VT , Oliveira ME , Feliciano MA , Vicente WR. 2014. Quantity of IL-2, IL-4, IL-10, INF-γ, TNF-α and KC-like cytokines in serum of bitches with pyometra in different stages of oestrous cycle and pregnancy. Reprod Domest Anim. 49:701–704. doi: 10.1111/rda.12360
- Maeda Y , Ohtsuka H , Tomioka M , Oikawa M. 2013. Effect of progesterone on Th1/Th2/Th17 and regulatory T cell-related genes in peripheral blood mononuclear cells during pregnancy in cows. Vet Res Commun. 37:43–49. doi: 10.1007/s11259-012-9545-7
- Maley SW , Buxton D , Macaldowie CN , Anderson IE , Wright SE , Bartley PM , Esteban-Redondo I , Hamilton CM , Storset AK , Innes EA. 2006. Characterization of the immune response in the placenta of cattle experimentally infected with Neospora caninum in early gestation. J Comp Pathol. 135:130–141. doi: 10.1016/j.jcpa.2006.07.001
- Martínez FF , Knubel CP , Sánchez MC , Cervi L , Motrán CC. 2012. Pregnancy-specific glycoprotein 1a activates dendritic cells to provide signals for Th17-, Th2-, and Treg-cell polarization. Eur J Immunol. 42:1573–1584. doi: 10.1002/eji.201142140
- Mattsson R , Ocklind G , Andersson M. 1984. Splenic macrophages during pregnancy in the mouse. Dev Comp Immunol. 8:443–450. doi: 10.1016/0145-305X(84)90051-X
- Mcnatty KP , Revefeim KJ , Young A. 1973. Peripheral plasma progesterone concentrations in sheep during the oestrous cycle. J Endocrinol. 58:219–225. doi: 10.1677/joe.0.0580219
- Mebius RE , Kraal G. 2005. Structure and function of the spleen. Nat Rev Immunol. 5:606–616. doi: 10.1038/nri1669
- Ott TL , Gifford CA. 2010. Effects of early conceptus signals on circulating immune cells: lessons from domestic ruminants. Am J Reprod Immunol. 64:245–254. doi: 10.1111/j.1600-0897.2010.00912.x
- Ozbilgin K , Turan A , Kahraman B , Atay C , Vatansever S , Uluer ET , Ozçakir T. 2015. Distribution of furin, TNF-α, and TGF-β2 in the endometrium of missed abortion and voluntary first trimester termination cases. Anal Quant Cytopathol Histpathol. 37:123–133.
- Pantaleo M , Piccinno M , Roncetti M , Mutinati M , Rizzo A , Sciorsci RL. 2013. Evaluation of serum concentrations of interleukin (IL)-4, IL-10, and IL-12 during pregnancy in bitches. Theriogenology. 79:970–973. doi: 10.1016/j.theriogenology.2013.01.017
- Prins JR , Gomez-Lopez N , Robertson SA. 2012. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 95:1–14. doi: 10.1016/j.jri.2012.05.004
- Raghupathy R , Makhseed M , Azizieh F , Omu A , Gupta M , Farhat R. 2000. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod. 15:713–718. doi: 10.1093/humrep/15.3.713
- Ruddle NH. 2014. Lymphotoxin and TNF: how it all began-a tribute to the travelers. Cytokine Growth Factor Rev. 25:83–89. doi: 10.1016/j.cytogfr.2014.02.001
- Saito S. 2000. Cytokine network at the feto-maternal interface. J Reprod Immunol. 47:87–103. doi: 10.1016/S0165-0378(00)00060-7
- Schoenborn JR , Wilson CB. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 96:41–101. doi: 10.1016/S0065-2776(07)96002-2
- Schroder K , Hertzog PJ , Ravasi T , Hume DA. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 75:163–189. doi: 10.1189/jlb.0603252
- Sokol CL , Barton GM , Farr AG , Medzhitov R. 2008. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 9:310–318. doi: 10.1038/ni1558
- Swirski FK , Nahrendorf M , Etzrodt M , Wildgruber M , Cortez-Retamozo V , Panizzi P , Figueiredo JL , Kohler RH , Chudnovskiy A , Waterman P , et al. 2009. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 325:612–616. doi: 10.1126/science.1175202
- Sykes L , MacIntyre DA , Yap XJ , Ponnampalam S , Teoh TG , Bennett PR. 2012a. Changes in the Th1:Th2 cytokine bias in pregnancy and the effects of the anti-inflammatory cyclopentenone prostaglandin 15-deoxy-Δ(12,14)-prostaglandin J2. Mediators Inflamm. 2012:416739.
- Sykes L , MacIntyre DA , Yap XJ , Teoh TG , Bennett PR. 2012b. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012:967629.
- Tuo W , MacMillan H , Günter N , Bazer FW , Brown WC. 1999. Upregulation of interleukin-4 and IFN-gamma expression by IFN-tau, a member of the type I IFN family. J Interferon Cytokine Res. 19:179–187. doi: 10.1089/107999099314324
- Williams Z. 2012. Inducing tolerance to pregnancy. N Engl J Med. 367:1159–1161. doi: 10.1056/NEJMcibr1207279
- Wong ML , Medrano JF. 2005. Real-time PCR for mRNA quantitation. Biotechniques. 39:75–85. doi: 10.2144/05391RV01
- Wyckoff J , Howland JL , Scott CM , Smith RA , Confer AW. 2005. Recombinant bovine interleukin 2 enhances immunity and protection induced by Brucella abortus vaccines in cattle. Vet Microbiol. 111:77–87. doi: 10.1016/j.vetmic.2005.09.004
- Yang L , Guo R , Yao X , Yan J , Bai Y , Zhang L. 2018a. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest Sci. 209:14–19. doi: 10.1016/j.livsci.2018.01.004
- Yang L , Liu Y , Lv W , Wang P , Wang B , Xue J , Zhang L. 2018b. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim Sci J. 89:1540–1548. doi: 10.1111/asj.13101
- Yang L , Wang Y , Ma X , Wang S , Zhang L. 2016. Changes in expression of Th1 and Th2 cytokines in bovine peripheral blood mononuclear cells during early pregnancy. Indian J Anim Res. 50:466–470.
- Yang L , Wang P , Mi H , Lv W , Liu B , Zhang L. 2019. Comparison of Th1 and Th2 cytokines production in ovine lymph nodes during early pregnancy. Theriogenology. 123:177–184. doi: 10.1016/j.theriogenology.2018.10.004
- Yang L , Zhang LY , Qiao HY , Liu N , Wang YX , Li SJ. 2014. Maternal immune regulation by conceptus during early pregnancy in the bovine. Asian J Anim Vet Adv. 9:610–620. doi: 10.3923/ajava.2014.610.620
- Yoo I , Han J , Kim M , Jang H , Sa S , Choi SH , Ka H. 2017. Expression and regulation of interleukin 6 and its receptor at the maternal-conceptus interface during pregnancy in pigs. Theriogenology. 96:85–91. doi: 10.1016/j.theriogenology.2017.04.007
- Zhang L , Xia Y , Tang F , Li SJ , Yang L , Wang B. 2015. The regulation of intrauterine immune cytokines and chemokines during early pregnancy in the bovine. Large Anim Rev. 21:23–31.
