ABSTRACT
This study aimed to evaluate the anti-post-weaning diarrhoea effect of a low dose coated-ZnO in weaned piglets in relation to intestinal permeability. A total of 72 cross-bred piglets, weaned at 27 d, were allocated to three dietary treatments as follows: basal diet without supplemental ZnO (control group); basal diet supplemented with 2250 mg/kg ZnO (ZnO group); basal diet supplemented with 562.5 mg/kg coated-ZnO (C-ZnO group). The trial lasted 21 days. Compared with control group, supplementation with coated-ZnO reduced (P < .05) diarrhoea index, plasma D-lactate level, and improved (P < .05) jejunal villus height and the expression of occludin and zonula occludens protein-1 in jejunal mucosa. In the C-ZnO group, intestinal total antioxidant capacity was enhanced (P < .05), and levels of malondialdehyde were decreased (P < .05) compared with the control group. The apoptotic index of intestinal epithelial cell was decreased (P < .05). Furthermore, the effects of the low dose coated-ZnO were similar to the high dose ZnO on the aforementioned parameters. Our results indicated that the low dose of coated-ZnO can alleviate diarrhoea by decreasing intestinal permeability as does a pharmacological dose of ZnO.
Introduction
Post-weaning diarrhoea is one of the most common causes of the reduced performance in weaning piglets. Zinc is intimately associated with gut development and immune functions due to its participating role in the synthesis of over 300 enzymes (Shen et al. Citation2014). The pharmacological concentration of inorganic Zn (2000–4000 mg/kg of Zn as ZnO) has been widely used in the diets of piglets in the swine industry, due to its effects on alleviating post-weaning diarrhoea and increasing growth performance (e.g. Hill et al. Citation2001). Such high levels of ZnO in weaned piglets results in severe environmental pollution caused by a large amount of Zn excretion (Carlson et al. Citation2004). Reducing dietary Zn level is a useful strategy to limit this environmental risk. It has been assumed that ZnO was the only inorganic form of Zn to resistant intestinal diseases (Schell and Kornegay Citation1996). However, ZnO in the diet is easy to be dissociated by the acidic gastric juice in the stomach and only a small amount of ZnO can reach the intestine. Thus, if ZnO can safely pass through the stomach, a lower dose of ZnO could be used in the diet (Shen et al. Citation2014).
Previously, we have conducted an experiment to evaluate the dissociation percentage of a coated-ZnO product in simulated gastric juice in vitro. The mineral particle of coated-ZnO is encapsulated with stearic acid, long-chain rice bran oil, metasilicic acid, carboxymethyl cellulose sodium, etc. The results of in vitro showed that the dissociation percentage of coated-ZnO in simulated gastric juice is 1.20%, while that of ZnO (uncoated) is 79.17%, which indicated that coating can well protect ZnO from being dissociated in the stomach (Liu et al. Citation2012). We had also evaluated the effects of coated-ZnO on the growth performance in weaning pigs (Duroc × Landrace × Yorkshire, average initial weight of 8.3 ± 0.3 kg weaned at 28 d age), and results showed that dietary supplementation of 562.5 mg/kg coated-ZnO was as effective as that of 2250 mg/kg ZnO in alleviating retarded growth of post-weaning pigs, and showed an obvious lower Zn residue (7148 vs. 16092 mg/kg) in faeces (Wang et al. Citation2013). The present study was continued to determine the effects of the low dose coated-Zn on post-weaning diarrhoea in pigs.
It is known that weaning induced changes in intestinal permeability to macromolecules could partly explain post-weaning diarrhoea (Boudry et al. Citation2004). Zhang and Guo (Citation2009) demonstrated that the protective effect of pharmacological levels of dietary ZnO in reducing diarrhoea might be associated with reduced intestinal permeability in weaning piglets. We hypothesized that supplementation of a low dose coated-ZnO, as a potential substitute for a high dose of ZnO, could also alleviate the incidence of diarrhoea in weaned piglets by decreasing intestinal permeability. Thus, the effects of coated-ZnO on intestinal permeability related indices in weaned piglets were determined in this study.
Materials and methods
Animals and dietary treatments
A total of 72 weaning piglets (Duroc × Landrace × Yorkshire) with an initial body weight of 8.46 (SEM = 0.23) kg at 27 (SEM = 1) d of age were randomly allotted to three groups on the basis of similar body weight, ancestry and sex. Each group had three pens of eight piglets (half castrated males and half females). The basal diet () with approximately 108.84 mg/kg Zn from ZnSO4 was formulated to meet or exceed the nutrient requirements recommended by the National Research Council (Citation2012). The three experimental diets were: (1) basal diet without supplemental ZnO (control group); (2) basal diet supplemented with 2250 mg/kg ZnO (ZnO group); (3) basal diet supplemented with 562.5 mg/kg coated-ZnO (C-ZnO group). The coated-ZnO contained 70% ZnO was provided by Hangzhou King Technology Feed Co., Ltd (Hangzhou, China). The crude protein, lysine, methionine, threonine, calcium, phosphorus, and zinc content of the basal diet were determined by methods of AOAC (Citation2000). No antibiotic was added to all diets. All pigs were given ad libitum access to water and the designated diet for 21 d.
Table 1. Compositions and nutrient levels of the basal diet (air-dried basis).
Diarrhoea incidence
The diarrhoea index was evaluated daily according to the previous faecal scoring system from 0 to 3 as follows: 0, normally shaped faeces; 1, shapeless faeces; 2, soft faeces; 3, liquid faeces (Castillo et al. Citation2008). The occurrence of diarrhoea was defined as the production of faeces at level 2 or 3 for two continuous days. Diarrhoea incidence (%) was defined as the number of pigs on a treatment with diarrhoea × diarrhoea days / (total number of pigs × 21 days) × 100%.
Slaughter and sample collection
After the feeding trial, six pigs from each treatment (two pigs per pen) were randomly selected for blood sampling via the jugular vein. The blood samples were collected into tubes containing sodium heparin, centrifugated at 3000g for 15 min at 4°C to obtain plasma, and then stored at −80°C until further analysis. The 18 selected pigs were then killed by electric stunning to collect tissue samples. Segments of (1-cm long) mid-jejunum were fixed in 4% neutral-buffered formalin solution for histology analysis. Jejunal mucosa samples were collected, washed with normal saline, frozen as aliquots in liquid N, and stored at −80°C for measurement of mRNA abundance and antioxidants.
Determination of intestinal permeability
Plasma levels of D-lactate and diamine oxidase were used as indices of intestinal permeability. The levels of D-lactic acid in plasma were determined by a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA) using a porcine D-lactic acid ELISA kit (R&D Systems, Minneapolis, MN, USA). Results were expressed as µg of D- lactic acid per mL of plasma. Plasma diamine oxidase activities were measured spectrophotometerically (UV-2000, Unico Instruments Co. Ltd., Shanghai, China) using diamine oxidase assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer. One unit diamine oxidase of activity equaled 1 nmoL of putrescine metabolized per hour at 37 °C and pH 7.2.
Observation of intestinal morphology
Jejunal samples were dehydrated, cleared, and embedded in paraffin. After being cut into 5-μm slices and stained with haematoxylin and eosin, specimens were examined under a light microscopy (Nikon Corp., Tokyo, Japan). The villus height was calculated from the tip of the villi to the villus crypt junction, and the crypt depth was defined as the depth of the invagination between adjacent villi. For each piglet, one slide was used and the average values were taken for a minimum of eight villi and crypts.
Semiquantitative RT–PCR analysis
Total RNA was isolated from jejunal mucosa samples by using a Trozol reagent (Invitrogen, Carlsbad, CA). The cDNA was synthesized via reverse transcription using the PrimeScripte RT Reagent Kit (Takara, Dalian, China) with a 1 μg RNA sample, according to the manufacturer’s instructions. Previous reported gene-specific primers for occluding (Xu et al. Citation2012), zonula occludens protein-1 (ZO-1; Xu et al. Citation2012) and E-cadherin (Song et al. Citation2010) were used in this study and shown in . Commercially available eukaryotic 18S rRNA primers were used as an endogenous control. Amplification reactions were performed in a total reaction volume of 25 µL which containing 12.5 μL 2 × Taq PCR MasterMix (including Mg2+, dNTP, and Taq DNA Polymerase), 1 µL of each primer, 2 µL cDNA, and double-distilled water. The cycling protocol was 5 min at 94°C, 30 cycles of 94°C for 30, 40 s at the annealing temperature (), extending at 72°C for 1 min, with a final extension at 72°C for 10 min. The obtained PCR products were run on a 2% agarose gel prestained with ethidium bromide and were visualized under UV light. The PCR products were quantified using Bandscan5.0 software. The intensity ratios of the target bands versus the 18S rRNA band indicated the relative expression level of the target gene.
Table 2. Gene-specific primers used in semiquantitative RT–PCR.
Determination of antioxidant indices in mucosa
Jejunal mucosa samples were thawed at 4°C and homogenized in 10 volumes of cold normal saline. The homogenates were then centrifuged at 30,000g for 10 min at 4°C and the supernatant was collected for analysing the contents of malondialdehyde (MDA), as well as the activities of total antioxidant capability (T-AOC), total superoxide dismutase (SOD), and catalase (CAT). These antioxidant indexes were performed spectrophotometerically (UV-2000, Unico Instruments Co. Ltd., Shanghai, China) using MDA, SOD, T-AOC, and CAT assay kits, which were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The supernatant total protein concentrations were determined using the Coomassie Brilliant Blue g-250 reagent with BSA as a standard, and the concentrations/activities of antioxidant indexes were expressed as units per milligram of protein.
Determination of apoptotic index
Formalin-fixed, paraffin wax-embedded jejunal samples were sectioned at 5-μm and analysed for apoptosis in mucosal epithelial cells with a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labelling (TUNEL) method as described by Han et al. (Citation2014). The number of TUNEL-stained epithelial cells in jejunal mucosa was counted under a light microscopy (Nikon Corp., Tokyo, Japan). The apoptotic index for each microscopic field was calculated as the number of positive TUNEL-stained epithelial cells divided by the total number of epithelial cells counted per field. For each piglet, one slide was used and the average values were taken for a minimum of eight fields.
Statistical analysis
Data were analysed using the one-way ANOVA procedure of SPSS (Version 17.0, SPSS, Chicago, IL), and Duncan’s multiple-range test was employed among groups. Values are presented as means ± SEM. P < .05 was considered statistically significant. Each pen was set as the experimental unit for diarrhoea incidence, but the individual piglet was the experimental unit for all other data.
Results
Weaning pigs fed with ZnO or C-ZnO diets significantly decreased the diarrhoea incidences compared with the control (P < .05, ). The effect of C-ZnO on diarrhoea was not significantly different from the ZnO treatment.
Figure 1. The diarrhoea incidences of weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 3. Bars without a common letter differ significantly (P < .05). Diarrhoea incidence (%) = number of pigs on a treatment with diarrhoea × diarrhoea days/(total number of pigs × 21 days) × 100%. Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO.
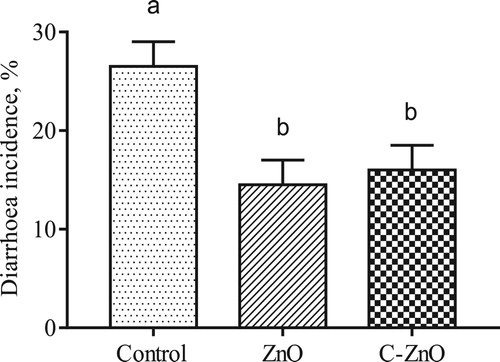
Intestinal permeability of weaned piglets, as reflected by plasma levels of D-lactate and diamine oxidase, is presented in . Dietary supplementation with ZnO or C-ZnO reduced (P < .05) plasma D-lactate concentrations when compared with the control group. ZnO or C-ZnO supplementation in the diet for weaning piglets did not affect the activity of diamine oxidase (P > .05). The effects of C-ZnO on intestinal permeability were similar to the ZnO-treated group.
Figure 2. Plasma d-lactate level (μg/mL) and diamine oxidase activity (U/mL) of weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 6. Bars without a common letter differ significantly (P < .05). Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO.
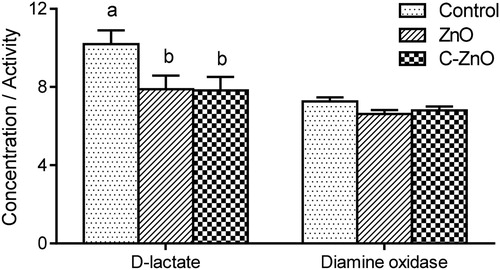
Morphological measurements of the jejunal mucosa of pigs are presented in . The highest value of villus height at the jejunal mucosa came from pigs in C-ZnO group, while the lowest value was from pigs in control group (P < .05). ZnO or C-ZnO had no effect on jejunal mucosa crypt depth and the villus height:crypt depth ratio. No differences in morphology of jejunal mucosa between the ZnO group and C-ZnO group were observed.
Figure 3. Representative haematoxylin and eosin-stained morphology and villus height, crypt depth and villus height:crypt depth ratio in jejunum of weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 6. Bars without a common letter differ significantly (P < .05). Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO.
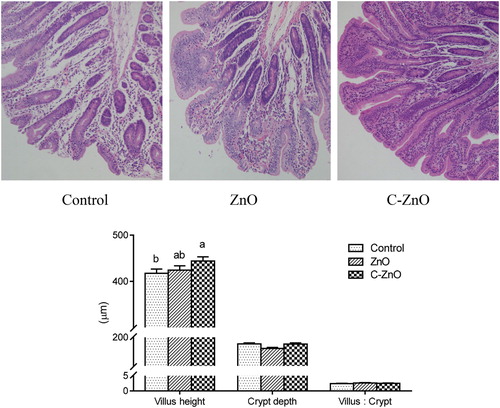
Added ZnO or C-ZnO to the diet of weaning piglets significantly enhanced (P < .05) mRNA levels for occludin and ZO-1 in the jejunal mucosa (), but did not affect the mRNA levels of E-cadherin (P > .05). Piglets fed the C-ZnO diet exhibited a higher (P < .05) level of ZO-1 than piglets fed the ZnO diet.
Figure 4. Gel electrophoresis of 18S rRNA, E-cadherin, occludin, and ZO-1 PCR products and expression patterns of E-cadherin, occludin, and ZO-1 mRNA of jejunal mucosa in weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 6. Bars without a common letter differ significantly (P < .05). Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO. 18S served as endogenous reference gene.
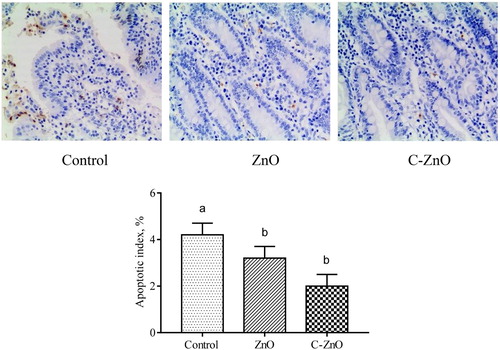
In the C-ZnO-treated group, intestinal T-AOC were enhanced (P < .05), whereas levels of MDA were decreased (P < .05) compared with the control group (), and the effects of C-ZnO were similar to the ZnO-treated group. ZnO or C-ZnO supplementation in the diet for weaning piglets did not affect the activity of T-SOD and CAT (P < .05). Additionally, supplementation with either ZnO or C-ZnO decreased the apoptotic index of intestinal epithelial cell compared with the control group ().
Figure 5. Jejunal mucosa antioxidant indices of weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 6. Bars without a common letter differ significantly (P < .05). Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO. Units for total antioxidant capability (T-AOC), total superoxide dismutase (SOD), catalase (CAT) are U/mg protein. Unit for malondialdehyde (MDA) is nmol/mg protein Control ZnO C-ZnO.
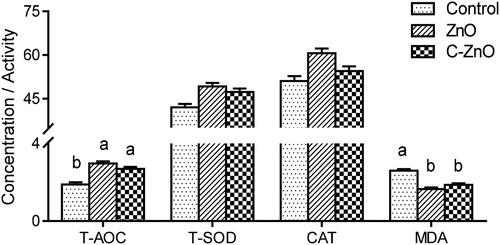
Figure 6. TUNEL-stained epithelial cells in jejunal mucosa and calculated apoptotic index of weanling pigs fed with Control, ZnO or C-ZnO diets for 21 days. Results are means ± SEM, n = 6. Bars without a common letter differ significantly (P < .05). Control, basal diet without ZnO supplemented; ZnO, diet supplemented with 2250 mg/kg ZnO, C-ZnO, diet supplemented with 562.5 mg/kg coated-ZnO.
Discussion
Previous works in our lab demonstrated that dietary supplementation of 562.5 mg/kg coated-ZnO was as effective as that of 2250 mg/kg ZnO in alleviating retarded growth of post-weaning pigs (Wang et al. Citation2013). In the current study, weaned pig diets supplemented with 562.5 mg/kg of coated-ZnO alleviates diarrhoea comparable to pharmacological levels of ZnO. The aforementioned results demonstrated that the use of coated-ZnO is an ideal method to reduce the additional levels of ZnO. In addition, no difference was detected between the ZnO and C-ZnO groups in any variable other than the ZO-1 mRNA expression measured in the present study. This observation probably confirmed the assumption that ZnO, mainly in its molecular form, functions at the intestine to reduce the incidence of diarrhoea.
It is known that the occurrence of diarrhoea is coincident with a pathological increase in intestinal permeability (Yu et al. Citation2012). Thus, to reveal the possible mechanisms for the anti-post-weaning diarrhoea effect of C-ZnO, the intestinal permeability related parameters were measured in the current study. Intestinal permeability was evaluated by testing levels of plasma D-lactate and diamine oxidase because they are proposed as quantitative and sensitive circulating markers for monitoring the extent of intestinal mucosal injury (Zhao et al. Citation2011). The integrity of the intestinal barrier was fundamental to the proper functioning of the epithelial cells and to prevent the entry of pathogenic bacteria, while injured intestinal barrier increased the epithelial permeability (Lu and Walker Citation2001). In the current study, both high-dose ZnO and low-dose coated-ZnO reduced plasma D-lactate concentration, which indicated protecting the intestinal barrier integrity.
Intestinal epithelium serves as not only the major digestive and absorptive site of nutrients but also the intestinal immune barrier in resisting the invasion of environmental pathogens. The structural changes in intestinal villus-crypt morphology as a result of stressors, may affect both secretory potential and permeability (Jeurissen et al. Citation2002). Weaning stress is often associated with villus atrophy and crypt hyperplasia (Montagne et al. Citation2007). An increase in the permeability has been shown to correlate closely with villous atrophy in the small intestine (Strobel et al. Citation1984). It has been reported that pharmacological does of ZnO can increase villus height and reduce crypt depth in the small intestine of weaned piglets (Li et al. Citation2001). Furthermore, Xia et al. (Citation2017) demonstrated that low dose of zinc oxide nanoparticles had comparable effects on improving jejunal morphology to a high dose of traditional ZnO. In the present study, supplementation with low does C-ZnO, as well as high does ZnO, had higher villus height at the jejunal mucosa as compared with the control. The improvements in small-intestinal morphology by feeding coated-ZnO might, at least partly, contribute to the reduced intestinal permeability.
Intestinal permeability had long been considered as an indicator of intestinal epithelial barrier function, which is primarily regulated by a well-organized system referred to as the tight junction (Kucharzik et al. Citation2001). Tight junction mainly consists of several unique proteins including the trans-membrane protein occludin, junctional adhesion molecule, like E-cadherin, and members of the claudin family as well as linker proteins such as ZO-1 (Ma et al. Citation2012). Changes in intestinal permeability during weaning might be due to a breakdown in tight junction integrity (Kansagra et al. Citation2003). Thus, to further clarify the molecular mechanism for the alteration of intestinal permeability in weaning piglets fed coated-ZnO, the changes in the expression of occludin, E-cadherin, and ZO-1 were determined in this study. The result of our work indicated that dietary supplementation with a low dose of C-ZnO or high dose of ZnO could in part prevent alterations in intestinal permeability during the weaning period by enhancing the expression of occludin and ZO-1. However, the finding that dietary addition of high dose ZnO was more effective than low dose C-ZnO for enhancing ZO-1 mRNA expression needs to be further investigated.
Mucosa oxidative stress has been documented as a causative agent in the induction and progression of post-weaning diarrhoea (Jiang et al. Citation2014). The accumulation of oxidative radical such as reactive oxygen species (ROS) can cause DNA damage and induce apoptosis of intestinal epithelial cells, which could then lead to increased intestinal permeability (Fan et al. Citation2017). Recently, Cao et al. (Citation2018) demonstrated that oxidative stress disrupted the intestinal barrier, increased intestinal permeability, and triggered diarrhoea in weaning piglets. Besides, it is reported that a dietary additive that can effectively repress oxidative stress could potentially relieve post-weaning diarrhoea (Michiels et al. Citation2012; Wang et al. Citation2015). Therefore, the intestinal antioxidant indices were evaluated to reveal the potential relationship between antioxidative stress of ZnO and tightening of intestinal permeability. The systemic antioxidants are responsible for the elimination of oxidative radicals, while MDA levels are often used as an indicator of ROS mediated damage (Kühn et al. Citation2002). Our results showed that dietary ZnO or C-ZnO significantly increased intestinal mucosa antioxidants (T-AOC) but decreased MDA levels. In combination with decreased apoptosis of intestinal epithelial cells by dietary ZnO or C-ZnO, it could be indicated that both high dose ZnO and low dose C-ZnO could efficiently tighten intestinal permeability by relieving the oxidative damage.
In conclusion, a low dose of coated-ZnO can tighten intestinal permeability and hence alleviate diarrhoea in weaned piglets by promoting intestinal homeostatic and architecture, protecting the intestinal mucosal barrier from damage, and enhancing intestinal antioxidant indices, as does a pharmacological dose of ZnO. Our results confirmed that the use of coated-ZnO is an ideal method to reduce the additional levels of ZnO for piglets.
Ethical approval
The protocol for the present experiment was approved by the Animal Care and Welfare Committee of Animal Science College, Zhejiang University (Hangzhou, China).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC . 2000. Official Methods of Analysis. 17th ed. Gaithersburg (MD) : Official Methods of Analysis of AOAC International.
- Boudry G , Péron V , Le Huërou-Luron I , Lallès JP , Sève B. 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 134(9):2256–2262. doi: 10.1093/jn/134.9.2256
- Cao S , Wu H , Wang C , Zhang Q , Jiao L , Lin F , Hu CH. 2018. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function and triggers mitophagy in piglets. J Animal Sci. 96(5):1795–1805. doi: 10.1093/jas/sky104
- Carlson MS , Boren CA , Wu C , Huntington CE , Bollinger DW , Veum TL. 2004. Evaluation of various inclusion rates of organic zinc either as a polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J Animal Sci. 82(5):1359–1366. doi: 10.2527/2004.8251359x
- Castillo M , Martín-Orúe SM , Taylor-Pickard JA , Pérez JF , Gasa J. 2008. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function. J Animal Sci. 86(1):94–101. doi: 10.2527/jas.2005-686
- Fan P , Tan Y , Jin K , Lin C , Xia S , Han B , Zhang F , Wu L , Ma X. 2017. Supplemental lipoic acid relieves post-weaning diarrhoea by decreasing intestinal permeability in rats. J Animal Physiol Animal Nutr. 101(1):136–146. doi: 10.1111/jpn.12427
- Han XY , Ma YF , Lv MY , Wu ZP , Qian LC. 2014. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Br J Nutr. 111(8):1405–1411. doi: 10.1017/S0007114513004042
- Hill GM , Mahan DC , Carter SD , Cromwell GL , Ewan RC , Harrold RL , Lewis AJ , Miller PS , Shurson GC , Veum TL. 2001. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Animal Sci. 79(4):934–941. doi: 10.2527/2001.794934x
- Jeurissen SH , Lewis F , Jd VDK , Mroz Z , Rebel JM , ter Huurne AA. 2002. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr Issues Intestinal Microbiol. 3(3):1–14.
- Jiang XR , Zhang HJ , Mantovani G , Alborali GL , Caputo JM , Savoini G , Dell’Orto V , Bontempo V. 2014. The effect of plant polyphenols on the antioxidant defence system of weaned piglets subjected to an Escherichia coli challenge. J Animal Feed Sci. 23(23):324–330. doi: 10.22358/jafs/65668/2014
- Kansagra K , Stoll B , Rognerud C , Niinikoski H , Ou CN , Harvey R , Burrin D. 2003. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 285(6):G1162–G1170. doi: 10.1152/ajpgi.00243.2003
- Kucharzik T , Walsh SV , Chen J , Parkos CA , Nusrat A. 2001. Neurophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 159(6):2001–2009. doi: 10.1016/S0002-9440(10)63051-9
- Kühn H , Borchert A. 2002. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radical Biol Med. 33(2):154–172. doi: 10.1016/S0891-5849(02)00855-9
- Li BT , Kessel AGV , Caine WR , Huang SX , Kirkwood RN. 2001. Small intestinal morphology and bacterial populations in ileal digesta and feces of newly weaned pigs receiving a high dietary level of zinc oxide. Can J Animal Sci. 81(4):511–516. doi: 10.4141/A01-043
- Liu LL , Li ZF , Chen Q , Lu JJ. 2012. Studies on the stability of the S-ZnO in vitro and its effects on growth performance in weaning pigs. Chin J Animal Sci. 47(23):54–58.
- Lu L , Walker WA. 2001. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 73(6):1124S–1130S. doi: 10.1093/ajcn/73.6.1124S
- Ma X , Fan PX , Li LS , Qiao SY , Zhang GL , Li DF. 2012. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Animal Sci. 90(Supplement 4):266–268. doi: 10.2527/jas.50965
- Michiels J , Skrivanova E , Missotten J , Ovyn A , Mrazek J , De Smet S , Dierick N. 2012. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: effects on performance, gut bacteria and morphology and plasma oxidative status. J Animal Physiol Animal Nutr. 96(6):1101–1111. doi: 10.1111/j.1439-0396.2011.01227.x
- Montagne L , Boudry G , Favier C , Le Huërou-Luron I , Lallès JP , Sève B. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 97(1):45–57. doi: 10.1017/S000711450720580X
- NRC . 2012. Nutrient requirements of swine. 11th rev. ed. Washigton (DC) : Natl. Acad. Press.
- Schell TC , Kornegay ET. 1996. Zinc concentration in tissues and performance of weanling pigs fed pharmacological levels of zinc from ZnO, Zn-methionine,Zn-lysine, or ZnSO4 . J Animal Sci. 74(7):1584–1593. doi: 10.2527/1996.7471584x
- Shen J , Chen Y , Wang Z , Zhou A , He M , Mao L , Zou H , Peng Q , Xue B , Wang L , et al. 2014. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br J Nutr. 111(12):2123–2134. doi: 10.1017/S0007114514000300
- Song CY , Gao B , Wu H , Wang XY , Chen GH , Mao J. 2010. Spatial and temporal expression of spermadhesin genes in reproductive tracts of male and female pigs and ejaculated sperm. Theriogenology. 73(5):551–559. doi: 10.1016/j.theriogenology.2009.09.030
- Strobel S , Brydon WG , Ferguson A. 1984. Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut. 25(11):1241–1246. doi: 10.1136/gut.25.11.1241
- Wang C , Xie P , Liu LL , Lu JJ , Zou XT. 2013. Effects of dietary capsulated zinc oxide on growth performance, blood metabolism and mineral concentrations in weaning piglets. Asian J Animal Vet Adv. 8(3):502–510. doi: 10.3923/ajava.2013.502.510
- Wang WH , Yang QY , Sun ZX , Chen XY , Yang C , Ma X. 2015. Advance of interactions between exogenous natural bioactive peptides and intestinal barrier and immune responses. Curr Protein Pept Sci. 16(5):297–343.
- Xia T , Lai W , Han M , Han M , Ma X , Zhang L. 2017. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget. 8(39):64878–64891. doi: 10.18632/oncotarget.17612
- Xu S , Lee J , Miyake M. 2012. Expression of zo-1 and occludin at mrna and protein level during preimplantation development of the pig parthenogenetic diploids. Zygote. 20(2):147–158. doi: 10.1017/S0967199410000705
- Yu LCH , Wang JT , Wei SC , Ni YH. 2012. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophysiol. 3(1):27–43. doi: 10.4291/wjgp.v3.i1.27
- Zhang B , Guo Y. 2009. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (zo-1) expression in weaning piglets. Br J Nutr. 102(5):687–693. doi: 10.1017/S0007114509289033
- Zhao Y , Qin G , Sun Z , Che D , Bao N , Zhang X. 2011. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int J Mol Sci. 12(12):8502–8512. doi: 10.3390/ijms12128502
