ABSTRACT
In the present study effects of a commercial probiotic, Ecotec on growth, thyroid hormone profile, hematology, immuno-biochemical responses, monocyte morphology and survival against pathogenic challenge in Labeo rohita juveniles have been investigated. Two different concentrations of the probiotic (t1: 8 × 107 CFU/g feed and t2: 16 × 107 CFU/g feed) were administered to the fish. Probiotic treated fish showed a high growth increment as compared to the control. Level of thyroid hormones T3 and T4 was found to be lowered while TSH level was high in probiotic treated groups. Total Leukocyte Count was significantly low in the probiotic supplemented groups. The Differential Leukocyte Count showed abundance of lymphocytes and monocytes in the control group while neutrophils and basophils were abundant in the probiotic treated groups. Biochemical parameters (Albumin and Globulin) were high in probiotic fed groups. Immunological paraameters (Super oxide dismutase, Catalase, Myeloperoxidase and Lysozyme) were significantly high in probiotic fed fish. Monocyte morphology in control group showed a pronounced phagocytic activity in comparison to probiotic treated groups. Infection with Aeromonas hydrophila showed that probiotic treated fish had a better survival rate. In conclusion Ecotec supplementation enhanced growth and improved immunity in Labeo rohita juveniles.
1. Introduction
Aquaculture is amongst the fastest developing food generating section in the world. Absurdly, disease outbreaks are common and are considered a major obstacle in sustainable aquaculture production (Verschuere et al. Citation2000). The conservative methods for disease control such as antibiotics and antimicrobial drugs have been used for quite some time but they have had limited success in preventing fish diseases. Moreover, there are some risk factors associated with their use: Firstly, the therapeutic use of antimicrobial agents in aquaculture, associated to those being used in human medication can be a major hazard to human wellbeing (Alderman and Hastings Citation1998; Witte et al. Citation1999). Additionally, the rising concerns of antibiotics in development of bacterial resistance, damage to microbial populations in aquatic environment, mixing of antibiotic particulates in aquaculture food products, and the suppression of aquatic animal’s immune system have made it very essential to find alternative ways of disease prevention in aquaculture (Sapkota et al. Citation2008).
Bacteria have been proposed as the biological control agent against fish diseases. Probiotics are known as live microbial feed additives which enter the host’s gastrointestinal tract, improve it’s intestinal microbial stability leading to improved growth and disease tolerance in the host (Gatesoupe Citation1999; Gomez-Gil et al. Citation2000). Some additional benefits of probiotics in aquaculture include improvement of water quality parameters, better nutrition of host species by production of digestive enzymes, competitive elimination of harmful bacteria, better survival rate and stress tolerance (Verschuere et al. Citation2000; Pirarat et al. Citation2006; Taoka et al. Citation2006; Yamada et al. Citation2018). Probiotics therefore, are a good strategy for controlling the microbial infections with a great potential to replace antibiotics. Different microbial strains being used as probiotics include: Lactobacillus acidophilus, L. bulgaricus, L. plantariu, Bifidobacterium, Streptococcus lactis, Saccharomyces cerevisiae, Enterococcus faecium, Pseudomonas fluorescens, Streptomyces spp. and Micrococcus spp. The mixtures of probiotics have been found to be more effective in disease control as compared to single cultures (Aly et al. Citation2008; Ridha and Azad Citation2016). Commercial probiotics are also being successfully used in aquaculture and have shown to provide better survival, weight gain and immunity enhancement (Ridha and Azad Citation2016).
Little data is found about the effect of probiotics on fish thyroid hormones which are believed to be important for varous physiological processes of the body. Thyroid hormones perform critical roles in the proper functioning of immune responses during stress (Dorshkind and Horseman Citation2001). Hematological and cell morphological analysis are important parameters to investigate the fish health status (Bahmani et al. Citation2001; Satheeshkumar et al. Citation2012). There is little data available about the roles of probiotics on the hematological parameters of Labeo rohita (L. rohita) which is a popular food fish in Asia. Furthermore, information about the effect of probiotics on immune cell morphology of L. rohita is scarce. Studies have shown in other organisms that probiotics cause some immuno-morphological changes in different cells (Isidro et al. Citation2014; Rieger et al. Citation2015). The present study was therefore, designed to investigate the effect probiotics on growth, thyroid hormones, hematology, immune cell morphology and survival agaist pathogenic challenge of L. rohita juveniles. The results of this study will be very helpful in understanding the growth and immunological responses of L. rohita to probiotics and thereby will enhance our knowledge for the healthy use of probiotics to flourish the aquaculture industry.
2. Materials and methods
2.1. Experimental fish
L. rohita juveniles were handled in compliance with the local animal welfare regulations (Ethical regulations, Lahore College for Women University, Lahore). L. rohita juveniles were obtained from Himalaya fish hatchery, Muridke, Lahore, Pakistan. Fish were kept in 60 l glass aquaria (6 aquaria per experiment) and were acclimatized to lab conditions for two weeks. During the acclimatization period fish were given basal feed (fish meal 28%; soybean meal 35%; maize 15%; wheat bran 10%; wheat 7%; vegetable oil 4%; vitamin premix 0.9%; mineral premix 0.1%) twice daily. Aerators were kept connected to fish tanks to maintain an optimum dissolved oxygen level. About 40% of water was exchanged every day. Water temperature, dissolved oxygen and pH during the experimental period were 26 ± 1.5°C, 7.3 ± 0.8 mg/l and 7 ± 0.9 respectively.
2.2. Probiotic treatment
Commercial probiotic ‘Ecotec’ (SEARLE, Pakistan) containing > 2 billion cfu freeze dried mixture of four different microbial strains: Lactobacillus acidophilus, LA-5, Bifidobacterium, BB-12, Streptococcus thermophiles, STY-31, and Lactobacillus delbrueckii ssp. Bulgaricus, LBY-27 was used in this study. Probiotic was mixed with the basal diet in two different proportions: 8 × 107 CFU/g feed and 16×107 CFU/g feed. Fish was randomly divided into three groups (n = 20 per group) in triplicate experiments (con: fish given only basal diet; t1: fish given probiotic 8 × 107 CFU/g feed; t2: fish given probiotic 16 × 107 CFU/g feed). The total period of the experimental study was eight weeks.
2.3. Growth measurements
Weight and length of each fish in all three groups were recorded at the beginning and the end of the experimental period. Following growth performance indicators were measured.
Weight gain: Final weight–Initial weight
Percentage weight gain: Final weight–Initial weight/Initial weight×100
Specific growth ratio: ln Final weight–ln Initial weight/time in days×100
Feed conversion ratio: Total feed used/weight gain
Condition factor: body weight/body length3×100
Length gain: Final length–initial length
2.4. Analysis of thyroid hormones
To measure the thyroid hormones serum samples were collected at the end of experiment. 10 fish were randomly selected from each group, anesthetized with clove oil and blood was collected from the caudal vessels. No anticoagulant was added and blood was kept at room temperature for 30 min to clot and then was centrifuged at 3000 rpm for 10 min. The supernatant serum was used for determination of Thyroid stimulating hormone (TSH), Triiodothyronine (T3) and Thyroxine (T4) by using ELISA kits (DiaMetra, Italy). All assays were validated for fish serum before use. ELISA assay were as follows: for TSH (sensitivity: 0.01 mIU/l; analytical range 0.2-20.0 mIU/l), for T3 ((sensitivity: 5 ng/dl; analytical range 50.0-750.0 ng/dl and for T4 (sensitivity: 0.4 µg/dl; analytical range 2.0-25.0 µg/dl).
2.5. Hematological studies
10 fish from each group were randomly selected from each group, anesthetized with clove oil and sacrificed for blood collection. Blood was obtained from the caudal vessels using EDTA solution as an anticoagulant. Total Leukocyte count (TLC) and Total erythrocyte count (TEC) were quantified by using hemocytometer. Hemoglobin (Hb) values were measured following Cyanmethemoglobin method. Briefly 20 µl of fresh blood was mixed with 5 ml of Drabkin fluid. The solution was allowed to stand at room temperature for 5 min and then absorbance of solution was measured by spectrophotometer (wavelength:540 nm). The concentration ofHb was measured by making a standard curve. For Differential Leukocyte count (DLC), blood smears were made from 5 fish per group immediately after blood collection. Smears were air dried for 1 h and then fixed in 95% methanol. Slides were then stained with Giemsa stain (Shah et al. Citation2009). A light microscope was used for DLC studies.
2.6. Biochemical analysis
Biochemical parameters were analysed from serum. Total protein (TP) was evaluated by using Total protein kit (Crescent Diagnostic, Saudi Arabia). This kit measures total protein by Photometric Clorimetri-Biuret method. Level of Albumin (ALB) was checked by Albumin kit (Crescent Diagnostic, Saudi Arabia). Globulin (GLB) was calculated by subtracting the value of ALB from TP. A/G ratio was calculated by dividing the values of ALB from GLB.
2.7. Immunological analysis
Serum Lysozyme (Lys) activity was measured by turbidimetric method as described by Sankaran and Gurnani (Citation1972). Briefly, lyophilized hen egg white Lys (Sigma Aldrich) was used as standard. Lys activity was expressed as units/ ml, one unit represents reduction in absorbance of 0.01/min. Myeloperoxidase (MPO) activity was determinedas descibed by Quade and Roth (Citation1997). 20 µl of serum was added in 96-well plates diluted with Hanks Balance Saline solution (HBSS, without CA2+ and Mg2+). Thirty-five microlitres of 20 mM 3,3′,5,5′-tetramethylbenzidine hydrochloride (Sigma Aldrich) and 5 mM of H2O2 were added and incubated till colour change. The reaction was stopped by adding 35 µl of 4M sulphuric acid (H2SO4). The OD was measured at 540 nm in micrplate reader. Activities of Superoxide dismutase (SOD) and Catalase (CAT) were evaluated using commercial kits (Sigma Aldrich).
2.8. Monocyte Morphometric studies
The morphology of monocytes was studied to compare the behaviour of cells in probiotic treated and untreated groups. The protocol followed for monocyte morphological study was that reported by (Luu et al. Citation2007) with slight modifications. The cells were examined and were photographed by Leica optical microscope (Leica DM 400M, Germany) at the magnification of 1000×.
2.9. In vivo challenge test
At the end of the feeding trial of probiotic, fish of each group were divided into two subgroups, one subgroup was intramuscularly challenged with 0.5 ml (2×106 cfu/ml) of Aeromonas hydrophila (A. Hydrophila). The other subgroup was injected with saline solution only. Fish was observed for 7 days post infection (dpi) during which survival rate of fish was observed. Relative percent survival (RPS) of challenged fish was calculated as: (1-% mortality of treated fish/ % mortality of control) * 100 (Amend Citation1981).
2.10. Statistical analysis
All the experiments were performed in triplicates. The experimental data was analysed using one way analysis of variance (ANOVA) followed by Tukey’s multiple range posthoc test (Graphpad Prism 4, San Diego, California, USA).
3. Results
3.1. Growth analysis
The weight and length of each fish was recorded at the end of experimental period. The weight of fish in both t1 and t2 groups was significantly high as compared to the control group (p < .05 and p < .01, respectively) ((a), ). Weight gain, percentage weight gain, specific growth ratio were significantly high in both probiotic treated groups (t1 and t2) than control. The feed conversion ratio was significantly low in probiotic supplemented groups than the control group. The condition factor in probiotic treated groups was also high in treated groups than control. The differences in different parameters were not significantly different between t1 and t2 groups. There was not significant difference in the length of fish between control and treated groups (p > .05) ((b), ).
Figure 1. Growth of fish recorded at the end of experimental period. (a) Scatter plot shows that weight of fish in probiotic treated groups t1 and t2 is significantly high as compared to the control group (con). (b) Scatter plot shows that the length of fish in probiotic treated groups is higher as compared to the control group. Asterisks indicate significant differences (* p < .05; ** p < .01) tested by one-way ANOVA with Tukey’s method as post-hoc test.
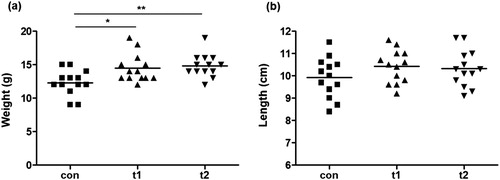
Table 1. Growth parameters of Labeo rohita: Con (control), t1 (Ecotec supplementation 8 × 107 CFU/g feed), t2 (Ecotec supplementation 16 × 107 CFU/g feed). Values with a different superscript in the same row are significantly different.
3.2. Thyroid hormone quantification
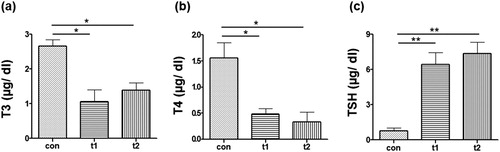
It was observed that level of both T3 and T4 was significantly low in probiotic treated fish. The T3 level in t1 and t2 groups was measured to be 1.06 ± 0.37 and 1.81 ± 0.31, respectively. The level of T3 in control group was 2.33 ± 0.49 ((a)). The level of T4 in t1 and t2 groups was 0.49 ± 0.10 and 0.33 ± 0.18, respectively, and it was significantly low (p < .05) as cmpared to the control group (1.56 ± 0.3) ((b)). Significant differences in TSH level were also also noted in probiotic treated and control fish (p < .01). Level of TSH in t1 and t2 was 6.44 ± 1 and 7.36 ± 0.96, respectively. T4 value in control group was 0.76 ± 0.28 ((c)).
Figure 2. Quantification of Thyroid hormones. Quantity of thyroid hormones in the serum was measured at the end of expermental period. (a) Amount of Triiodothyronine (T3) in control (con) was significantly high as compared to probiotic treated groups (t1 and t2). (b) Amount of Thyroxine (T4) in con was significantly high as compared to t1 and t2. (c) Thyroid stimulating hormone (TSH) was significantly low in con as as compared to t1 and t2. Data are the mean ± SEM of three replicates. Asterisks indicate significant differences (* p < .05; ** p < .01) tested by one-way ANOVA analysis with Tukey’s method as post-hoc test.
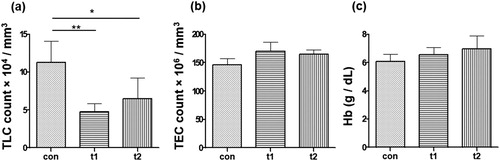
3.3. Hematological parameters
TLC in control group (10 × 104 /mm3) was higher than TLC in the probiotic treated groups (t1: 5 × 104 /mm3, t2: 6 × 104 /mm3) (p < .01; p < .05, respectively) ((a)). There was not significant difference in TEC in probiotic treated and control groups (con: 146 × 106 /mm3, t1: 170 × 106 /mm3, t2: 165 × 106 /mm3) (p > .05) ((b)). Probiotic treatment caused slight increase in the Hb level (con: 6 g /dl, t1: 6.5 g /dl, t2: 6.9 g /dl) ((c)) but the difference was not significant (p > .05).
Figure 3. Total Leukocyte Count (TLC), Total Erythrocyte Count (TEC) and Haemoglobin (Hb). (a) TLC was done after eight weeks of probiotic treatment. Total number of leukocytes in control (con) are significantly high as compared to probiotic treated groups t1 and t2. (b) The number of erythrocytes showed no significant difference between control and treated groups. (c) Hb was not significantly different between con and probiotic treated groups. Data are the mean ± SEM of three replicates. Asterisks indicate significant differences (* p < .05; ** p < .01) tested by one-way ANOVA analysis with Tukey’s method as post-hoc test.
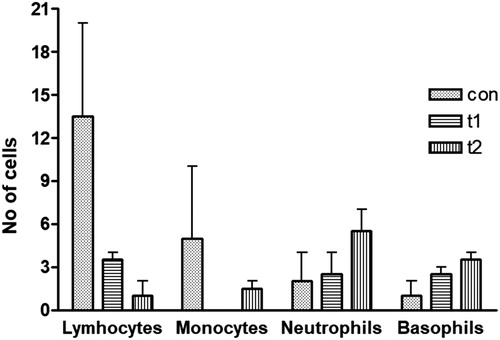
In the DLC data it was observed that the number of lymphocytes was higher in control group than the treated groups (con: n = 13, t1: n = 4, t2: n = 1) (). The monocyte number was also high in the control group (n = 5) than probiotic supplemented groups (t1: n = 0, t2: n = 2). The number of neutrophils was higher in the probiotic treated groups (t1: n = 3, t2: n = 6) as compared to the control group (n = 2). Basophils were found to be lower in control group (n = 1) than the probiotic treated groups (t1: n = 3, t2: n = 4). Thus it was concluded that probiotc triggered a low number of lymphocyte and monocyte populations and a high number of neutrophil and basophils in L. rohita juveniles ().
Figure 4. Differential leukocyte count (DLC). Lymphocytes and monocytes were higher in no. in the control group (con) as compared to the probiotic treated groups (t1 and t2). However, the neutrophils and basophils were abundant in probiotic treated groups as compared to the control group. Data are the mean ± SEM of two independent replicates.
3.4. Biochemical parameters
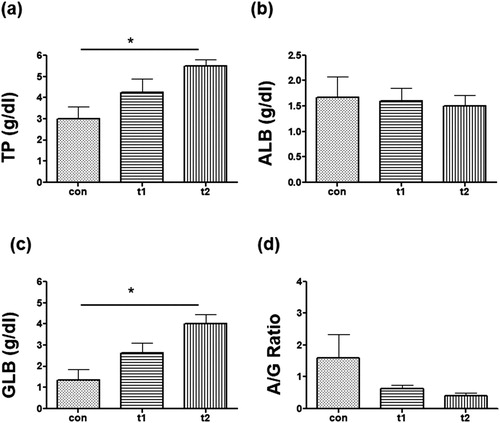
Total protein was increased in probiotic treted groups (con: 3.0, t1: 4.2, t2: 5.5) ((a)). The GLB level was also increased in probiotic fed groups (con: 1.3.0, t1: 2.6, t2: 4.0) ((b)). Albumin was slightly decreased or unaltered in the probiotic groups (con: 1.6, t1: 1.6, t2: 1.5) ((c)). A/G ratio was decreased after probiotic treatament (con: 1.5, t1: 0.6, t2: 0.3) ((d)).
Figure 5. Biochemical parameters. (a) Total Protein (TP) level was high in probiotic supplemented groups (t1 and t2) as as compared to control (con). (b) Albumin (ALB) level was not much different between con and probiotic treated groups. (c) Globulin (GLB) was significantly high in t2. (d) A/G ratio of probiotic treated groups was low than the con group. Data are the mean ± SEM of three replicates. Asterisks indicate significant differences (* p < .05) tested by one-way ANOVA analysis with Tukey’s method as post-hoc test.
3.5. Immunological parameters
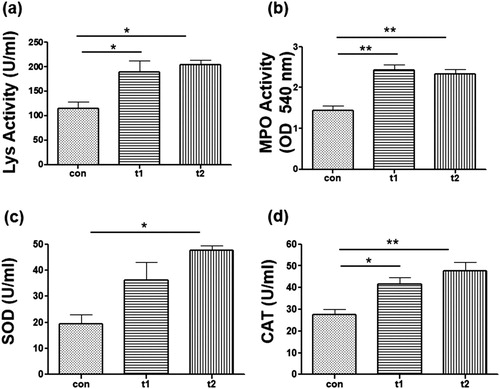
Lys activity in probiotic administered groups was significantly high than control group (con: 115, t1: 189.3, t2: 203.7) ((a)). The probiotic treated groups showed significantly high activities of MPO (con: 1.4, t1: 2.4, t2: 2.3) ((b)). Fish fed with probiotics also showed high activity of SOD (con: 19.3, t1: 36.3, t2: 47.6) ((c)). CAT level was also seen to be higher in probiotic treated groups (con: 27.6, t1: 41.6, t2: 47.6) ((d)).
Figure 6. Immunological parameters. (a) Lysozyme (Lys) activity was significantly high in probiotic supplemented groups (t1 and t2) as as compared to control (con). (b) Myeloperoxidase (MPO) activity was significantly different between con, t1 and t2. (c) Super oxide dismutase (SOD) level waas high in ti and t2 and was significantly high in t2 than con. (d) Catalase (CAT) activity was significantly high in t1 and t2 groups than the con group. Data are the mean ± SEM of three replicates. Asterisks indicate significant differences (* p < .05; ** p < .01) tested by one-way ANOVA analysis with Tukey’s method as post-hoc test.
3.6. Morphology of the monocytes
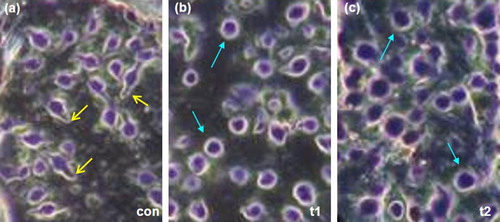
The morphology of the monocytes was studied to see their response to probiotics. It was observed that the cells in the control groups showed phagocytotic morphology characterized by elongated shapes with surface processes ((a)). On the other hand cells in the treated groups t1 and t2 were found to be round in shape and showed less processes ((b,c)).
Figure 7. Representative images of morphology of the monocytes after probiotic treatment. (a) Morphology of the monocytes in the control group. (b) Morphology of the monocytes in the t1 group. (c) Morphology of the monocytes in the t2 group. The control group showed more elongated macrophages bearing processes (highligted by yellow arrows), Monocytes in the probiotic treated groups (t1, t2) showed a round morphology (highlighted by green arrows).
3.7. In vivo challenge study
There was 100% survival in all the three experimental groups injected with salinsolution. However, the RPS of A. Hydrophila challenged fish was high in t1 and t2 treatedfish than the control (). The RPS of fish previously treated with t1 and t2 was 46% and 67%, respectively.
Table 2. Relative Percent Survival of Labeo rohita fed with probiotic containing diet for eight weeks and challenged with Aeromonas Hydrophila (A. hydrophila): Con (control), t1 (Ecotec supplementation 8 × 107 CFU/g feed), t2 (Ecotec supplementation 16 × 107 CFU/g feed), dpi (days post infection).
4. Discussion
Probiotics are considered as eco-friendly, safe alternatives to antibiotics in aquaculture because probiotics not only help in fighting against pathogens but also improve feed utilization, growth and survival of fish (Huynh et al. Citation2017; Hoseinifar et al. Citation2018). Colonization of probiotic bacteria in animals’gut reduces the chance of pathogenic bacterial infections. This confers a health benefit to the organisms with a capacity to defend against various diseases (Asaduzzaman et al. Citation2018; Butt and Volkoff Citation2019). The present study was performed to investigate the role of a commercial probiotic Ecotec on the growth, thyroid hormones, immunological parameters, hemato-morphology and response to pathogens of L. rohita fish. The growth of fish was measuredat the end of the experimental period. Although the length did not show significant differences between groups, the weight of fish in probiotic treated groups was significantly high than control untreated group (). Growth enhancement in fish upon probiotic treatment has been reported by various studies (Taoka et al. Citation2006; Park et al. Citation2016; Adel et al. Citation2017; Mohammadian et al. Citation2019). The increased growth rate in probiotic treated fish can be attributed to better feed untilization and digestibility. El-Haroun et al. (Citation2006) reporeted growth stimulation in Nile tilapia when given a commercial feed additive Biogen which contained B. Subtilis. The mechanism described was the generation of large number of digestive enzymes (lipase, amylase and protease) which had a positive effect on growth. Another probiotic Biogreen has also been reported to cause significantdifferences on specific growth ratio of Cyprinus carpio Frys (Eleraky et al. Citation2014). They found an increase in total fat and protein content in the probiotic treated group which can be due to high feed intake in probiotic treated fish. Thyroid hormones, secreted from the hypothalamic-pituitary-thyroid axis, are responsible for body metablosim, growth, behaviour, immunoregulation and stress reponses in fish (Dorshkind and Horseman Citation2001; Peter Citation2011; Shkil et al. Citation2019). Therefore, variation T3, T4 and TSH levels can be used to monitor essential biological functions of fish. We found a decrease in T3 and T4 levels in the probiotic treated groups (). An optimum level of T3 for proper growth and immune system stimulation is necessary. Depressed growth, lowered immune response and NBT activity has been reported in L.rohita when given a high oral administration of T3 (Sahoo Citation2003). Elevated levels of thyroid hormones can cause ‘hypothyroidism’ in fish producing severe irregularities in nervous and/or metabolic systems (Vancamp et al. Citation2018; Shkil et al. Citation2019). Higher levels of T3/T4 might cause a negative impact on fish specific immune response than the non-specific one resulting in high susceptibility to infections. Therefore, a low or optimum concentration of T3 ensures proper growth rate and immune functioning as was seen in our probiotic treated fish. T3 has been reported to increase the primary antibody response and infammation. More T3 production is therefore, in line with the high production of antibody producing plasma cells-lymphocyte and monocytes in the control group (DLC data). TSH level was increased after probiotic treatment. High levels of TSH in the blood can theoretically be a good marker of thyroid health. Saravanan et al. Citation2010 observed an induction of T3 and low level of TSH in L. rohita after exposure to sublethal concentration of endosulfan. Monitering of TSH levels helps to control thyroid diseases beacuse in the blood levels of TSH can theoretically be considred a good indicator of thyroid health (Soldin et al. Citation2013).
Evaluation of Haematological parameters such as TLC, TEC and Hb are routinely used as biomarker to monitor the physiological and immunological status of fish (Dahiya et al. Citation2012; Sihag and Sharma Citation2012). Variation in LTC in probiotic fed fish was also reported by other researchers (Dahiya et al. Citation2012; Sihag and Sharma Citation2012; Nazir et al. Citation2018). Wenobserved that probiotic treated fish had a low number of leukocytes as compared to the control fish. Number of leukocytes have been reported to rise upon bacterial infections in fish (Amrevuawho et al. Citation2014). A high number of TLCs in the control group thus indicate a stressful condition in the control group. Probiotic treated groups might have helped fish to competitively fight the harmful bacteria more efficiently, thereby causing a low and less demand of leukocytes as depicted in our results. The number of erythrocytes as well as Hb were slightly high in the probiotic treated groups. The increase in TEC and Hb levels in probiotic treated fish explains that there are a pretty good number of oxygen carrying cells and high Hb level in the fish which helps its metabolic activities. Similar results of TEC increase after probiotic treatment in aquaculture have been reported by other studies as well (Irianto and Austin Citation2002; Kumar et al. Citation2006; Reda and Selim Citation2015; Nazir et al. Citation2018). Thus, our TLC, TEC and Hb results suggest a more robust immunological and physiological status of the Ecotec treated groups than the control group.
Lymphocytes and monocytes showed more abundance in the control groups as compared to the Ecotec treated groups (). The high number of lymphocytes and monocytes in the control group may indicate a challenged adaptive immune system and an infection triggered innate immunity, respectively. On the other hand neutrophils and basophils number was higher in the probiotic treated groups. Neutrophils kill pathogens by lytic enzymes and oxidative mechanisms during which several toxic reactive oxygen intermediates are produced (Mölne et al. Citation2000; Jahangiri and Esteban Citation2018). Probiotic triggered increase in the number of neutrophils represents a powerful protective status of fish in probiotic treated groups by which any incoming infection would be attacked more robustly. Basophils which are rapidly activated in lymphoid and peripheral tissues after parasitic infections are a good defence mechanism (Voehringer Citation2009; Hoseinifar et al. Citation2018). Basophils play a role in type 2 immunity (Mitre and Nutman Citation2006; Min et al. Citation2012). The elevated levels of basophils in probiotic treated fish thus indicates the role of probiotics in provoking a protective intrinsic immunity.
Total protein (composed of albumin, globulin, fibrinogen) reflects the physiological condition and innate resistance of an organism. Globulin/immunoglobulins are important defence system of an organism until the components of specific immunity develop. Here, fish supplemented with Ecotec showed higher levels of total protein and globulin. This is in agreement with the previous findings that probiotics enhance the total protein and globulins in tilapia and catla (Zhou et al. Citation2010; Das et al. Citation2013). A/G Ratio decreased in probiotic fed groups. Low A/G ratio is an indicator of better immunological status of fish (Kumar et al. Citation2006).
Ecotec showed imrovement in immunological parameters of L. Rohita in the present study. SOD and CAT are important enzyme of the innate immune system and play important roles in proper immune functioning. It also helps in destroying the super oxide anions from the tissues. CAT also protects cells from the oxidative damage caused by Reactive oxygen species. MPO is another important enzyme of the immune processes and helps the organisms in fighting against infections. Lys is a member of non-specific humoral immunity. It is produced by phagocytic cells (monoctes and neutrophils) and has bactericidal properties. SOD, CAT, MPO and Lys activities enhanced in the present study. SOD and CAT activites have previously been described to increase in rainbow trout, tilapia and carp after treatment with probiotics (Zhou et al. Citation2010; Gupta et al. Citation2016; Park et al. Citation2016). Overall the improvement in immune responses might be considered as a reason for better growth rate in probiotic supplemented groups.
Monocytes have a highly developed phagocytic activity against microbial challenges (Dale et al. Citation2008; Kanwal et al. Citation2014). In the morphological study of monocytes it was observed that the cells in the control group showed a more phagocytic phenotype characterized by their elongated shape and processes (). The phagocytic morphology of the monocytes reflected their active state which also explains for their higher number in the control group (DLC data) than probiotic supplemented groups. More phagocytic activity in the control group denoted regular microbial challenges in the control fish as compared to probiotic treated groups.
A high relative percent survival rate was observed in probiotic administered groups than the control group after A. Hydrophila challenge. These findings corelate with the results of Kumar et al. (Citation2006) who observed that survival rate of L. Rohita was high in B. Subtilis supplemented group. Similar findings of the growth and survival increase in larvae of sea bass have been reported by Tovar-Ramírez et al. (Citation2004) upon supplementation of probiotic (1.1% live yeast). Liu et al. (Citation2013) also reported resistance of Lactobacillus fed juvenile hybrid tilapia to A.hydophila challenge. Enehnaced imminity by probiotics agiants A. Hydrophilica in zebrafish was recently repoted by Lin et al. (Citation2019). Better survival rate to microbial challenges can be attributed to better immunogical status of the probiotic supplemented fish.
5. Conclusion
The results of this study revealed that probiotic, Ecotec led to a positive effect on fish growth, thyroid hormone regulation, hematological, biochemical and immunological indices and survival against pathogens. These results are thus promising in presenting that probiotic supplemented fish has a more healty status as compared to the control untreated fish. Based on these findings probiotics can be presented as a safe alternative to chemotherapeutic agents/antibiotics. This will reduce the consumption of chemotherapeutic agents which can harm animal intestinal microbiota leading to generation of resistant bacterial strains causing several long-term execrable impacts on animal and human health.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Zakia Kanwal http://orcid.org/0000-0002-0539-3056
Additional information
Funding
References
- Adel M , Lazado CC , Safari R , Sakineh Yeganeh S , Zorriehzahra MJ. 2017. Aqualase, a yeast-based in-feed probiotic, modulates intestinal microbiota, immunity and growth of rainbow trout Oncorhynchus mykiss. Aquac Res. 48(4):1815–1826. doi: 10.1111/are.13019
- Alderman D , Hastings T. 1998. Antibiotic use in aquaculture: development of antibiotic resistance–potential for consumer health risks. Int J Food Sci Tech. 33:139–155. doi: 10.1046/j.1365-2621.1998.3320139.x
- Aly SM , Abd-El-Rahman AM , John G , Mohamed MF. 2008. Characterization of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture. 277:1–6. doi: 10.1016/j.aquaculture.2008.02.021
- Amend DF. 1981. Potency testing of fish vaccines. Dev Biol Stand. 49:447–454.
- Amrevuawho OM , Akinyemi AA , Ezeri OGN , Bankole OM , Takeet OV. 2014. Pathological study of Clarias gariepinus (Burchell, 1822) sub-adult artificially infected with Pseudomonas aeruginosa. Braz J Aquat Sci Tech. 18:65–69. doi: 10.14210/bjast.v18n2.p65-69
- Asaduzzaman M , Sofia E , Shakil A , Haque NF , Khan MNA , Ikeda D , Kinoshita S , Abol-Munafi AB. 2018. Host gut-derived probiotic bacteria promote hypertrophic muscle progression and upregulate growth-related gene expression of slow-growing Malaysian Mahseer Tor tambroides. Aquaculture Rep. 9:37–45. doi: 10.1016/j.aqrep.2017.12.001
- Bahmani M , Kazemi R , Donskaya P. 2001. A comparative study of some hematological features in young reared sturgeons (Acipenser persicus and Huso huso). Fish Physiol Biochem. 24:135–140. doi: 10.1023/A:1011911019155
- Butt RL , Volkoff H. 2019. Gut microbiota and energy homeostasis in fish. Front Endocrinol. 10:9. doi: 10.3389/fendo.2019.00009
- Dahiya T , Sihag RC , Gahlawat SK. 2012. Effect of probiotics on the haematological parameters of Indian Magur (Clarius batrachus L.). J Fish Aquat Sci. 7:279–290. doi: 10.3923/jfas.2012.279.290
- Dale DC , Boxer L , Liles WC. 2008. The phagocytes: neutrophils and monocytes. Blood. 112:935–945. doi: 10.1182/blood-2007-12-077917
- Das A , Nakhro K , Chowdhury S , Kamilya D. 2013. Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (catla catla). Fish Shellfish Immunol. 35:1547–1553. doi: 10.1016/j.fsi.2013.08.022
- Dorshkind K , Horseman ND. 2001. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays. 23:288–294. doi: 10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P
- El-Haroun ER , Goda AM , Kabir CMA. 2006. Effect of dietary probiotic biogen supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquacult Res. 37(14):1473–1480. doi: 10.1111/j.1365-2109.2006.01584.x
- Eleraky W , Yahya M , Rasha MR , Eletreby S. 2014. Evaluation of prebiotic and probiotic dietary supplementation on growth performance and some blood parameters of Cyprinus carpio Frys. Egypt J Aquat Biol Fish. 18(2):29–38.
- Gatesoupe F. 1999. The use of probiotics in aquaculture. Aquaculture. 180:147–165. doi: 10.1016/S0044-8486(99)00187-8
- Gomez-Gil B , Roque A , Turnbull JF. 2000. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture. 191:259–270. doi: 10.1016/S0044-8486(00)00431-2
- Gupta A , Gupta P , Dhawan A. 2016. Paenibacillus polymyxa as a water additive improved immuneresponse of Cyprinus carpio and disease resistance against Aeromonas hydrophila. Aquaculture Reports. 4:86–92. doi: 10.1016/j.aqrep.2016.07.002
- Hoseinifar SH , Sun Y-Z , Wang A , Zhou Z. 2018. Probiotics as Means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol. 9:2429. doi: 10.3389/fmicb.2018.02429
- Huynh TG , Shiu YL , Nguyen TP , Truong QP , Chen JC , Liu CH. 2017. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunol. 64:367–382. doi: 10.1016/j.fsi.2017.03.035
- Irianto A , Austin B. 2002. Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. 25:333–342. doi: 10.1046/j.1365-2761.2002.00375.x
- Isidro RA , Bonilla FJ , Pagan H , Cruz ML , Lopez P , Godoy L , Hernandez S , Loucil-Alicea RY , Rivera-Amill V , Yamamura Y , et al. 2014. The probiotic mixture VSL# 3 alters the morphology and secretion profile of both polarized and unpolarized human macrophages in a polarization-dependent manner. J Clin Cell Immunol. 5:1000227.
- Jahangiri L , Esteban MA. 2018. Administration of probiotics in the water in Finfish aquaculture systems: A Review. Fishes. 3(3):33. doi: 10.3390/fishes3030033
- Kanwal Z , Wiegertjes GF , Veneman WJ , Meijer AH , Spaink HP. 2014. Comparative studies of Toll-like receptor signalling using zebrafish. Dev Comp Immunol. 46:35–52. doi: 10.1016/j.dci.2014.02.003
- Kumar R , Mukherjee SC , Prasad KP , Pal AK. 2006. Evaluation of Bacillus subtilis as a probiotic to Indian major carp Labeo rohita (Ham.). Aquac Res. 37:1215–1221. doi: 10.1111/j.1365-2109.2006.01551.x
- Lin Y-S , Saputra F , Chen Y-C , Hu S-Y. 2019. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol. 86:410–419. doi: 10.1016/j.fsi.2018.11.047
- Liu W , Ren P , He S , Xu L , Yang Y , Gu Z , et al. 2013. Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish Shellfish Immun. 35:54–62. doi: 10.1016/j.fsi.2013.04.010
- Luu NT , Madden J , Calder PC , Grimble RF , Shearman CP , Chan T , Dastur N , Howell WM , Rainger GE , Nash GB. 2007. Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J Nutr. 137:2769–2774. doi: 10.1093/jn/137.12.2769
- Min B , Brown MA , LeGros G. 2012. Understanding the roles of basophils: breaking Dawn. Immunology. 135:192–197. doi: 10.1111/j.1365-2567.2011.03530.x
- Mitre E , Nutman T. 2006. Basophils, basophilia and helminth infections. Chem Immunol Allergy. 90:141–156.
- Mohammadian T , Nasirpour M , Tabandeh MR , Heidary AA , Ghanei-Motlagh R , Hosseini SS. 2019. Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol. 86:269–279. doi: 10.1016/j.fsi.2018.11.052
- Mölne L , Verdrengh M , Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 68:6162–6167. doi: 10.1128/IAI.68.11.6162-6167.2000
- Nazir I , Chauhan RS , Arya P. 2018. Evaluation of immunostimulatory effect of feed probiotic biosyn on Labeo rohita. J Pharmacognosy Phytochem 2018. 7(1):2611–2614.
- Park Y , Lee S , Hong J , Kim D , Moniruzzaman M , Bai S. 2016. Use of probiotics to enhance growth, stimulate immunity and confer disease resistance to Aeromonas salmonicida in rainbow trout (Oncorhynchus mykiss). Aquac Res. 48(6):2672–2682. doi: 10.1111/are.13099
- Peter MC. 2011. The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol. 172:198–210. doi: 10.1016/j.ygcen.2011.02.023
- Pirarat N , Kobayashi T , Katagiri T , Maita M , Endo M. 2006. Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunop. 113:339–347. doi: 10.1016/j.vetimm.2006.06.003
- Quade MJ , Roth JA. 1997. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol. 58:239–248. doi: 10.1016/S0165-2427(97)00048-2
- Reda RM , Selim KM. 2015. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquacult Int. 23:203–217. doi: 10.1007/s10499-014-9809-z
- Ridha MT , Azad IS. 2016. Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquac Res. 47:2757–2767. doi: 10.1111/are.12726
- Rieger J , Janczyk P , Hünigen H , Neumann K , Plendl J. 2015. Intraepithelial lymphocyte numbers and histomorphological parameters in the porcine gut after Enterococcus faecium NCIMB 10415 feeding in a Salmonella Typhimurium challenge. Vet Immunol Immunop. 164:40–50. doi: 10.1016/j.vetimm.2014.12.013
- Sahoo K. 2003. Immunostimulating effect of triiodothyronine: dietary administration of triiodothyronine in rohu (Labeo rohita) enhances immunity and resistance to Aeromonas hydrophila infection. J Appl Ichthyol. 19:118–122. doi: 10.1046/j.1439-0426.2003.00349.x
- Sankaran K , Gurnani S. 1972. On the variation in the catalytic activity of lysozyme in fishes. Indian J Biochem Biophys. 9:162–165.
- Sapkota A , Sapkota AR , Kucharski M , Burke J , McKenzie S , Walker P , Lawrence R. 2008. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int. 34:1215–1226. doi: 10.1016/j.envint.2008.04.009
- Saravanan TS , Rajesh P , Sundaramoorthy M. 2010. Studies on effects of chronic exposure of endosulfan to Labeo rohita. J Environ Biol. 31:755–758.
- Satheeshkumar P , Ananthan G , Kumar DS , Jagadeesan L. 2012. Haematology and biochemical parameters of different feeding behaviour of teleost fishes from Vellar estuary, India. Comp Clin Pathol. 21:1187–1191. doi: 10.1007/s00580-011-1259-7
- Shah AW , Parveen M , Mir SH , Sarwar S , Yousuf A. 2009. Impact of helminth parasitism on fish haematology of Anchar Lake. Kashmir Pak J Nutr. 8:42–45. doi: 10.3923/pjn.2009.42.45
- Shkil F , Siomava N , Voronezhskay E , Diogo R. 2019. Effects of hyperthyroidism in the development of the appendicular skeleton and muscles of zebrafish, with notes on evolutionary developmental pathology (Evo-Devo-Path). Sci Rep. 9:5413. doi: 10.1038/s41598-019-41912-9
- Sihag RC , Sharma P. 2012. Probiotics: the new ecofriendly alternative measures of disease control for sustainable aquaculture. J Fish Aquat Sci. 7:72–103. doi: 10.3923/jfas.2012.72.103
- Soldin OP , Chung SH , Colie CC. 2013. The use of TSH in determining thyroid disease: how does it impact the practice of medicine in pregnancy? J Thyroid Res. 2013:148157. doi: 10.1155/2013/148157
- Taoka Y , Maeda H , Jo JY , Jeon MN , Bai SC , Lee WJ , Yuge K , Koshio S. 2006. Growth, stress tolerance and non-specific immune response of Japanese flounder, Paralichthys olivaceus to probiotics in a closed recirculating system. Fish Sci. 72:310–321. doi: 10.1111/j.1444-2906.2006.01152.x
- Tovar-Ramírez D , Zambonino-Infante J , Cahu C , Gatesoupe FJ , Vázque-Juárwz R. 2004. Influence of dietary live yeast on European sea bass (Dicentrarchus labrax) larval development. Aquacult. 234:415–427. doi: 10.1016/j.aquaculture.2004.01.028
- Vancamp P , Houbrechts AM , Darras VM. 2018. Insights from zebrafsh defciency models to understand the impact of local thyroid hormone regulator action on early development. Gen Comp Endocrinol. doi:10.1016/j.ygcen.2018.09.011.
- Verschuere L , Rombaut G , Sorgeloos P , Verstraete W. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 64:655–671. doi: 10.1128/MMBR.64.4.655-671.2000
- Voehringer D. 2009. The role of basophils in helminth infection. Trends Parasitol. 25:551–556. doi: 10.1016/j.pt.2009.09.004
- Witte W , Klare I , Werner G. 1999. Selective pressure by antibiotics as feed additives. Infection. 27:S35–S38. doi: 10.1007/BF02561669
- Yamada Y , Endou M , Morikawa S , Shima J , Komatshzaki N. 2018. Lactic acid bacteria isolated from Japanese Fermented fish (Funa-Sushi) inhibit Mesangial proliferative glomerulonephritis by alcohol intake with stress. J Nutr Metab. 2018:8. doi: 10.1155/2018/6491907
- Zhou X , Tian Z , Wang Y , Li W. 2010. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem. 36:501–509. doi: 10.1007/s10695-009-9320-z
